Deck 8: Periodic Properties of the Elements
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/120
Play
Full screen (f)
Deck 8: Periodic Properties of the Elements
1
Choose the orbital diagram that represents the ground state of N.
A)
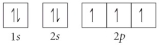
B)
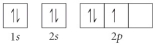
C)
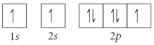
D)

E)

A)

B)

C)

D)

E)


2
Give the ground state electron configuration for Cd.
A)[Kr] 5s2 5d10
B)[Kr] 5s2 4d10 5p2
C)[Kr] 4d10
D)[Kr] 5s2 4d8
E)[Kr] 5s2 4d10
A)[Kr] 5s2 5d10
B)[Kr] 5s2 4d10 5p2
C)[Kr] 4d10
D)[Kr] 5s2 4d8
E)[Kr] 5s2 4d10
[Kr] 5s2 4d10
3
Give the ground state electron configuration for Pb.
A)[Xe] 6s2 6p2
B)[Xe] 6s2 5d10 6p2
C)[Xe] 6s2 5f14 6d10 6p2
D)[Xe] 6s2 4f14 5d10 6p2
E)[Xe] 6s2 5f14 5d10 6p2
A)[Xe] 6s2 6p2
B)[Xe] 6s2 5d10 6p2
C)[Xe] 6s2 5f14 6d10 6p2
D)[Xe] 6s2 4f14 5d10 6p2
E)[Xe] 6s2 5f14 5d10 6p2
[Xe] 6s2 4f14 5d10 6p2
4
Choose the valence orbital diagram that represents the Si.
A)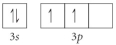
B)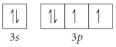
C)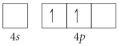
D)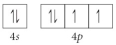
E)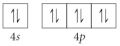
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
5
Choose the valence orbital diagram that represents the ground state of Zn.
A)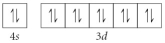
B)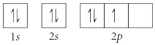
C)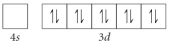
D)
E)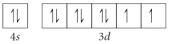
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
6
How many unpaired electrons are present in the ground state Ge atom?
A)0
B)3
C)1
D)2
E)4
A)0
B)3
C)1
D)2
E)4

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
7
Write out the orbital diagram that represents the ground state of As.How many unpaired electrons are there?
A)0
B)4
C)3
D)2
E)1
A)0
B)4
C)3
D)2
E)1

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
8
Give the ground state electron configuration for Sr.
A)[Kr] 5s2 4d2
B)[Kr] 5s2 4d10 5p2
C)[Kr] 5s2
D)[Kr] 5s2 5d10 5p2
E)[Kr] 5s2 4d10
A)[Kr] 5s2 4d2
B)[Kr] 5s2 4d10 5p2
C)[Kr] 5s2
D)[Kr] 5s2 5d10 5p2
E)[Kr] 5s2 4d10

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
9
How many unpaired electrons are present in the ground state P atom?
A)0
B)3
C)1
D)2
E)4
A)0
B)3
C)1
D)2
E)4

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
10
Give the ground state electron configuration for I.
A)[Kr] 5s2 4d10 5p6
B)[Kr] 5s2 4d10 5p5
C)[Kr] 4d10 5p6
D)[Kr] 5s2 5p6
E)[Kr] 5s2 5d10 5p6
A)[Kr] 5s2 4d10 5p6
B)[Kr] 5s2 4d10 5p5
C)[Kr] 4d10 5p6
D)[Kr] 5s2 5p6
E)[Kr] 5s2 5d10 5p6

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
11
Which of the following statements is true?
A)An orbital that penetrates into the region occupied by core electrons is less shielded from nuclear charge than an orbital that does not penetrate and therefore has a lower energy.
B)An orbital that penetrates into the region occupied by core electrons is more shielded from nuclear charge than an orbital that does not penetrate and therefore has a lower energy.
C)It is possible for two electrons in the same atom to have identical values for all four quantum numbers.
D)Two electrons in the same orbital can have the same spin.
E)None of the above are true.
A)An orbital that penetrates into the region occupied by core electrons is less shielded from nuclear charge than an orbital that does not penetrate and therefore has a lower energy.
B)An orbital that penetrates into the region occupied by core electrons is more shielded from nuclear charge than an orbital that does not penetrate and therefore has a lower energy.
C)It is possible for two electrons in the same atom to have identical values for all four quantum numbers.
D)Two electrons in the same orbital can have the same spin.
E)None of the above are true.

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
12
When filling degenerate orbitals,electrons fill them singly at first,with parallel spins.This is know as
A)Aufbau principle.
B)Heisenberg uncertainty principle.
C)Hund's rule.
D)Pauli exclusion principle.
A)Aufbau principle.
B)Heisenberg uncertainty principle.
C)Hund's rule.
D)Pauli exclusion principle.

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
13
Give the set of four quantum numbers that could represent the last electron added (using the Aufbau principle)to the Cl atom.
A)n = 3,l = 1,ml = 1,ms = +
B)n = 3,l = 0,ml = 1,ms = -
C)n = 3,l = 2,ml =1 ,ms = +
D)n = 2,l = 1,ml = 1,ms = -
E)n = 3,l =2 ,ml = 1,ms = -
A)n = 3,l = 1,ml = 1,ms = +

B)n = 3,l = 0,ml = 1,ms = -

C)n = 3,l = 2,ml =1 ,ms = +

D)n = 2,l = 1,ml = 1,ms = -

E)n = 3,l =2 ,ml = 1,ms = -


Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
14
Identify the element that has a ground state electronic configuration of [Ar] 4s2 3d10 4p1.
A)Al
B)In
C)Ga
D)B
A)Al
B)In
C)Ga
D)B

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
15
No two electrons can have the same four quantum number is known as
A)Pauli exclusion principle.
B)Hund's rule.
C)Aufbau principle.
D)Heisenberg uncertainty principle.
A)Pauli exclusion principle.
B)Hund's rule.
C)Aufbau principle.
D)Heisenberg uncertainty principle.

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
16
Give the set of four quantum numbers that represent the last electron added (using the Aufbau principle)to the Zn atom.
A)n = 4,l = 3,ml = 3,ms = -
B)n = 3,l = 2,ml = 2,ms = -
C)n = 3,l = 1,ml = 1,ms = +
D)n = 3,l = 3,ml = 2,ms = -
E)n = 4,l = 2,ml = 0,ms = +
A)n = 4,l = 3,ml = 3,ms = -

B)n = 3,l = 2,ml = 2,ms = -

C)n = 3,l = 1,ml = 1,ms = +

D)n = 3,l = 3,ml = 2,ms = -

E)n = 4,l = 2,ml = 0,ms = +


Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
17
Identify the element that has a ground state electronic configuration of [Kr] 5s2 4d5.
A)Tc
B)Mn
C)Nb
D)Ru
A)Tc
B)Mn
C)Nb
D)Ru

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
18
Give the set of four quantum numbers that could represent the last electron added (using the Aufbau principle)to the Sr atom.
A)n = 5,l = 0,ml = 0,ms = -
B)n = 4,l = 1,ml = 1,ms = -
C)n = 5,l = 1,ml = 0,ms = +
D)n = 4,l = 1,ml = -1,ms = +
E)n = 5,l = 1,ml =1 ,ms = -
A)n = 5,l = 0,ml = 0,ms = -

B)n = 4,l = 1,ml = 1,ms = -

C)n = 5,l = 1,ml = 0,ms = +

D)n = 4,l = 1,ml = -1,ms = +

E)n = 5,l = 1,ml =1 ,ms = -


Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
19
How many unpaired electrons are present in the ground state Kr atom?
A)1
B)2
C)0
D)3
E)5
A)1
B)2
C)0
D)3
E)5

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
20
Give the ground state electron configuration for Se.
A)[Ar] 4s2 3d10 4p4
B)[Ar] 4s2 4d10 4p4
C)[Ar] 4s2 3d10 4p6
D)[Ar] 4s2 3d10
E)[Ar] 3d10 4p4
A)[Ar] 4s2 3d10 4p4
B)[Ar] 4s2 4d10 4p4
C)[Ar] 4s2 3d10 4p6
D)[Ar] 4s2 3d10
E)[Ar] 3d10 4p4

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
21
How many of the following elements have 2 unpaired electrons in the ground state?
C O Ti Si
A)1
B)2
C)3
D)4
C O Ti Si
A)1
B)2
C)3
D)4

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
22
Choose the statement that is true.
A)Outer electrons efficiently shield one another from nuclear charge.
B)Core electrons effectively shield outer electrons from nuclear charge.
C)Valence electrons are most difficult of all electrons to remove.
D)Core electrons are the easiest of all electrons to remove.
E)All of the above are true.
A)Outer electrons efficiently shield one another from nuclear charge.
B)Core electrons effectively shield outer electrons from nuclear charge.
C)Valence electrons are most difficult of all electrons to remove.
D)Core electrons are the easiest of all electrons to remove.
E)All of the above are true.

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
23
How many valence electrons does an atom of Ti possess?
A)2
B)4
C)6
D)8
E)0
A)2
B)4
C)6
D)8
E)0

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
24
Give the complete electronic configuration for Mn.
A)1s2 2s2 2p6 3s2 3p6 4s2 4d5
B)1s2 2s2 2p6 3s2 3p6 4s1 3d6
C)1s2 2s2 2p6 3s2 3p6 4s2 3d5
D)1s2 2s2 2p6 3s2 3p6 4s2 4p5
A)1s2 2s2 2p6 3s2 3p6 4s2 4d5
B)1s2 2s2 2p6 3s2 3p6 4s1 3d6
C)1s2 2s2 2p6 3s2 3p6 4s2 3d5
D)1s2 2s2 2p6 3s2 3p6 4s2 4p5

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
25
Place the following in order of increasing radius.
Br⁻ Na+ Rb+
A)Br⁻ < Rb⁺ < Na⁺
B)Na⁺ < Br⁻ < Rb+
C)Rb⁺ < Br⁻ < Na⁺
D)Br⁻ < Na⁺ < Rb⁺
E)Rb⁺ < Na⁺ < Br⁻
Br⁻ Na+ Rb+
A)Br⁻ < Rb⁺ < Na⁺
B)Na⁺ < Br⁻ < Rb+
C)Rb⁺ < Br⁻ < Na⁺
D)Br⁻ < Na⁺ < Rb⁺
E)Rb⁺ < Na⁺ < Br⁻

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
26
Predict the charge for the most stable ion of nitrogen.
A)-3
B)-2
C)1
D)0
E)+3
A)-3
B)-2
C)1
D)0
E)+3

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
27
Place the following elements in order of decreasing atomic radius. Xe Rb Ar
A)Ar > Xe > Rb
B)Xe > Rb > Ar
C)Ar > Rb > Xe
D)Rb > Xe > Ar
E)Rb > Ar > Xe
A)Ar > Xe > Rb
B)Xe > Rb > Ar
C)Ar > Rb > Xe
D)Rb > Xe > Ar
E)Rb > Ar > Xe

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
28
Place the following elements in order of increasing atomic radius.
P Ba Cl
A)Ba < P < Cl
B)P < Cl < Ba
C)Cl < P < Ba
D)Cl < Ba < P
E)Ba < Cl < P
P Ba Cl
A)Ba < P < Cl
B)P < Cl < Ba
C)Cl < P < Ba
D)Cl < Ba < P
E)Ba < Cl < P

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
29
How many valence electrons do the alkali metals possess?
A)1
B)2
C)7
D)6
E)8
A)1
B)2
C)7
D)6
E)8

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
30
Identify the number of valence electrons for Mg.
A)8
B)7
C)5
D)2
A)8
B)7
C)5
D)2

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
31
Place the following in order of decreasing radius.
Te2⁻ F⁻ O2⁻
A)F⁻ > O2⁻ > Te2⁻
B)F⁻ > Te2⁻ > O2⁻
C)Te2⁻ > O2⁻ > F⁻
D)Te2⁻ > F⁻ > O2⁻
E)O2⁻ > F⁻ > Te2⁻
Te2⁻ F⁻ O2⁻
A)F⁻ > O2⁻ > Te2⁻
B)F⁻ > Te2⁻ > O2⁻
C)Te2⁻ > O2⁻ > F⁻
D)Te2⁻ > F⁻ > O2⁻
E)O2⁻ > F⁻ > Te2⁻

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
32
How many valence electrons does an atom of S have?
A)3
B)1
C)2
D)4
E)6
A)3
B)1
C)2
D)4
E)6

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
33
Place the following in order of increasing atomic radius.
As O Br
A)As < Br < O
B)O < As < Br
C)Br < As < O
D)As < O < Br
E)O < Br < As
As O Br
A)As < Br < O
B)O < As < Br
C)Br < As < O
D)As < O < Br
E)O < Br < As

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
34
How many valence electrons does an atom of C have?
A)1
B)4
C)2
D)3
A)1
B)4
C)2
D)3

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
35
How many valence electrons does an atom of Ba possess?
A)2
B)1
C)8
D)6
E)3
A)2
B)1
C)8
D)6
E)3

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
36
How many valence electrons does an atom of Cu possess?
A)2
B)9
C)11
D)3
E)1
A)2
B)9
C)11
D)3
E)1

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
37
Place the following in order of increasing radius.
Ca2+ S2⁻ Cl⁻
A)Ca2⁺ < Cl⁻ < S2-
B)Cl⁻ < Ca2⁺ < S2⁻
C)S2⁻ < Cl⁻ < Ca2⁺
D)Ca2⁺ < S2⁻ < Cl⁻
E)Cl⁻ < S2⁻ < Ca2⁺
Ca2+ S2⁻ Cl⁻
A)Ca2⁺ < Cl⁻ < S2-
B)Cl⁻ < Ca2⁺ < S2⁻
C)S2⁻ < Cl⁻ < Ca2⁺
D)Ca2⁺ < S2⁻ < Cl⁻
E)Cl⁻ < S2⁻ < Ca2⁺

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
38
How many valence electrons does an atom of Al possess?
A)1
B)2
C)5
D)3
E)8
A)1
B)2
C)5
D)3
E)8

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
39
How many valence electrons do the halogens possess?
A)5
B)6
C)2
D)1
E)7
A)5
B)6
C)2
D)1
E)7

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
40
How many of the following elements have 1 unpaired electron in the ground state?
B Al S Cl
A)1
B)2
C)3
D)4
B Al S Cl
A)1
B)2
C)3
D)4

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
41
Choose the ground state electron configuration for Ti2+.
A)[Ar] 3d2
B)[Ar] 4s2
C)[Ar] 4s2 3d2
D)[Ar] 4s2 3d4
E)[Ar] 3d4
A)[Ar] 3d2
B)[Ar] 4s2
C)[Ar] 4s2 3d2
D)[Ar] 4s2 3d4
E)[Ar] 3d4

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
42
Give the ground state electron configuration for the ion of Ba.
A)[Kr] 5s2 5p6
B)[Kr] 5s2 4d10 5p6 6s2 6p2
C)[Kr] 5s2 4d10 5p6 6s1
D)[Kr] 5s2 4d10 5p6 6s2
E)[Kr] 5s2 4d10 5p6
A)[Kr] 5s2 5p6
B)[Kr] 5s2 4d10 5p6 6s2 6p2
C)[Kr] 5s2 4d10 5p6 6s1
D)[Kr] 5s2 4d10 5p6 6s2
E)[Kr] 5s2 4d10 5p6

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
43
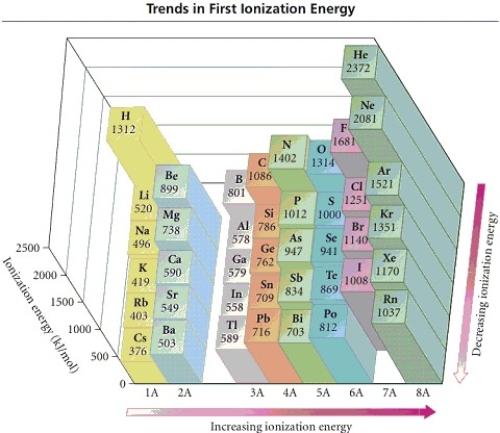
Refer to the figure.Place the following in order of decreasing IE1.
Cs Mg Ar
A)Cs > Mg > Ar
B)Mg > Ar > Cs
C)Ar > Mg > Cs
D)Cs > Ar > Mg
E)Mg > Cs > Ar

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
44
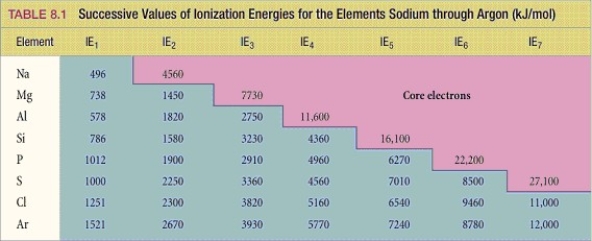
What period 3 element has the following ionization energies (all in kJ/mol)?
IE1 = 1012 IE2 = 1900 IE3= 2910 IE4= 4960 IE5= 6270 IE6 = 22,200
A)Si
B)S
C)P
D)Cl
E)Mg

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
45
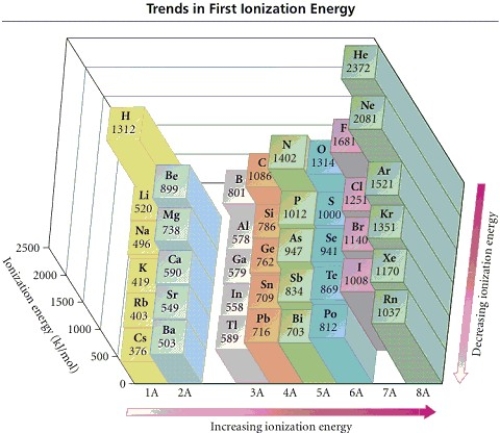
Refer to the figure.Place the following in order of increasing IE1.
N F As
A)N < As < F
B)As < N < F
C)F < N < As
D)As < F < N
E)F < AS < N

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
46
How many of the following species are diamagnetic?
Cs Zr2+ Al3+ Hg2+
A)1
B)3
C)0
D)2
E)4
Cs Zr2+ Al3+ Hg2+
A)1
B)3
C)0
D)2
E)4

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
47
Choose the paramagnetic species from below.
A)Ca
B)O2⁻
C)Cd2⁺
D)Zn
E)Nb3⁺
A)Ca
B)O2⁻
C)Cd2⁺
D)Zn
E)Nb3⁺

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
48
Choose the ground state electron configuration for Zn2+.
A)[Ar] 4s2 3d8
B)[Ar] 3d10
C)[Ar] 4s2 3d6
D)[Ar]
E)[Ar] 3d8
A)[Ar] 4s2 3d8
B)[Ar] 3d10
C)[Ar] 4s2 3d6
D)[Ar]
E)[Ar] 3d8

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
49
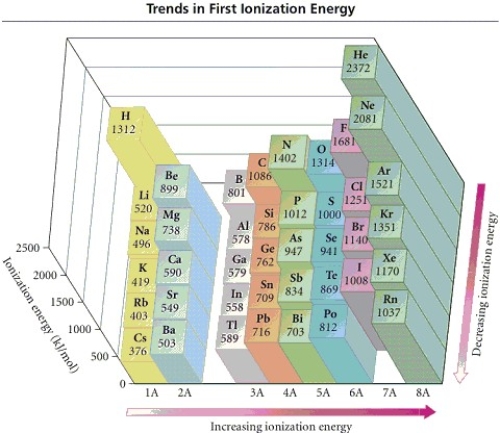
Refer to the figure.Place the following in order of increasing IE1.
K Ca Rb
A)Ca < K < Rb
B)Rb < Ca < K
C)Ca < Rb < K
D)Rb < K < Ca
E)K < Ca < Rb

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
50
Give the ground state electron configuration for Mg2+.
A)1s2 2s2 2p6 3s2
B)1s2 2s2 2p6
C)1s2 2s2 2p6 3s2 3p2
D)1s2 2s2 2p6 3s2 3p6
E)1s2 2s2 2p6 3s1
A)1s2 2s2 2p6 3s2
B)1s2 2s2 2p6
C)1s2 2s2 2p6 3s2 3p2
D)1s2 2s2 2p6 3s2 3p6
E)1s2 2s2 2p6 3s1

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
51
Choose the ground state electron configuration for Zr2+.
A)[Kr] 5s2
B)[Kr] 5s2 4d2
C)[Kr] 4d2
D)[Kr]
E)[Kr] 5s2 4d4
A)[Kr] 5s2
B)[Kr] 5s2 4d2
C)[Kr] 4d2
D)[Kr]
E)[Kr] 5s2 4d4

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
52
How many of the following species are paramagnetic?
Sc3+ Br⁻ Mg2+ Se
A)0
B)2
C)1
D)4
E)3
Sc3+ Br⁻ Mg2+ Se
A)0
B)2
C)1
D)4
E)3

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
53
Which reaction below represents the second ionization of Sr?
A)Sr(g)→ Sr⁺(g)+ e⁻
B)Sr2⁺(g)+ e⁻ → Sr⁺(g)
C)Sr⁺(g)+ e⁻ → Sr(g)
D)Sr⁻(g)+ e⁻ → Sr2⁻(g)
E)Sr⁺(g)→ Sr2⁺(g)+ e⁻
A)Sr(g)→ Sr⁺(g)+ e⁻
B)Sr2⁺(g)+ e⁻ → Sr⁺(g)
C)Sr⁺(g)+ e⁻ → Sr(g)
D)Sr⁻(g)+ e⁻ → Sr2⁻(g)
E)Sr⁺(g)→ Sr2⁺(g)+ e⁻

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
54
Give the ground state electron configuration for Se2⁻.
A)[Ar] 4s2 3d10 4p4
B)[Ar] 4s2 3d10 4p2
C)[Ar] 4s2 4p6
D)[Ar] 4s2 3d10 4p6
E)[Ar] 4s2 3d8 4p6
A)[Ar] 4s2 3d10 4p4
B)[Ar] 4s2 3d10 4p2
C)[Ar] 4s2 4p6
D)[Ar] 4s2 3d10 4p6
E)[Ar] 4s2 3d8 4p6

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
55
Place the following in order of decreasing metallic character.
P As K
A)P > As > K
B)As > P > K
C)K > P > As
D)As > K > P
E)K > As > P
P As K
A)P > As > K
B)As > P > K
C)K > P > As
D)As > K > P
E)K > As > P

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
56
Choose the paramagnetic species from below.
A)Ti4+
B)O
C)Ar
D)All of the above are paramagnetic.
E)None of the above are paramagnetic.
A)Ti4+
B)O
C)Ar
D)All of the above are paramagnetic.
E)None of the above are paramagnetic.

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
57
Which reaction below represents the first ionization of O?
A)O⁺(g)+ e⁻ → O(g)
B)O(g)+ e⁻ → O⁻(g)
C)O⁻(g)→ O(g)+ e⁻
D)O(g)→ O⁺(g)+ e⁻
E)O⁻(g)+ e⁻ → O2⁻(g)
A)O⁺(g)+ e⁻ → O(g)
B)O(g)+ e⁻ → O⁻(g)
C)O⁻(g)→ O(g)+ e⁻
D)O(g)→ O⁺(g)+ e⁻
E)O⁻(g)+ e⁻ → O2⁻(g)

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
58
Choose the diamagnetic species from below.
A)Sn2⁺
B)Br
C)P
D)Cr
E)None of the above are diamagnetic.
A)Sn2⁺
B)Br
C)P
D)Cr
E)None of the above are diamagnetic.

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
59
Give the ground state electron configuration for Br⁻.
A)[Ar] 4s2 3d10 4p6
B)[Ar] 4s2 3d10 4p5
C)[Ar] 4s2 4p6
D)[Ar] 4s2 4d10 4p6
E)[Ar] 4s2 3d10 4p4
A)[Ar] 4s2 3d10 4p6
B)[Ar] 4s2 3d10 4p5
C)[Ar] 4s2 4p6
D)[Ar] 4s2 4d10 4p6
E)[Ar] 4s2 3d10 4p4

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
60
Give the ground state electron configuration for Rb+.
A)[Ar ]4s2 4p6
B)[Kr] 5s1
C)[Ar] 4s2 3d10 4p6
D)[Kr] 5s2
E)[Kr] 5s2 4d2
A)[Ar ]4s2 4p6
B)[Kr] 5s1
C)[Ar] 4s2 3d10 4p6
D)[Kr] 5s2
E)[Kr] 5s2 4d2

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
61
Match the following.
-number of unpaired electrons in Cr2+
A)0
B)2
C)electrons in the outermost shell
D)1
E)electrons in completed shells
-number of unpaired electrons in Cr2+
A)0
B)2
C)electrons in the outermost shell
D)1
E)electrons in completed shells

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
62
Match the following.
number of unpaired electrons in Na
A)0
B)2
C)electrons in the outermost shell
D)1
E)electrons in completed shells
number of unpaired electrons in Na
A)0
B)2
C)electrons in the outermost shell
D)1
E)electrons in completed shells

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
63
Choose the valence orbital diagram that represents the ground state of Zr.
A)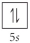
B)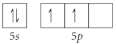
C)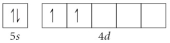
D)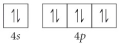
E)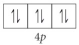
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
64
Why does the size of the transition elements stay roughly the same as you move across a period?

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
65
Match the following.
core electrons
A)0
B)2
C)electrons in the outermost shell
D)1
E)electrons in completed shells
core electrons
A)0
B)2
C)electrons in the outermost shell
D)1
E)electrons in completed shells

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
66
Which reaction below represents the second electron affinity of S?
A)S(g)+ e⁻ → S⁻(g)
B)S⁻(g)+ e⁻ → S2⁻(g)
C)S(g)→ S⁺(g)+ e⁻
D)S⁻(g)→ S(g)+ e⁻
E)S2⁻(g)→ S⁻(g)+ e⁻
A)S(g)+ e⁻ → S⁻(g)
B)S⁻(g)+ e⁻ → S2⁻(g)
C)S(g)→ S⁺(g)+ e⁻
D)S⁻(g)→ S(g)+ e⁻
E)S2⁻(g)→ S⁻(g)+ e⁻

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
67
Choose the valence orbital diagram that represents the ground state of Se2⁻.
A)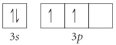
B)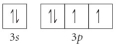
C)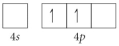
D)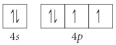
E)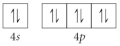
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
68
Choose the ground state electron configuration for Cr3+.
A)[Ar] 4s1 3d2
B)[Ar]
C)[Ar] 4s2 3d6
D)[Ar] 3d3
E)[Ar] 4s2 3d1
A)[Ar] 4s1 3d2
B)[Ar]
C)[Ar] 4s2 3d6
D)[Ar] 3d3
E)[Ar] 4s2 3d1

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
69
Give the set of four quantum numbers that could represent the electron lost to form the K+ ion from the K atom.
A)n = 3,l = 1,ml = 1,ms = -
B)n = 4,l = 1,ml = 1,ms = +
C)n = 4,l = 4,ml = 0,ms = -
D)n = 4,l = 0,ml = 0,ms = +
E)n = 3,l = 0,ml = 1,ms = +
A)n = 3,l = 1,ml = 1,ms = -

B)n = 4,l = 1,ml = 1,ms = +

C)n = 4,l = 4,ml = 0,ms = -

D)n = 4,l = 0,ml = 0,ms = +

E)n = 3,l = 0,ml = 1,ms = +


Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
70
Place the following in order of increasing metallic character.
Br Cs Se
A)Br < Se < Cs
B)Se < Br < Cs
C)Cs < Br < Se
D)Cs < Se < Br
E)Br < Cs < Se
Br Cs Se
A)Br < Se < Cs
B)Se < Br < Cs
C)Cs < Br < Se
D)Cs < Se < Br
E)Br < Cs < Se

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
71
Give the set of four quantum numbers that could represent the electron gained to form the Br- ion from the Br atom.
A)n = 4,l = 2,ml = 1,ms = -
B)n = 4,l = 0,ml = 1,ms = +
C)n = 4,l = 1,ml = 1,ms = -
D)n = 3,l = 2,ml = 2,ms = +
E)n = 5,l = 1,ml = -1 ,ms = +
A)n = 4,l = 2,ml = 1,ms = -

B)n = 4,l = 0,ml = 1,ms = +

C)n = 4,l = 1,ml = 1,ms = -

D)n = 3,l = 2,ml = 2,ms = +

E)n = 5,l = 1,ml = -1 ,ms = +


Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
72
Give the set of four quantum numbers that could represent the electron lost to form the Rb+ ion from the Rb atom.
A)n = 6,l = 0,ml = 0,ms = -
B)n = 4,l = 1,ml = 1,ms = -
C)n = 5,l = 1,ml = 0,ms = +
D)n = 4,l = 1,ml = 0,ms = -
E)n = 5,l = 0,ml = 0,ms = +
A)n = 6,l = 0,ml = 0,ms = -

B)n = 4,l = 1,ml = 1,ms = -

C)n = 5,l = 1,ml = 0,ms = +

D)n = 4,l = 1,ml = 0,ms = -

E)n = 5,l = 0,ml = 0,ms = +


Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
73
Below is a list of successive ionization energies (in kJ/mol)for a period 3 element.Identify the element and explain how you came to that conclusion.
IE2 = 2250 IE3 = 3360 IE4= 4560 IE5= 7010 IE6= 8500 IE7 = 27,100
IE2 = 2250 IE3 = 3360 IE4= 4560 IE5= 7010 IE6= 8500 IE7 = 27,100

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
74
List the noble gas that has the highest ionization energy.

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
75
Why do Li,Na,and K have similar chemical properties?

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
76
Match the following.
valence electrons
A)0
B)2
C)electrons in the outermost shell
D)1
E)electrons in completed shells
valence electrons
A)0
B)2
C)electrons in the outermost shell
D)1
E)electrons in completed shells

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
77
Which reaction below represents the electron affinity of Li?
A)Li(g)+ e⁻ → Li⁻(g)
B)Li(g)→ Li⁺(g)+ e⁻
C)Li(g)+ e⁻ → Li⁺(g)
D)Li⁺(g)→ Li(g)+ e⁻
E)Li⁺(g)+ e⁻ → Li(g)
A)Li(g)+ e⁻ → Li⁻(g)
B)Li(g)→ Li⁺(g)+ e⁻
C)Li(g)+ e⁻ → Li⁺(g)
D)Li⁺(g)→ Li(g)+ e⁻
E)Li⁺(g)+ e⁻ → Li(g)

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
78
Why is the first ionization energy of sulfur smaller than the first ionization energy of phosphorus?

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
79
Match the following.
number of unpaired electrons in Zn2+
A)0
B)2
C)electrons in the outermost shell
D)1
E)electrons in completed shells
number of unpaired electrons in Zn2+
A)0
B)2
C)electrons in the outermost shell
D)1
E)electrons in completed shells

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
80
Define ionization energy.

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck


