Deck 16: Temperature and Heat
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/110
Play
Full screen (f)
Deck 16: Temperature and Heat
1
Object 1 has three times the specific heat capacity and four times the mass of Object 2.The two objects are heated from the same initial temperature,T0,to the same final temperature Tf.During this process,if Object 1 absorbs heat Q,the amount of heat absorbed by Object 2 will be
A)12Q.
B)6Q.
C) Q.
Q.
D)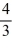 Q.
Q.
E)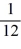 Q.
Q.
A)12Q.
B)6Q.
C)
 Q.
Q.D)
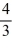 Q.
Q.E)
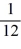 Q.
Q.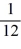 Q.
Q. 2
A solid cylindrical bar conducts heat at a rate of 25 W from a hot to a cold reservoir under steady state conditions.If both the length and the diameter of this bar are doubled,the rate at which it will conduct heat between these reservoirs will be
A)200 W.
B)100 W.
C)50 W.
D)25 W.
E)12.5 W.
A)200 W.
B)100 W.
C)50 W.
D)25 W.
E)12.5 W.
50 W.
3
The coefficient of linear expansion for aluminum is 1.8 × 10-6 K-1.What is its coefficient of volume expansion?
A)9.0 × 10-6 K-1
B)5.8 × 10-18 K-1
C)5.4 × 10-6 K-1
D)3.6 × 10-6 K-1
E)0.60 × 10-6 K-1
A)9.0 × 10-6 K-1
B)5.8 × 10-18 K-1
C)5.4 × 10-6 K-1
D)3.6 × 10-6 K-1
E)0.60 × 10-6 K-1
5.4 × 10-6 K-1
4
An architect is interested in estimating the rate of heat loss,ΔQ/Δt,through a sheet of insulating material as a function of the thickness of the sheet.Assuming fixed temperatures on the two faces of the sheet and steady state heat flow,which one of the graphs shown in the figure best represents the rate of heat transfer as a function of the thickness of the insulating sheet? 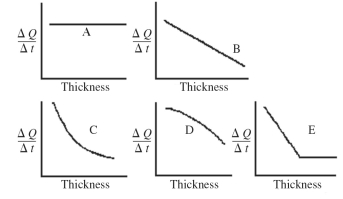
A)A
B)B
C)C
D)D
E)E

A)A
B)B
C)C
D)D
E)E

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
5
An object having a fixed emissivity of 0.725 radiates heat at a rate of 10 W when it is at an absolute temperature T.If its temperature is doubled to 2T,at what rate will it now radiate?
A)20 W
B)40 W
C)80 W
D)160 W
E)320 W
A)20 W
B)40 W
C)80 W
D)160 W
E)320 W

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
6
Two metal rods are to be used to conduct heat from a region at 100°C to a region at 0°C as shown in the figure.The rods can be placed in parallel,as shown on the left,or in series,as on the right.When steady state flow is established,the heat conducted in the series arrangement is 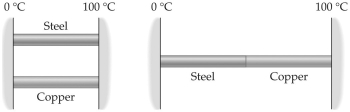
A)greater than the heat conducted with the rods in parallel.
B)the same as the heat conducted with the rods in parallel.
C)less than the heat conducted with the rods in parallel.

A)greater than the heat conducted with the rods in parallel.
B)the same as the heat conducted with the rods in parallel.
C)less than the heat conducted with the rods in parallel.

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
7
Which one of the following quantities is the smallest unit of heat energy?
A)calorie
B)kilocalorie
C)Btu
D)joule
A)calorie
B)kilocalorie
C)Btu
D)joule

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
8
Two metal spheres are made of the same material and have the same diameter,but one is solid and the other is hollow.If their temperature is increased by the same amount,
A)the solid sphere becomes bigger than the hollow one.
B)the hollow sphere becomes bigger than the solid one.
C)the two spheres remain of equal size.
D)the solid sphere becomes denser and the hollow one less dense.
E)the solid sphere becomes less dense and the hollow one denser.
A)the solid sphere becomes bigger than the hollow one.
B)the hollow sphere becomes bigger than the solid one.
C)the two spheres remain of equal size.
D)the solid sphere becomes denser and the hollow one less dense.
E)the solid sphere becomes less dense and the hollow one denser.

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
9
If,with steady state heat flow established,you double the thickness of a wall built from solid uniform material,the rate of heat loss for a given temperature difference across the thickness will
A)become one-half its original value.
B)also double.
C)become one-fourth its original value.
D)become 1/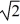 of its original value.
of its original value.
E)become four times its original value.
A)become one-half its original value.
B)also double.
C)become one-fourth its original value.
D)become 1/
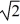 of its original value.
of its original value.E)become four times its original value.

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
10
On a cold day,a piece of metal feels much colder to the touch than a piece of wood.This is due to the difference in which one of the following physical properties of these materials?
A)density
B)specific heat
C)emissivity
D)thermal conductivity
E)mass
A)density
B)specific heat
C)emissivity
D)thermal conductivity
E)mass

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
11
The process in which heat flows by the mass movement of molecules from one place to another is known as
A)conduction.
B)convection.
C)radiation.
A)conduction.
B)convection.
C)radiation.

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
12
A thermally isolated system is made up of a hot piece of aluminum and a cold piece of copper,with the aluminum and the copper in thermal contact.The specific heat capacity of aluminum is more than double that of copper.Which object experiences the greater magnitude gain or loss of heat during the time the system takes to reach thermal equilibrium?
A)the aluminum
B)the copper
C)Neither one;both of them experience the same size gain or loss of heat.
D)It is impossible to tell without knowing the masses.
E)It is impossible to tell without knowing the volumes.
A)the aluminum
B)the copper
C)Neither one;both of them experience the same size gain or loss of heat.
D)It is impossible to tell without knowing the masses.
E)It is impossible to tell without knowing the volumes.

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
13
The temperature in your classroom is closest to
A)68 K.
B)68°C.
C)50°C.
D)295 K.
A)68 K.
B)68°C.
C)50°C.
D)295 K.

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
14
A thermally isolated system is made up of a hot piece of aluminum and a cold piece of copper,with the aluminum and the copper in thermal contact.The specific heat capacity of aluminum is more than double that of copper.Which object experiences the greater temperature change during the time the system takes to reach thermal equilibrium?
A)the copper
B)the aluminum
C)Neither one;both of them experience the same size temperature change.
D)It is impossible to tell without knowing the masses.
E)It is impossible to tell without knowing the volumes.
A)the copper
B)the aluminum
C)Neither one;both of them experience the same size temperature change.
D)It is impossible to tell without knowing the masses.
E)It is impossible to tell without knowing the volumes.

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
15
Which two temperature changes are equivalent?
A)1 K = 1°F
B)1°F = 1°C
C)1°C = 1 K
D)none of the above
A)1 K = 1°F
B)1°F = 1°C
C)1°C = 1 K
D)none of the above

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
16
Consider a flat steel plate with a hole through its center as shown in the figure.When the temperature of the plate is increased,the hole will 
A)expand only if it takes up more than half the plate's surface area.
B)contract if it takes up less than half the plate's surface area.
C)always contract as the plate expands into it.
D)always expand with the plate.
E)remain the same size as the plate expands around it.

A)expand only if it takes up more than half the plate's surface area.
B)contract if it takes up less than half the plate's surface area.
C)always contract as the plate expands into it.
D)always expand with the plate.
E)remain the same size as the plate expands around it.

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
17
As shown in the figure,a bimetallic strip,consisting of metal G on the top and metal H on the bottom,is rigidly attached to a wall at the left.The coefficient of linear thermal expansion for metal G is greater than that of metal H.If the strip is uniformly heated,it will 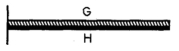
A)curve upward.
B)curve downward.
C)remain horizontal,but get longer.
D)remain horizontal,but get shorter.
E)bend in the middle.

A)curve upward.
B)curve downward.
C)remain horizontal,but get longer.
D)remain horizontal,but get shorter.
E)bend in the middle.

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
18
If you wanted to know how much the temperature of a particular piece of material would rise when a known amount of heat was added to it,which of the following quantities would be most helpful to know?
A)initial temperature
B)specific heat
C)density
D)coefficient of linear expansion
E)thermal conductivity
A)initial temperature
B)specific heat
C)density
D)coefficient of linear expansion
E)thermal conductivity

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
19
Object 1 has three times the specific heat capacity and four times the mass of Object 2.The two objects are given the same amount of heat.If the temperature of Object 1 changes by an amount ΔT,the change in temperature of Object 2 will be
A)ΔT.
B) ΔT.
ΔT.
C)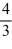 ΔT.
ΔT.
D)6ΔT.
E)12ΔT.
A)ΔT.
B)
 ΔT.
ΔT.C)
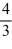 ΔT.
ΔT.D)6ΔT.
E)12ΔT.

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
20
If the absolute temperature of an object is tripled,the thermal power radiated by this object (assuming that its emissivity and size are not affected by the temperature change)will
A)increase by a factor of 3.
B)increase by a factor of 9.
C)increase by a factor of 18.
D)increase by a factor of 27.
E)increase by a factor of 81.
A)increase by a factor of 3.
B)increase by a factor of 9.
C)increase by a factor of 18.
D)increase by a factor of 27.
E)increase by a factor of 81.

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
21
Express -40°C in °F.
A)-72°F
B)-54°F
C)-40°F
D)4.4°F
A)-72°F
B)-54°F
C)-40°F
D)4.4°F

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
22
Nitrogen boils at -196°C.What is the corresponding temperature in the Fahrenheit scale?
A)-315°F
B)-196°F
C)-346°F
D)-290°F
E)-321°F
A)-315°F
B)-196°F
C)-346°F
D)-290°F
E)-321°F

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
23
A person running in place on an exercise machine for 10 min uses up 17 kcal (food calories).Another person exercises by repeatedly lifting two 2.5-kg weights a distance of 50 cm.How many repetitions of this exercise are equivalent to 10 minutes of running in place? Assume that the person uses negligible energy in letting down the weights after each lift.(1 cal = 4.186 J)
A)730
B)1450
C)1500
D)2200
E)2900
A)730
B)1450
C)1500
D)2200
E)2900

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
24
The temperature changes from 35°F during the night to 75°F during the day.What is the temperature change on the Celsius scale?
A)72°C
B)40°C
C)32°C
D)22°C
A)72°C
B)40°C
C)32°C
D)22°C

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
25
A hole in a brass plate has a diameter of 1.200 cm at 20°C.What is the diameter of the hole when the plate is heated to 220°C? The coefficient of linear thermal expansion for brass is 19 × 10-6 K-1.
A)1.205 cm
B)1.195 cm
C)1.200 cm
D)1.210 cm
A)1.205 cm
B)1.195 cm
C)1.200 cm
D)1.210 cm

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
26
A steel bridge is 1000 m long at -20°C in winter.What is the change in length when the temperature rises to 40°C in summer? The average coefficient of linear expansion of this steel is 11 × 10-6 K-1.
A)0.33 m
B)0.44 m
C)0.55 m
D)0.66 m
A)0.33 m
B)0.44 m
C)0.55 m
D)0.66 m

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
27
Platinum melts at 3215°F.What is the corresponding temperature in the Kelvin scale?
A)2041 K
B)2135 K
C)2207 K
D)2296 K
E)3215 K
A)2041 K
B)2135 K
C)2207 K
D)2296 K
E)3215 K

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
28
At what,if any,temperature are the numerical readings on the Fahrenheit and Celsius scales the same?
A)-30°
B)-40°
C)-50°
D)-60°
E)They can never read the same because they are based on different zeroes.
A)-30°
B)-40°
C)-50°
D)-60°
E)They can never read the same because they are based on different zeroes.

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
29
Express a body temperature 98.6°F in Celsius degrees.
A)37.0°C
B)45.5°C
C)66.6°C
D)72.6°C
A)37.0°C
B)45.5°C
C)66.6°C
D)72.6°C

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
30
A person consumes a snack containing 14 food calories (14 kcal).What is the power this food produces if it is to be "burned off" due to exercise in 6 hours? (1 cal = 4.186 J)
A)2.7 W
B)9,763 W
C)0.6 W
D)0.0027 W
A)2.7 W
B)9,763 W
C)0.6 W
D)0.0027 W

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
31
An aluminum rod 17.400 cm long at 20°C is heated to 100°C.What is its new length? Aluminum has a linear expansion coefficient of 25 × 10-6 K-1.
A)17.435 cm
B)17.365 cm
C)0.348 cm
D)0.0348 cm
A)17.435 cm
B)17.365 cm
C)0.348 cm
D)0.0348 cm

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
32
At room temperature,a typical person loses energy to the surroundings at the rate of 62 W.If this energy loss has to be made up by an equivalent food intake,how many kilocalories (food calories)does this person need to consume every day just to make up this heat loss? (1 cal = 4.186 J)
A)1000 kcal
B)1100 kcal
C)1300 kcal
D)1500 kcal
E)1600 kcal
A)1000 kcal
B)1100 kcal
C)1300 kcal
D)1500 kcal
E)1600 kcal

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
33
The coefficient of linear expansion of steel is 12 × 10-6 K-1.What is the change in length of a 25-m steel bridge span when it undergoes a temperature change of 40 K from winter to summer?
A)1.2 cm
B)1.4 cm
C)1.6 cm
D)1.8 cm
E)2.0 cm
A)1.2 cm
B)1.4 cm
C)1.6 cm
D)1.8 cm
E)2.0 cm

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
34
A large vat contains 1.000 L of water at 20°C.What volume will this water occupy when it is heated up to 80°C? Water has a volume expansion coefficient of 210 × 10-6 K-1.
A)1.600 L
B)1.013 L
C)0.987 L
D)0.9987 L
A)1.600 L
B)1.013 L
C)0.987 L
D)0.9987 L

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
35
By what primary heat transfer mechanism does the sun warm the earth?
A)convection
B)conduction
C)radiation
D)All of the above processes are equally important in combination.
A)convection
B)conduction
C)radiation
D)All of the above processes are equally important in combination.

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
36
By what length will a slab of concrete that is originally 18 m long contract when the temperature drops from 24°C to -16°C? The coefficient of linear thermal expansion for this concrete is 1.0 × 10-5 K-1.
A)0.50 cm
B)0.72 cm
C)1.2 cm
D)1.5 cm
A)0.50 cm
B)0.72 cm
C)1.2 cm
D)1.5 cm

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
37
The weather outside is frightful.The temperature is -22°F.What is the corresponding temperature in the Celsius scale?
A)-35°C
B)-30°C
C)-22°C
D)-20°C
E)-12°C
A)-35°C
B)-30°C
C)-22°C
D)-20°C
E)-12°C

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
38
Oxygen condenses into a liquid at approximately 90 K.What temperature,in degrees Fahrenheit,does this correspond to?
A)-193°F
B)-217°F
C)-265°F
D)-297°F
A)-193°F
B)-217°F
C)-265°F
D)-297°F

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
39
A temperature change of 20°C corresponds to a Fahrenheit temperature change of
A)68°F.
B)11°F.
C)36°F.
D)18°F.
A)68°F.
B)11°F.
C)36°F.
D)18°F.

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
40
A quantity of mercury occupies 400.0 cm3 at 0°C.What volume will it occupy when heated to 50°C? Mercury has a volume expansion coefficient of 180 × 10-6 K-1.
A)450 cm3
B)409.7 cm3
C)403.6 cm3
D)401.8 cm3
A)450 cm3
B)409.7 cm3
C)403.6 cm3
D)401.8 cm3

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
41
A machine part consists of 0.10 kg of iron (of specific heat 470 J/kg ∙ K )and 0.16 kg of copper (of specific heat 390 J/kg ∙ K).How much heat must be added to the gear to raise its temperature from 18°C to 53°C?
A)910 J
B)3800 J
C)4000 J
D)4400 J
A)910 J
B)3800 J
C)4000 J
D)4400 J

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
42
The coefficient of linear expansion of copper is 17 × 10-6 K-1.A sheet of copper has a round hole with a radius of 3.0 m cut out of it.If the sheet is heated and undergoes a change in temperature of 80 K,what is the change in the radius of the hole?
A)It decreases by 4.1 mm.
B)It increases by 4.1 mm.
C)It decreases by 8.2 mm.
D)It increases by 8.2 mm.
E)It does not change.
A)It decreases by 4.1 mm.
B)It increases by 4.1 mm.
C)It decreases by 8.2 mm.
D)It increases by 8.2 mm.
E)It does not change.

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
43
A 4.0-kg aluminum block is originally at 10°C.If 160 kJ of heat is added to the block,what is its final temperature? The specific heat capacity of aluminum is 910 J/kg ∙ K.
A)24°C
B)34°C
C)44°C
D)54°C
A)24°C
B)34°C
C)44°C
D)54°C

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
44
The coefficient of volume expansion of a certain olive oil is 0.68 × 10-3 K-1.A 1.0-L glass beaker is filled to the brim with olive oil at room temperature.The beaker is placed on a range and the temperature of the oil and beaker increases by 25°C.As a result,0.0167 L of olive oil spills over the top of the beaker.Which of the following values is closest to the coefficient of linear expansion of the glass from which the beaker is made?
A)1 × 10-6 K-1
B)4 × 10-6 K-1
C)1 × 10-5 K-1
D)2 × 10-5 K-1
E)3 × 10-5 K-1
A)1 × 10-6 K-1
B)4 × 10-6 K-1
C)1 × 10-5 K-1
D)2 × 10-5 K-1
E)3 × 10-5 K-1

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
45
The coefficient of linear expansion of copper is 17 × 10-6 K-1 and that of steel is 12 × 10-6 K-1.At 12°C a steel rod has a diameter of 2.540 cm and a copper pipe has a diameter of 2.536 cm.Which one of the following quantities is closest to the temperature to which the copper pipe must be heated in order for the unheated steel rod to fit snugly in the copper pipe?
A)53°C
B)81°C
C)93°C
D)105°C
E)143°C
A)53°C
B)81°C
C)93°C
D)105°C
E)143°C

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
46
An aluminum rod is 10.0 cm long and a steel rod is 80.0 cm long when both rods are at a temperature of 15°C.Both rods have the same diameter.The rods are now joined end-to-end to form a rod 90.0 cm long.If the temperature is now raised from 15°C to 90°C,what is the increase in the length of the joined rod? The coefficient of linear expansion of aluminum is 2.4 × 10-5 K-1 and that of steel is 1.2 × 10-5 K-1.
A)0.90 mm
B)0.81 mm
C)0.72 mm
D)0.63 mm
E)0.99 mm
A)0.90 mm
B)0.81 mm
C)0.72 mm
D)0.63 mm
E)0.99 mm

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
47
A steel pipe 36.0 m long,installed when the temperature was 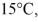
Is used to transport superheated steam at a temperature of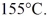
Steel's coefficient of linear expansion is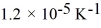
.The pipe is allowed to expand freely when the steam is transported.What is the increase in the length of the pipe when it is used with the superheated steam?
A)60 mm
B)57 mm
C)54 mm
D)64 mm
E)67 mm
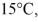
Is used to transport superheated steam at a temperature of
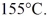
Steel's coefficient of linear expansion is
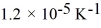
.The pipe is allowed to expand freely when the steam is transported.What is the increase in the length of the pipe when it is used with the superheated steam?
A)60 mm
B)57 mm
C)54 mm
D)64 mm
E)67 mm

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
48
Suppose that a rigid aluminum wire were to be strung out in a loop that just fits snugly around the equator (assuming a perfectly spherical Earth with a radius of 6.37 × 106 m).If the temperature of the wire is increased by 0.50°C,and the increase in length is distributed equally over the entire length,how far off the ground will the wire loop be if it remained centered on the earth? The coefficient of linear expansion of aluminum is 24 × 10-6 K-1.
A)7.6 mm
B)76 mm
C)76 cm
D)76 m
E)760 m
A)7.6 mm
B)76 mm
C)76 cm
D)76 m
E)760 m

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
49
The density of water at 0°C is 999.84 kg/m3 and at 4°C it is 999.96 kg/m3.A 1.0-L container,full to the brim with water at 4.0°C is placed in the refrigerator.By the time that the temperature of the water reaches 0.0°C,what volume of water has spilled from the container,assuming that the contraction of the container is negligible?
A)1.1 × 10-7 m3
B)1.2 × 10-7 m3
C)1.3 × 10-7 m3
D)1.4 × 10-7 m3
E)1.5 × 10-7 m3
A)1.1 × 10-7 m3
B)1.2 × 10-7 m3
C)1.3 × 10-7 m3
D)1.4 × 10-7 m3
E)1.5 × 10-7 m3

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
50
The coefficient of linear expansion of copper is 17 × 10-6 K-1.A block of copper 30 cm wide,45 cm long,and 10 cm thick is heated from 0°C to 100°C What is the change in the volume of the block?
A)2.3 × 10-5 m3
B)4.6 × 10-5 m3
C)5.2 × 10-5 m3
D)6.9 × 10-5 m3
E)14 × 10-5 m3
A)2.3 × 10-5 m3
B)4.6 × 10-5 m3
C)5.2 × 10-5 m3
D)6.9 × 10-5 m3
E)14 × 10-5 m3

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
51
How much heat is required to raise the temperature of a 225-g lead ball from 15.0°C to 25.0°C? The specific heat of lead is 128 J/kg ∙ K.
A)725 J
B)576 J
C)145 J
D)217 J
E)288 J
A)725 J
B)576 J
C)145 J
D)217 J
E)288 J

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
52
The coefficient of linear expansion of aluminum is 24.0 × 10-6 K-1,and the density of aluminum at 0°C is 2.70 × 103 kg/m3.What is the density of aluminum at 300°C?
A)3.93 × 103 kg/m3
B)2.73 × 103 kg/m3
C)2.70 × 103 kg/m3
D)2.67 × 103 kg/m3
E)2.64 × 103 kg/m3
A)3.93 × 103 kg/m3
B)2.73 × 103 kg/m3
C)2.70 × 103 kg/m3
D)2.67 × 103 kg/m3
E)2.64 × 103 kg/m3

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
53
A glass flask has a volume of 500 mL at a temperature of 20° C.The flask contains 492 mL of mercury at an equilibrium temperature of 20°C.The temperature is raised until the mercury reaches the 500 mL reference mark.At what temperature does this occur? The coefficients of volume expansion of mercury and glass are 18 × 10-5 K-1 (mercury)and 2.0 × 10-5 K-1 (glass).
A)120°C
B)110°C
C)100°C
D)140°C
E)130°C
A)120°C
B)110°C
C)100°C
D)140°C
E)130°C

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
54
The volume coefficient of thermal expansion for gasoline is 950 × 10-6 K-1.By how many cubic centimeters does the volume of 1.00 L of gasoline change when the temperature rises from 30°C to 50°C?
A)6.0 cm3
B)12 cm3
C)19 cm3
D)37 cm3
A)6.0 cm3
B)12 cm3
C)19 cm3
D)37 cm3

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
55
For the mercury in a thermometer to expand from 4.00 cm3 to 4.10 cm3,what change in temperature is necessary? The mercury has a volume expansion coefficient of 1.80 × 10-4 K-1.
A)400°C
B)140°C
C)14°C
D)8.2°C
A)400°C
B)140°C
C)14°C
D)8.2°C

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
56
The coefficient of linear expansion of aluminum is 24 × 10-6 K-1 and the coefficient of volume expansion of olive oil is 0.68 × 10-3 K-1.A novice cook,in preparation of some pesto,fills a 1.00-L aluminum pot to the brim and heats the oil and the pot from an initial temperature of 15°C to 190°C.To his consternation some olive oil spills over the top.How much?
A)0.11 L
B)0.12 L
C)0.13 L
D)0.14 L
E)0.15 L
A)0.11 L
B)0.12 L
C)0.13 L
D)0.14 L
E)0.15 L

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
57
The coefficient of linear expansion of copper is 17 × 10-6 K-1 and that of steel is 12 × 10-6 K-1.At 12°C a steel rod has a diameter of 2.540 cm and a copper pipe has a diameter of 2.536 cm.If they are heated together to a higher temperature,which one of the following quantities is closest to the common temperature at which the steel rod will fit snugly in the copper pipe?
A)310°C
B)330°C
C)340°C
D)350°C
E)380°C
A)310°C
B)330°C
C)340°C
D)350°C
E)380°C

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
58
If 150 kcal of heat raises the temperature of 2.0 kg of a material by 400°F,what is the specific heat capacity of the material?
A)1.35 kcal/kg ∙ °C
B)0.75 kcal/kg ∙ °C
C)0.34 kcal/kg ∙ °C
D)0.19 kcal/kg ∙ °C
A)1.35 kcal/kg ∙ °C
B)0.75 kcal/kg ∙ °C
C)0.34 kcal/kg ∙ °C
D)0.19 kcal/kg ∙ °C

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
59
A brass rod is 69.5 cm long and an aluminum rod is 49.3 cm long when both rods are at an initial temperature of 0° C.The rods are placed in line with a gap of 1.2 cm between them,as shown in the figure.The distance between the far ends of the rods is maintained at 120.0 cm throughout.The temperature of both rods is raised equally until they are barely in contact.At what temperature does contact occur? The coefficients of linear expansion of brass and aluminum are 2.0 × 10-5 K-1 (brass)and 2.4 × 10-5 K-1 (aluminum). 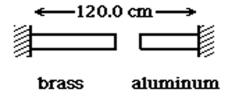
A)470°C
B)440°C
C)420°C
D)490°C
E)510°C

A)470°C
B)440°C
C)420°C
D)490°C
E)510°C

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
60
A mercury thermometer has a glass bulb of interior volume 0.100 cm3 at 10°C.The glass capillary tube above the bulb has an inner cross-sectional area of 0.012 mm2.The coefficient of volume expansion of mercury is 1.8 × 10-4 K-1.If the expansion of the glass is negligible,how much will the mercury rise in the capillary tube when the temperature rises from 5°C to 35°C if the bulb was full at 5°C?
A)0.45 mm
B)4.5 mm
C)45 mm
D)45 cm
A)0.45 mm
B)4.5 mm
C)45 mm
D)45 cm

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
61
In grinding a steel knife,the metal can get as hot as 400°C.If the blade has a mass of 80 g,what is the minimum amount of water needed at 20°C if the water is to remain liquid and not rise above 100°C when the hot blade is quenched in it? The specific heat of the steel is 0.11 cal/g ∙ °C and the specific heat of water is 1.0 cal/g ∙ °C.
A)22 g
B)33 g
C)44 g
D)55 g
A)22 g
B)33 g
C)44 g
D)55 g

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
62
A glass beaker of unknown mass contains 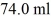
Of water.The system absorbs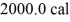
Of heat and the temperature rises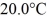
As a result.What is the mass of the beaker? The specific heat of glass is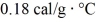
,and that of water is 1.0 cal/g ∙ °C.
A)140 g
B)560 g
C)540 g
D)270,000 g
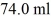
Of water.The system absorbs
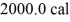
Of heat and the temperature rises
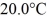
As a result.What is the mass of the beaker? The specific heat of glass is
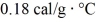
,and that of water is 1.0 cal/g ∙ °C.
A)140 g
B)560 g
C)540 g
D)270,000 g

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
63
A 6.5-g iron meteor hits the earth at a speed of 295 m/s.If its kinetic energy is entirely converted to heat in the meteor,by how much will its temperature rise? The specific heat of iron is 113 cal/kg ∙ °C,and 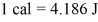
.
A)92.0°C
B)57,100°C
C)0.147°C
D)384°C
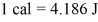
.
A)92.0°C
B)57,100°C
C)0.147°C
D)384°C

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
64
A lab student drops a 400.0-g piece of metal at 120.0°C into a cup containing 450.0 g of water at 15.0°C.After waiting for a few minutes,the student measures that the final temperature of the system is 40.0°C.What is the specific heat of the metal,assuming that no significant heat is exchanged with the surroundings or the cup? The specific heat of water is 4186 J/kg ∙ K.
A)1470 J/kg ∙ K
B)2830 J/kg ∙ K
C)3420 J/kg ∙ K
D)3780 J/kg ∙ K
E)4280 J/kg ∙ K
A)1470 J/kg ∙ K
B)2830 J/kg ∙ K
C)3420 J/kg ∙ K
D)3780 J/kg ∙ K
E)4280 J/kg ∙ K

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
65
A 5.00-g lead BB moving at 44.0 m/s penetrates a wood block and comes to rest inside the block.If half of its kinetic energy is absorbed by the BB,what is the change in the temperature of the BB? The specific heat of lead is 128 J/kg ∙ K.
A)0.940 K
B)1.10 K
C)1.26 K
D)2.78 K
E)3.78 K
A)0.940 K
B)1.10 K
C)1.26 K
D)2.78 K
E)3.78 K

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
66
An aluminum electric tea kettle with a mass of 500 g is heated with a 500-W heating coil.How long will it take to heat up 1.0 kg of water from 18°C to 98°C in the tea kettle? The specific heat of aluminum is 900 J/kg ∙ K and that of water is 4186 J/kg ∙ K.
A)5.0 minutes
B)7.0 minutes
C)12 minutes
D)15 minutes
E)18 minutes
A)5.0 minutes
B)7.0 minutes
C)12 minutes
D)15 minutes
E)18 minutes

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
67
A person tries to heat up her bath water by adding 5.0 L of water at 80°C to 60 L of water at 30°C.What is the final temperature of the bath water?
A)34°C
B)36°C
C)38°C
D)40°C
A)34°C
B)36°C
C)38°C
D)40°C

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
68
It is necessary to determine the specific heat of an unknown object.The mass of the object is 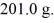
It is determined experimentally that it takes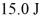
To raise the temperature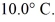
What is the specific heat of the object?
A)7.46 J/kg ∙ K
B)1,500 J/kg ∙ K
C)0.00130 J/kg ∙ K
D)3,020,000 J/kg ∙ K
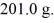
It is determined experimentally that it takes
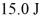
To raise the temperature
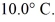
What is the specific heat of the object?
A)7.46 J/kg ∙ K
B)1,500 J/kg ∙ K
C)0.00130 J/kg ∙ K
D)3,020,000 J/kg ∙ K

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
69
If 50 g of lead (of specific heat 0.11 kcal/kg ∙ °C)at 100°C is put into 75 g of water (of specific heat 1.0 kcal/kg ∙ °C)at 0°C.What is the final temperature of the mixture?
A)2.0°C
B)6.8°C
C)25°C
D)50°C
A)2.0°C
B)6.8°C
C)25°C
D)50°C

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
70
A container of 114.0 g of water is heated using 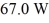
Of power,with perfect efficiency.How long will it take to raise the temperature of the water from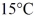
To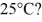
The specific heat capacity of the container is negligible,and the specific heat capacity of water is 4.186 × 103 J/kg ∙ C.
A)71 s
B)4.1 s
C)17 s
D)320,000 s
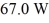
Of power,with perfect efficiency.How long will it take to raise the temperature of the water from
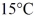
To
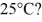
The specific heat capacity of the container is negligible,and the specific heat capacity of water is 4.186 × 103 J/kg ∙ C.
A)71 s
B)4.1 s
C)17 s
D)320,000 s

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
71
A lab assistant pours 330 g of water at 45°C into an 855-g aluminum container that is at an initial temperature of 10°C.The specific heat of aluminum is 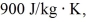
And that of water is 4186 J/kg ∙ K.What is the final temperature of the system,assuming no heat is exchanged with the surroundings?
A)28°C
B)32°C
C)31°C
D)33°C
E)35°C
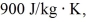
And that of water is 4186 J/kg ∙ K.What is the final temperature of the system,assuming no heat is exchanged with the surroundings?
A)28°C
B)32°C
C)31°C
D)33°C
E)35°C

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
72
In a flask,114.0 g of water is heated using 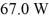
Of power,with perfect efficiency.How long will it take to raise the temperature of the water from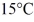
To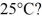
The specific heat of water is 4186 J/kg ∙ K.
A)71 s
B)4.1 s
C)17 s
D)320,000 s
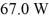
Of power,with perfect efficiency.How long will it take to raise the temperature of the water from
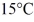
To
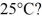
The specific heat of water is 4186 J/kg ∙ K.
A)71 s
B)4.1 s
C)17 s
D)320,000 s

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
73
A lab assistant drops a 400.0-g piece of metal at 100.0°C into a 100.0-g aluminum cup containing 500.0 g of water at 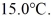
In a few minutes,she measures the final temperature of the system to be 40.0°C.What is the specific heat of the 400.0-g piece of metal,assuming that no significant heat is exchanged with the surroundings? The specific heat of this aluminum is 900.0 J/kg ∙ K and that of water is 4186 J/kg ∙ K.
A)1900 J/kg ∙ K
B)2270 J/kg ∙ K
C)3300 J/kg ∙ K
D)3800 J/kg ∙ K
E)4280 J/kg ∙ K
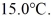
In a few minutes,she measures the final temperature of the system to be 40.0°C.What is the specific heat of the 400.0-g piece of metal,assuming that no significant heat is exchanged with the surroundings? The specific heat of this aluminum is 900.0 J/kg ∙ K and that of water is 4186 J/kg ∙ K.
A)1900 J/kg ∙ K
B)2270 J/kg ∙ K
C)3300 J/kg ∙ K
D)3800 J/kg ∙ K
E)4280 J/kg ∙ K

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
74
A 200-L electric water heater uses 2.0 kW.Assuming no heat loss,how many hours would it take to heat the water in this tank from 23°C to 75°C? The specific heat of water is 4186 J/kg ∙ K and its density is 1000 kg/m3.
A)5.0.hours
B)6.0 hours
C)7.0 hours
D)8.0 hours
A)5.0.hours
B)6.0 hours
C)7.0 hours
D)8.0 hours

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
75
On his honeymoon,James Joule attempted to explore the relationships between various forms of energy by measuring the rise of temperature of water which had fallen down a waterfall on Mount Blanc.What maximum temperature rise would one expect for a waterfall with a vertical drop of 20 m? The specific heat of water is 4186 J/kg ∙ K.
A)0.047°C
B)0.053°C
C)0.064°C
D)0.071°C
A)0.047°C
B)0.053°C
C)0.064°C
D)0.071°C

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
76
A camper is about to drink his morning coffee.He pours 400 grams of coffee,initially at 75°C into a 250-g aluminum cup,initially at 16°C.What is the equilibrium temperature of the coffee-cup system,assuming no heat is lost to the surroundings? The specific heat of aluminum is 900 J/kg ∙ K,and the specific heat of coffee is essentially the same as that of water,which is 4186 J/kg ∙ K.
A)45°C
B)62°C
C)65°C
D)68°C
E)71°C
A)45°C
B)62°C
C)65°C
D)68°C
E)71°C

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
77
The water flowing over Niagara Falls drops a distance of 50 m.If all the gravitational potential energy is converted to thermal energy,by what temperature does the water rise? The specific heat of water is 4186 J/kg ∙ K.
A)0.10°C
B)0.12°C
C)0.37°C
D)0.42°C
A)0.10°C
B)0.12°C
C)0.37°C
D)0.42°C

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
78
A 90-g aluminum calorimeter contains 390 g of water at an equilibrium temperature of 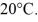
A
Piece of metal,initially at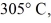
Is added to the calorimeter.The final temperature at equilibrium is 32° C.Assume there is no external heat exchange.The specific heat capacities of aluminum and water are 910 J/kg ∙ K (aluminum)and 4190 J/kg ∙ K (water).What is the specific heat capacity of the 160-g piece of metal?
A)470 J/kg ∙ K
B)430 J/kg ∙ K
C)350 J/kg ∙ K
D)310 J/kg ∙ K
E)510 J/kg ∙ K
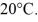
A

Piece of metal,initially at
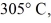
Is added to the calorimeter.The final temperature at equilibrium is 32° C.Assume there is no external heat exchange.The specific heat capacities of aluminum and water are 910 J/kg ∙ K (aluminum)and 4190 J/kg ∙ K (water).What is the specific heat capacity of the 160-g piece of metal?
A)470 J/kg ∙ K
B)430 J/kg ∙ K
C)350 J/kg ∙ K
D)310 J/kg ∙ K
E)510 J/kg ∙ K

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
79
A carpenter is driving a 15.0-g steel nail into a board.His 1.00-kg hammer is moving at 8.50 m/s when it strikes the nail.Half of the kinetic energy of the hammer is transformed into heat in the nail and does not flow out of the nail.What is the increase in temperature of the nail after the three blows that the carpenter needs to drive the nail in completely? The specific heat of steel is 448 J/kg ∙ K.
A)8.1 K
B)3.6 K
C)1.8 K
D)2.7 K
E)7.7 K
A)8.1 K
B)3.6 K
C)1.8 K
D)2.7 K
E)7.7 K

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
80
A 920-g empty iron kettle is put on a stove.How much heat in joules must it absorb to raise its temperature form 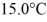
To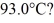
The specific heat for iron is 113 cal/kg ∙ °C,and 1 cal = 4.186 J.
A)33,900 J
B)40,500 J
C)8,110 J
D)40,100 J
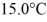
To
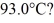
The specific heat for iron is 113 cal/kg ∙ °C,and 1 cal = 4.186 J.
A)33,900 J
B)40,500 J
C)8,110 J
D)40,100 J

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck


