Deck 12: Solutions
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/127
Play
Full screen (f)
Deck 12: Solutions
1
Which of the following statements is true?
A)The solubility of a gas in water increases with increasing pressure.
B)The solubility of a gas in water decreases with increasing pressure.
C)The solubility of a gas in water increases with decreasing pressure.
D)The solubility of a gas in water decreases with decreasing pressure.
E)None of the above statements are true.
A)The solubility of a gas in water increases with increasing pressure.
B)The solubility of a gas in water decreases with increasing pressure.
C)The solubility of a gas in water increases with decreasing pressure.
D)The solubility of a gas in water decreases with decreasing pressure.
E)None of the above statements are true.
The solubility of a gas in water increases with increasing pressure.
2
Which of the following ions should have the most exothermic ΔHhydration?
A)Na⁺
B)Mg2⁺
C)Al3⁺
D)Ca2⁺
E)Sr2⁺
A)Na⁺
B)Mg2⁺
C)Al3⁺
D)Ca2⁺
E)Sr2⁺
Al3⁺
3
Which of the following compounds will be most soluble in ethanol (CH3CH2OH)?
A)trimethylamine (N(CH3)3)
B)acetone (CH3COCH3)
C)ethylene glycol (HOCH2CH2OH)
D)hexane (CH3CH2CH2CH2CH2CH3)
E)None of these compounds should be soluble in methanol.
A)trimethylamine (N(CH3)3)
B)acetone (CH3COCH3)
C)ethylene glycol (HOCH2CH2OH)
D)hexane (CH3CH2CH2CH2CH2CH3)
E)None of these compounds should be soluble in methanol.
ethylene glycol (HOCH2CH2OH)
4
Give the term for amount of solute in mole per mass of solvent in kg.
A)molality
B)molarity
C)mole fraction
D)mole percent
E)mass percent
A)molality
B)molarity
C)mole fraction
D)mole percent
E)mass percent

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
5
A solution is formed at room temperature by vigorously dissolving enough of the solid solute so that some solid remains at the bottom of the solution.Which statement below is true?
A)The solution is considered unsaturated.
B)The solution is considered supersaturated.
C)The solution is considered saturated.
D)The solution would be considered unsaturated if it were cooled a bit to increase the solubility of the solid.
E)None of the above are true.
A)The solution is considered unsaturated.
B)The solution is considered supersaturated.
C)The solution is considered saturated.
D)The solution would be considered unsaturated if it were cooled a bit to increase the solubility of the solid.
E)None of the above are true.

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
6
Choose the situation below that would result in a ΔHsolution near 0.
A)When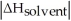
>>
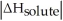
B)When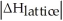
>
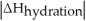
C)When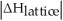
<
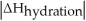
D)When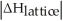
Is close to
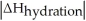
E)There isn't enough information to determine.
A)When
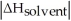
>>
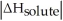
B)When
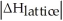
>
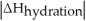
C)When
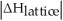
<
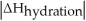
D)When
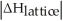
Is close to
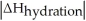
E)There isn't enough information to determine.

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
7
Determine the solubility of CO2 in soda water at 25°C if the pressure of CO2 is 5.2 atm.The Henry's law constant for carbon dioxide in water at this temperature is 3.4 × 10-2 M/atm.
A)0.15 M
B)0.57 M
C)0.65 M
D)0.18 M
E)0.29 M
A)0.15 M
B)0.57 M
C)0.65 M
D)0.18 M
E)0.29 M

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
8
Identify the gas that is dissolved in carbonated sodas.
A)carbon dioxide
B)oxygen
C)nitrogen
D)carbon monoxide
E)hydrogen
A)carbon dioxide
B)oxygen
C)nitrogen
D)carbon monoxide
E)hydrogen

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
9
Determine the solubility of N2 in water exposed to air at 25°C if the atmospheric pressure is 1.2 atm.Assume that the mole fraction of nitrogen is 0.78 in air and the Henry's law constant for nitrogen in water at this temperature is 6.1 × 10-4 M/atm.
A)1.5 × 10-4 M
B)6.5 × 10-4 M
C)5.7 × 10-4 M
D)1.8 × 10-4 M
E)3.6 × 10-4 M
A)1.5 × 10-4 M
B)6.5 × 10-4 M
C)5.7 × 10-4 M
D)1.8 × 10-4 M
E)3.6 × 10-4 M

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
10
Which of the following statements is true?
A)The solubility of a solid is not dependent on either temperature or pressure.
B)The solubility of a solid is highly dependent on pressure.
C)The solubility of a solid is highly dependent on both pressure and temperature.
D)The solubility of a solid is highly dependent on temperature.
E)None of the above.
A)The solubility of a solid is not dependent on either temperature or pressure.
B)The solubility of a solid is highly dependent on pressure.
C)The solubility of a solid is highly dependent on both pressure and temperature.
D)The solubility of a solid is highly dependent on temperature.
E)None of the above.

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
11
A solution containing less than the equilibrium amount of solvent is called ________.
A)an unsaturated solution
B)a solute
C)a supersaturated solution
D)a saturated solution.
E)a solvent
A)an unsaturated solution
B)a solute
C)a supersaturated solution
D)a saturated solution.
E)a solvent

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
12
Choose the situation below that would result in an endothermic ΔHsolution.
A)When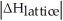 >
> 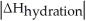
B)When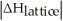 <
<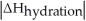
C)When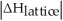 Is close to
Is close to 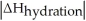
D)When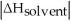 >>
>>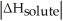
E)There isn't enough information to determine.
A)When
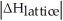 >
> 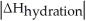
B)When
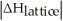 <
<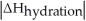
C)When
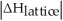 Is close to
Is close to 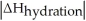
D)When
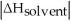 >>
>>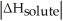
E)There isn't enough information to determine.

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
13
Give the intermolecular force that is responsible for the solubility of ethanol in water.
A)dispersion forces
B)dipole-dipole forces
C)hydrogen bonding
D)ion-dipole forces
E)ionic forces
A)dispersion forces
B)dipole-dipole forces
C)hydrogen bonding
D)ion-dipole forces
E)ionic forces

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
14
Which of the following compounds is most soluble in hexane (CH3CH2CH2CH2CH2CH2CH3)?
A)methanol
B)ethanol
C)1-propanol
D)1-butanol
E)1-pentanol
A)methanol
B)ethanol
C)1-propanol
D)1-butanol
E)1-pentanol

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
15
Determine DHlattice for KBr if the ΔHsolution (KBr)= +19.9 kJ/mol and the
ΔHhydration (KBr)= -670.kJ/mol.
A)+650 kJ/mol
B)-650 kJ/mol
C)+690 kJ/mol
D)-710 kJ/mol
E)-690 kJ/mol
ΔHhydration (KBr)= -670.kJ/mol.
A)+650 kJ/mol
B)-650 kJ/mol
C)+690 kJ/mol
D)-710 kJ/mol
E)-690 kJ/mol

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
16
Choose the situation below that would result in an exothermic ΔHsolution.
A)When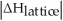 >
> 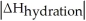
B)When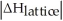 Is close to
Is close to 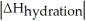
C)When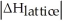 <
< 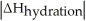
D)When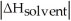 >>
>> 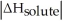
E)There isn't enough information to determine.
A)When
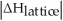 >
> 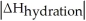
B)When
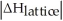 Is close to
Is close to 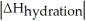
C)When
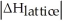 <
< 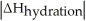
D)When
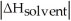 >>
>> 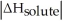
E)There isn't enough information to determine.

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
17
Which of the following compounds will be most soluble in pentane (C5H12)?
A)pentanol (CH3CH2CH2CH2CH2OH)
B)benzene (C6H6)
C)acetic acid (CH3CO2H)
D)ethyl methyl ketone (CH3CH2COCH3)
E)None of these compounds should be soluble in pentane.
A)pentanol (CH3CH2CH2CH2CH2OH)
B)benzene (C6H6)
C)acetic acid (CH3CO2H)
D)ethyl methyl ketone (CH3CH2COCH3)
E)None of these compounds should be soluble in pentane.

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
18
Which of the following statements is true?
A)In general,the solubility of a solid in water decreases with increasing temperature.
B)In general,the solubility of a gas in water decreases with increasing temperature.
C)The solubility of a gas in water usually increases with decreasing pressure.
D)The solubility of an ionic solid in water decreases with increasing temperature.
E)None of the above statements are true.
A)In general,the solubility of a solid in water decreases with increasing temperature.
B)In general,the solubility of a gas in water decreases with increasing temperature.
C)The solubility of a gas in water usually increases with decreasing pressure.
D)The solubility of an ionic solid in water decreases with increasing temperature.
E)None of the above statements are true.

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
19
Which of the following should have the largest Henry's law constant (kH)in water?
A)Ar
B)CO
C)Xe
D)CH3CH3
E)CO2
A)Ar
B)CO
C)Xe
D)CH3CH3
E)CO2

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
20
Choose the statement below that is true.
A)A solution will form between two substances if the solute-solvent interactions are of comparable strength to the solute-solute and solvent-solvent interactions.
B)A solution will form between two substances if the solute-solvent interactions are small enough to be overcome by the solute-solute and solvent-solvent interactions.
C)A solution will form between two substances if the solute-solute interactions are strong enough to overcome the solvent-solvent interactions.
D)A solution will form between two substances only if the solvent-solvent interactions are weak enough to overcome the solute-solvent interactions.
E)None of the above are true.
A)A solution will form between two substances if the solute-solvent interactions are of comparable strength to the solute-solute and solvent-solvent interactions.
B)A solution will form between two substances if the solute-solvent interactions are small enough to be overcome by the solute-solute and solvent-solvent interactions.
C)A solution will form between two substances if the solute-solute interactions are strong enough to overcome the solvent-solvent interactions.
D)A solution will form between two substances only if the solvent-solvent interactions are weak enough to overcome the solute-solvent interactions.
E)None of the above are true.

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
21
What mass (in g)of NH3 must be dissolved in 475 g of methanol to make a 0.250 m solution?
A)2.02 g
B)4.94 g
C)1.19 g
D)8.42 g
E)1.90 g
A)2.02 g
B)4.94 g
C)1.19 g
D)8.42 g
E)1.90 g

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
22
What mass of ethane (CH3CH3)is contained in 50.0 mL of a 13.9% by mass solution of ethane in hexane? The density of the solution is 0.611 g/mL.
A)2.35 g
B)8.49 g
C)11.8 g
D)6.95 g
E)4.25 g
A)2.35 g
B)8.49 g
C)11.8 g
D)6.95 g
E)4.25 g

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
23
How many moles of KF are contained in 347 g of water in a 0.175 m KF solution?
A)1.65 × 10-2 mol KF
B)5.04 × 10-2 mol KF
C)6.07 × 10-2 mol KF
D)3.22 × 10-2 mol KF
E)1.98 × 10-2 mol KF
A)1.65 × 10-2 mol KF
B)5.04 × 10-2 mol KF
C)6.07 × 10-2 mol KF
D)3.22 × 10-2 mol KF
E)1.98 × 10-2 mol KF

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
24
Which of the following concentration units are temperature dependent?
A)mole fraction
B)molality
C)mass percent
D)molarity
E)none of the above
A)mole fraction
B)molality
C)mass percent
D)molarity
E)none of the above

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
25
A solution is prepared by dissolving 76.3 g NaI in 545 g of water.Determine the mole fraction of NaI if the final volume of the solution is 576 mL.
A)6.04 × 10-3
B)1.65 × 10-2
C)1.40 × 10-3
D)1.32 × 10-2
E)8.84 × 10-2
A)6.04 × 10-3
B)1.65 × 10-2
C)1.40 × 10-3
D)1.32 × 10-2
E)8.84 × 10-2

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
26
Determine the Henry's law constant for ammonia in water at 25°C if an ammonia pressure of
0.022 atm produces a solution with a concentration of 1.3 M.
A)59 M/atm
B)0.017 M/atm
C)0.029 M/atm
D)35 M/atm
E)0.038 M/atm
0.022 atm produces a solution with a concentration of 1.3 M.
A)59 M/atm
B)0.017 M/atm
C)0.029 M/atm
D)35 M/atm
E)0.038 M/atm

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
27
Calculate the mole fraction of total ions in an aqueous solution prepared by dissolving 0.400 moles of MgCl2 in 850.0 g of water.
A)0.00841
B)0.0254
C)0.00848
D)0.0248
E)0.0167
A)0.00841
B)0.0254
C)0.00848
D)0.0248
E)0.0167

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
28
A solution is prepared by dissolving 98.6 g of NaCl in enough water to form 875 mL of solution.Calculate the mass % of the solution if the density of the solution is 1.06 g/mL.
A)11.3%
B)12.7%
C)9.4%
D)10.6%
E)11.9%
A)11.3%
B)12.7%
C)9.4%
D)10.6%
E)11.9%

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
29
How many moles of KF are contained in 244 mL of 0.135 m KF solution? The density of the solution is 1.22 g/mL.
A)4.31 × 10-2 mol KF
B)4.02 × 10-2 mol KF
C)3.29 × 10-2 mol KF
D)2.32 × 10-2 mol KF
E)1.67 × 10-2 mol KF
A)4.31 × 10-2 mol KF
B)4.02 × 10-2 mol KF
C)3.29 × 10-2 mol KF
D)2.32 × 10-2 mol KF
E)1.67 × 10-2 mol KF

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
30
A solution is prepared by dissolving 38.6 g sucrose (C12H22O11)in 495 g of water.Determine the mole fraction of sucrose if the final volume of the solution is 508 mL.
A)4.09 × 10-3
B)7.80 × 10-2
C)1.28 × 10-3
D)7.23 × 10-2
E)2.45 × 10-3
A)4.09 × 10-3
B)7.80 × 10-2
C)1.28 × 10-3
D)7.23 × 10-2
E)2.45 × 10-3

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
31
Determine the partial pressure of oxygen necessary to form an aqueous solution that is 4.1 × 10-4 M O2 at 25°C.The Henry's law constant for oxygen in water at 25°C is 1.3 × 10-3 M/atm.
A)1.9 atm
B)0.53 atm
C)0.24 atm
D)0.77 atm
E)0.32 atm
A)1.9 atm
B)0.53 atm
C)0.24 atm
D)0.77 atm
E)0.32 atm

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
32
A 1.00 L sample of water contains 0.0036 g of Cl⁻ ions.Determine the concentration of chloride ions in ppm if the density of the solution is 1.00 g/mL.
A)2.8 ppm
B)7.2 ppm
C)3.6 ppm
D)1.8 ppm
E)5.4 ppm
A)2.8 ppm
B)7.2 ppm
C)3.6 ppm
D)1.8 ppm
E)5.4 ppm

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
33
Identify the colligative property.
A)vapor pressure lowering
B)freezing point depression
C)boiling point elevation
D)osmotic pressure
E)all of the above
A)vapor pressure lowering
B)freezing point depression
C)boiling point elevation
D)osmotic pressure
E)all of the above

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
34
What mass of CuCl2 is contained in 75.85 g of a 22.4% by mass solution of CuCl2 in water.
A)17.0 g
B)5.89 g
C)29.5 g
D)33.9 g
E)11.2 g
A)17.0 g
B)5.89 g
C)29.5 g
D)33.9 g
E)11.2 g

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
35
Calculate the molality of a solution that is prepared by mixing 25.5 mL of CH3OH (d = 0.792 g/mL)and 387 mL of CH3CH2CH2OH (d = 0.811 g/mL).
A)0.630 m
B)0.812 m
C)1.57 m
D)1.89 m
E)4.98 m
A)0.630 m
B)0.812 m
C)1.57 m
D)1.89 m
E)4.98 m

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
36
Calculate the molality of a solution formed by dissolving 27.8 g of LiI in 500.0 mL of water.
A)0.254 m
B)0.394 m
C)0.556 m
D)0.241 m
E)0.415 m
A)0.254 m
B)0.394 m
C)0.556 m
D)0.241 m
E)0.415 m

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
37
A 4.55 L sample of water contains 0.115 g of sodium ions.Determine the concentration of sodium ions in ppm if the density of the solution is 1.00 g/mL.
A)52.3 ppm
B)13.2 ppm
C)12.7 ppm
D)25.3 ppm
E)36.5 ppm
A)52.3 ppm
B)13.2 ppm
C)12.7 ppm
D)25.3 ppm
E)36.5 ppm

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
38
A solution is prepared by dissolving 49.3 g of KBr in enough water to form 473 mL of solution.Calculate the mass % of KBr in the solution if the density is 1.12 g/mL.
A)10.4%
B)8.57%
C)10.1%
D)11.7%
E)9.31%
A)10.4%
B)8.57%
C)10.1%
D)11.7%
E)9.31%

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
39
Determine the molality of a solution prepared by dissolving 0.500 moles of CaF2 in 11.5 moles H2O.
A)1.88 m
B)4.35 m
C)5.31 m
D)4.14 m
E)2.41 m
A)1.88 m
B)4.35 m
C)5.31 m
D)4.14 m
E)2.41 m

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
40
Calculate the mass of oxygen (in mg)dissolved in a 5.00 L bucket of water exposed to a pressure of 1.13 atm of air.Assume the mole fraction of oxygen in air to be 0.21 and the Henry's law constant for oxygen in water at this temperature to be 1.3 × 10-3 M/atm.
A)49.4 mg
B)23.5 mg
C)9.87 mg
D)27.3 mg
E)13.7 mg
A)49.4 mg
B)23.5 mg
C)9.87 mg
D)27.3 mg
E)13.7 mg

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
41
Commercial grade HCl solutions are typically 39.0% (by mass)HCl in water.Determine the molarity of the HCl,if the solution has a density of 1.20 g/mL.
A)7.79 M
B)10.7 M
C)12.8 M
D)9.35 M
E)13.9 M
A)7.79 M
B)10.7 M
C)12.8 M
D)9.35 M
E)13.9 M

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
42
Place the following solutions in order of increasing osmotic pressure.
I.0.15 M C2H6O2
II.0.15 M MgCl2
III.0.15 M NaCl
A)III < I < II
B)II < III < I
C)I < II < III
D)II < I < III
E)I < III < II
I.0.15 M C2H6O2
II.0.15 M MgCl2
III.0.15 M NaCl
A)III < I < II
B)II < III < I
C)I < II < III
D)II < I < III
E)I < III < II

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
43
Give the reason that antifreeze is added to a car radiator.
A)The freezing point is lowered and the boiling point is elevated.
B)The freezing point is elevated and the boiling point is lowered.
C)The freezing point and the boiling point are elevated.
D)The freezing point and the boiling point are lowered.
E)None of the above
A)The freezing point is lowered and the boiling point is elevated.
B)The freezing point is elevated and the boiling point is lowered.
C)The freezing point and the boiling point are elevated.
D)The freezing point and the boiling point are lowered.
E)None of the above

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
44
Choose the aqueous solution that has the highest boiling point.These are all solutions of nonvolatile solutes and you should assume ideal van't Hoff factors where applicable.
A)0.100 m NaNO3
B)0.100 m Li2SO4
C)0.200 m C3H8O3
D)0.060 m Na3PO4
E)They all have the same boiling point.
A)0.100 m NaNO3
B)0.100 m Li2SO4
C)0.200 m C3H8O3
D)0.060 m Na3PO4
E)They all have the same boiling point.

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
45
The boiling point elevation of an aqueous sucrose solution is found to be 0.39°C.What mass of sucrose (molar mass = 342.30 g/mol)would be needed to dissolve in 500.0 g of water?
Kb (water)= 0.512°C/m.
A)261 g sucrose
B)528 g sucrose
C)762 g sucrose
D)223 g sucrose
E)130.g sucrose
Kb (water)= 0.512°C/m.
A)261 g sucrose
B)528 g sucrose
C)762 g sucrose
D)223 g sucrose
E)130.g sucrose

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
46
Determine the molality of an aqueous solution prepared by dissolving 0.112 moles of LiCl in 13.7 moles of H2O.
A)0.454 m
B)0.220 m
C)0.818 m
D)0.122 m
E)0.153 m
A)0.454 m
B)0.220 m
C)0.818 m
D)0.122 m
E)0.153 m

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
47
Calculate the boiling point of a solution of 500.0 g of ethylene glycol (C2H6O2)dissolved in 500.0 g of water.Kf = 1.86°C/m and Kb = 0.512°C/m.Use 100°C as the boiling point of water.
A)108°C
B)92°C
C)130°C
D)70°C
E)8.3°C
A)108°C
B)92°C
C)130°C
D)70°C
E)8.3°C

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
48
Determine the freezing point depression of a solution that contains 30.7 g glycerin (C3H8O3,molar mass = 92.09 g/mol)in 376 mL of water.Some possibly useful constants for water are Kf = 1.86°C/m and Kb = 0.512°C/m .
A)0.887°C
B)1.65°C
C)3.33°C
D)4.95°C
E)0.654°C
A)0.887°C
B)1.65°C
C)3.33°C
D)4.95°C
E)0.654°C

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
49
A solution is 0.0433 m LiF.What is the molarity of the solution if the density is 1.10 g/mL?
A)0.0441 M
B)0.0390 M
C)0.0519 M
D)0.0476 M
E)0.0417 M
A)0.0441 M
B)0.0390 M
C)0.0519 M
D)0.0476 M
E)0.0417 M

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
50
Calculate the freezing point of a solution of 500.0 g of ethylene glycol (C2H6O2)dissolved in 500.0 g of water.Kf = 1.86°C/m and Kb = 0.512°C/m.
A)30.0°C
B)-30.0°C
C)8.32°C
D)-8.32°C
E)70.2°C
A)30.0°C
B)-30.0°C
C)8.32°C
D)-8.32°C
E)70.2°C

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
51
Determine the vapor pressure of a solution at 25°C that contains 76.6 g of glucose (C6H12O6)in 250.0 mL of water.The vapor pressure of pure water at 25°C is 23.8 torr.
A)70.8 torr
B)72.9 torr
C)23.1 torr
D)22.9 torr
E)7.29 torr
A)70.8 torr
B)72.9 torr
C)23.1 torr
D)22.9 torr
E)7.29 torr

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
52
An aqueous solution is 0.387 M in HCl.What is the molality of the solution if the density is 1.23 g/mL?
A)0.115 m
B)0.387 m
C)0.315 m
D)0.411 m
E)0.318 m
A)0.115 m
B)0.387 m
C)0.315 m
D)0.411 m
E)0.318 m

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
53
Choose the aqueous solution with the lowest vapor pressure.These are all solutions of nonvolatile solutes and you should assume ideal van't Hoff factors where applicable.
A)0.120 m C2H6O2
B)0.040 m (NH4)2SO4
C)0.060 m K2CO3
D)0.030 m LiC2H3O2
E)They all have the same vapor pressure.
A)0.120 m C2H6O2
B)0.040 m (NH4)2SO4
C)0.060 m K2CO3
D)0.030 m LiC2H3O2
E)They all have the same vapor pressure.

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
54
Choose the aqueous solution with the highest vapor pressure.These are all solutions of nonvolatile solutes and you should assume ideal van't Hoff factors where applicable.
A)0.50 m C3H8O3
B)0.50 m C2H6O2
C)0.50 m C6H12O6
D)0.50 m C12H22O11
E)They all have about the same vapor pressure.
A)0.50 m C3H8O3
B)0.50 m C2H6O2
C)0.50 m C6H12O6
D)0.50 m C12H22O11
E)They all have about the same vapor pressure.

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
55
Choose the aqueous solution that has the highest boiling point.These are all solutions of nonvolatile solutes and you should assume ideal van't Hoff factors where applicable.
A)0.100 m AlCl3
B)0.100 m NaCl
C)0.100 m MgCl2
D)0.100 m C6H12O6
E)They all have the same boiling point.
A)0.100 m AlCl3
B)0.100 m NaCl
C)0.100 m MgCl2
D)0.100 m C6H12O6
E)They all have the same boiling point.

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
56
Choose the solvent below that would show the greatest boiling point elevation when used to make a 0.10 m nonelectrolyte solution.
A)CCl4,Kb = 29.9°C/m
B)C6H6,Kb = 5.12°C/m
C)CH3CH2OCH2CH3,Kb = 1.79°C/m
D)CH3CH2OH,Kb = 1.99°C/m
E)CHCl3,Kb = 4.70°C/m
A)CCl4,Kb = 29.9°C/m
B)C6H6,Kb = 5.12°C/m
C)CH3CH2OCH2CH3,Kb = 1.79°C/m
D)CH3CH2OH,Kb = 1.99°C/m
E)CHCl3,Kb = 4.70°C/m

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
57
Determine the freezing point of a solution that contains 78.8 g of naphthalene (C10H8,molar mass = 128.16 g/mol)dissolved in 722 mL of benzene (d = 0.877 g/mL).Pure benzene has a melting point of 5.50°C and a freezing point depression constant of 4.90°C/m.
A)4.76°C
B)4.17°C
C)0.74°C
D)1.33°C
E)1.68°C
A)4.76°C
B)4.17°C
C)0.74°C
D)1.33°C
E)1.68°C

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
58
Commercial grade HCl solutions are typically 39.0% (by mass)HCl in water.Determine the molality of the HCl,if the solution has a density of 1.20 g/mL.
A)39.0 m
B)17.5 m
C)6.39 m
D)10.7 m
E)9.44 m
A)39.0 m
B)17.5 m
C)6.39 m
D)10.7 m
E)9.44 m

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
59
Determine the vapor pressure of a solution at 55°C that contains 34.2 g NaCl in 375 mL of water.The vapor pressure of pure water at 55°C is 118.1 torr.
A)115 torr
B)87.1 torr
C)108 torr
D)112 torr
E)92.8 torr
A)115 torr
B)87.1 torr
C)108 torr
D)112 torr
E)92.8 torr

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
60
Determine the boiling point of a solution that contains 78.8 g of naphthalene (C10H8,molar mass = 128.16 g/mol)dissolved in 722 mL of benzene (d = 0.877 g/mL).Pure benzene has a boiling point of 80.1°C and a boiling point elevation constant of 2.53°C/m.
A)2.2°C
B)2.5°C
C)82.3°C
D)80.4°C
E)82.6°C
A)2.2°C
B)2.5°C
C)82.3°C
D)80.4°C
E)82.6°C

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
61
What happens to a supersaturated solution of potassium acetate once it is cooled and a small crystal of solid potassium acetate is added?

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
62
Match the following.
0.020 m NH4Cl
A)largest van't Hoff factor
B)solution of ionic compound with highest freezing point
C)solution with ΔTb = 0.026°C
D)highest boiling point
E)solution that is most strongly dependent upon pressure
0.020 m NH4Cl
A)largest van't Hoff factor
B)solution of ionic compound with highest freezing point
C)solution with ΔTb = 0.026°C
D)highest boiling point
E)solution that is most strongly dependent upon pressure

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
63
Identify the compound that would have the highest osmotic pressure when dissolved in water.
A)NaCl
B)K2SO4
C)MgCl2
D)FeCl3
E)MgSO4
A)NaCl
B)K2SO4
C)MgCl2
D)FeCl3
E)MgSO4

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
64
Choose the aqueous solution below with the highest freezing point.These are all solutions of nonvolatile solutes and you should assume ideal van't Hoff factors where applicable.
A)0.200 m Ca(ClO4)2
B)0.200 m K3PO3
C)0.200 m HOCH2CH2OH
D)0.200 m Ba(NO3)2
E)These all have the same freezing point.
A)0.200 m Ca(ClO4)2
B)0.200 m K3PO3
C)0.200 m HOCH2CH2OH
D)0.200 m Ba(NO3)2
E)These all have the same freezing point.

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
65
Match the following.
0.050 m NaCl
A)largest van't Hoff factor
B)solution of ionic compound with highest freezing point
C)solution with ΔTb = 0.026°C
D)highest boiling point
E)solution that is most strongly dependent upon pressure
0.050 m NaCl
A)largest van't Hoff factor
B)solution of ionic compound with highest freezing point
C)solution with ΔTb = 0.026°C
D)highest boiling point
E)solution that is most strongly dependent upon pressure

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
66
Match the following.
0.050 m C6H12O6 (aqueous)
A)largest van't Hoff factor
B)solution of ionic compound with highest freezing point
C)solution with ΔTb = 0.026°C
D)highest boiling point
E)solution that is most strongly dependent upon pressure
0.050 m C6H12O6 (aqueous)
A)largest van't Hoff factor
B)solution of ionic compound with highest freezing point
C)solution with ΔTb = 0.026°C
D)highest boiling point
E)solution that is most strongly dependent upon pressure

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
67
Define molality.

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
68
A compound is found to have a molar mass of 598 g/mol.If 35.8 mg of the compound is dissolved in enough water to make 175 mL of solution at 25°C,what is the osmotic pressure of the resulting solution?
A)3.42 torr
B)6.36 torr
C)5.01 torr
D)5.99 torr
E)8.36 torr
A)3.42 torr
B)6.36 torr
C)5.01 torr
D)5.99 torr
E)8.36 torr

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
69
Explain why the van't Hoff factor for MgCl2 is less than it's predicted value.

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
70
The boiling point of an aqueous 1.83 m (NH4)2SO4 (molar mass = 132.15 g/mol)solution is 102.5°C.Determine the value of the van't Hoff factor for this solute if the Kb for water is 0.512°C/m.
A)3.0
B)3.6
C)1.8
D)2.7
E)2.3
A)3.0
B)3.6
C)1.8
D)2.7
E)2.3

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
71
Why isn't pentanol (CH3CH2CH2CH2CH2OH)very soluble in water?

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
72
Define osmosis.

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
73
Match the following.
0.010 m Al(NO3)3
A)largest van't Hoff factor
B)solution of ionic compound with highest freezing point
C)solution with ΔTb = 0.026°C
D)highest boiling point
E)solution that is most strongly dependent upon pressure
0.010 m Al(NO3)3
A)largest van't Hoff factor
B)solution of ionic compound with highest freezing point
C)solution with ΔTb = 0.026°C
D)highest boiling point
E)solution that is most strongly dependent upon pressure

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
74
How does a solution of two volatile components with strong solute-solvent attractions deviate from Raoult's law? Why?

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
75
Choose the aqueous solution below with the lowest freezing point.These are all solutions of nonvolatile solutes and you should assume ideal van't Hoff factors where applicable.
A)0.075 m NaI
B)0.075 m (NH4)3PO4
C)0.075 m NaBrO4
D)0.075 m LiCN
E)0.075 m KNO2
A)0.075 m NaI
B)0.075 m (NH4)3PO4
C)0.075 m NaBrO4
D)0.075 m LiCN
E)0.075 m KNO2

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
76
Place the following aqueous solutions of nonvolatile,nonionic compounds in order of decreasing osmotic pressure.
I.0.011 M sucrose
II.0.00095 M glucose
III.0.0060 M glycerin
A)I > III > II
B)I > II > III
C)II > III > I
D)III > I > II
E)II > I > III
I.0.011 M sucrose
II.0.00095 M glucose
III.0.0060 M glycerin
A)I > III > II
B)I > II > III
C)II > III > I
D)III > I > II
E)II > I > III

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
77
Match the following.
0.0050 m CO2
A)largest van't Hoff factor
B)solution of ionic compound with highest freezing point
C)solution with ΔTb = 0.026°C
D)highest boiling point
E)solution that is most strongly dependent upon pressure
0.0050 m CO2
A)largest van't Hoff factor
B)solution of ionic compound with highest freezing point
C)solution with ΔTb = 0.026°C
D)highest boiling point
E)solution that is most strongly dependent upon pressure

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
78
A 150.0 mL sample of an aqueous solution at 25°C contains 15.2 mg of an unknown nonelectrolyte compound.If the solution has an osmotic pressure of 8.44 torr,what is the molar mass of the unknown compound?
A)223 g/mol
B)294 g/mol
C)341 g/mol
D)448 g/mol
E)195 g/mol
A)223 g/mol
B)294 g/mol
C)341 g/mol
D)448 g/mol
E)195 g/mol

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
79
Calculate the osmotic pressure,in torr,of a solution containing 3.00 mg of a sugar (342 g/mole)in 15.0 mL of water at 25°C.

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck
80
What is a semipermeable membrane?

Unlock Deck
Unlock for access to all 127 flashcards in this deck.
Unlock Deck
k this deck


