Deck 6: Neutralizing the Threat of Acid Rain
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/68
Play
Full screen (f)
Deck 6: Neutralizing the Threat of Acid Rain
1
Which substance has the highest pH?
A)orange juice
B)rain
C)a sulfuric acid solution
D)a lye solution
A)orange juice
B)rain
C)a sulfuric acid solution
D)a lye solution
a lye solution
2
Which reaction most accurately represents the dissociation of nitrous acid (HNO2)in water?
A)HNO2 + H2O → H3O+ + NO2¯
B)HNO2 + H2O → H3O2+ + NO¯
C)HNO2 + H2O → HN + HO+ + HO2¯
D)HNO2 + H2O → H3O+ + N + O2¯
A)HNO2 + H2O → H3O+ + NO2¯
B)HNO2 + H2O → H3O2+ + NO¯
C)HNO2 + H2O → HN + HO+ + HO2¯
D)HNO2 + H2O → H3O+ + N + O2¯
HNO2 + H2O → H3O+ + NO2¯
3
Which anthropogenic pollutants are implicated in the formation of most acidic precipitation?
A)carbon oxides
B)phosphoric acid and hydrochloric acid
C)ozone and carbon monoxide
D)nitrogen oxides and sulfur oxides
A)carbon oxides
B)phosphoric acid and hydrochloric acid
C)ozone and carbon monoxide
D)nitrogen oxides and sulfur oxides
nitrogen oxides and sulfur oxides
4
Which concentration is consistent with a basic solution?
A)MH+ = 3.2 × 10¯3 M
B)MOH- = 3.6 × 10¯9 M
C)MH+ = 9.7 × 10¯4 M
D)MOH- = 3.4 × 10¯3 M
A)MH+ = 3.2 × 10¯3 M
B)MOH- = 3.6 × 10¯9 M
C)MH+ = 9.7 × 10¯4 M
D)MOH- = 3.4 × 10¯3 M

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
5
The Clean Air Act Amendments of 1990 authorize the assignment of permits to corporations allowing a predetermined level of sulfur dioxide emissions.Permit holders that keep emissions below the permit level may
A)charge a higher price for generated power.
B)sell their unused emission rights to another corporation.
C)receive tax rebates.
D)receive a lower emission permit in the future.
A)charge a higher price for generated power.
B)sell their unused emission rights to another corporation.
C)receive tax rebates.
D)receive a lower emission permit in the future.

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
6
When dissolved in water,hydrogen bromide (HBr)forms hydrobromic acid.Determine the hydroxide ion concentration in a 4,500 mL solution containing 3.78 g hydrogen bromide;Kw = 1.00 × 10¯14.
A)[OH¯] = 9.63 × 10¯13 M
B)[OH¯] = 2.14 × 10¯13 M
C)[OH¯] = 4.67 × 10¯2 M
D)[OH¯] = 0.0104 M
A)[OH¯] = 9.63 × 10¯13 M
B)[OH¯] = 2.14 × 10¯13 M
C)[OH¯] = 4.67 × 10¯2 M
D)[OH¯] = 0.0104 M

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
7
A proton released by an acid in aqueous solution quickly reacts with water to form a hydronium ion.What product is formed when a proton reacts with ammonia (NH3)?
A)NH3+
B)NH4
C)NH2¯
D)NH4+
A)NH3+
B)NH4
C)NH2¯
D)NH4+

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
8
Calculate the pH of a solution prepared by dissolving 1.2 g of potassium hydroxide (KOH)in 1,250 mL of water.
A)0.017
B)1.77
C)9.22
D)12.23
A)0.017
B)1.77
C)9.22
D)12.23

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
9
Which chemical equation shows the dissociation of magnesium hydroxide?
A)Mg(OH)2 → Mg2+ + 2OH¯
B)MgOH → Mg2+ + OH2¯
C)Mg(OH)3 → Mg3+ + 3OH¯
D)Mg(OH)2 → Mg2+ + H2O + O2-
A)Mg(OH)2 → Mg2+ + 2OH¯
B)MgOH → Mg2+ + OH2¯
C)Mg(OH)3 → Mg3+ + 3OH¯
D)Mg(OH)2 → Mg2+ + H2O + O2-

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
10
Which chemical equation shows the dissociation of 2 protons from trihydrogen phosphate (phosphoric acid)?
A)H3PO4 → H2+ + HPO4¯
B)H3PO4 → H22+ + PO42¯
C)H3PO4 → 2H+ + HPO42¯
D)H3PO4 → H33+ + PO43¯
A)H3PO4 → H2+ + HPO4¯
B)H3PO4 → H22+ + PO42¯
C)H3PO4 → 2H+ + HPO42¯
D)H3PO4 → H33+ + PO43¯

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
11
Sulfur oxides have been implicated as important contributors to the problem of acid rain.What is the principal anthropogenic source of these compounds?
A)transportation
B)coal fired power plants
C)lightning
D)volcanoes
A)transportation
B)coal fired power plants
C)lightning
D)volcanoes

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
12
What atmospheric component is responsible for the natural acidity of rain?
A)nitrogen
B)ozone
C)oxygen
D)carbon dioxide
A)nitrogen
B)ozone
C)oxygen
D)carbon dioxide

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
13
The pH of lemon juice is approximately 2.40.At this pH,the hydronium ion concentration is closest to which concentration?
A)2.5 × 10¯12 M
B)4.0 × 10¯3 M
C)0.38 M
D)5.6 × 10¯4 M
A)2.5 × 10¯12 M
B)4.0 × 10¯3 M
C)0.38 M
D)5.6 × 10¯4 M

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
14
Evaluate the ratio MH+ (pH 3)/ MH+ (pH 7).
A)0.0001
B)0.001
C)0.429
D)10,000
A)0.0001
B)0.001
C)0.429
D)10,000

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
15
The formation of nitric oxide (NO,a precursor to nitric acid)from oxygen and nitrogen is ordinarily a very slow process.What accounts for its more rapid formation in automobile exhaust?
A)the extreme conditions involved in gasoline combustion
B)the presence of nitrogen compounds in gasoline
C)catalysts present in fuel that facilitate its formation
D)well-designed catalytic converters
A)the extreme conditions involved in gasoline combustion
B)the presence of nitrogen compounds in gasoline
C)catalysts present in fuel that facilitate its formation
D)well-designed catalytic converters

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
16
Coal is not a pure chemical compound,but its elemental composition can be approximated by the formula C135H96NO9S.What is the approximate percentage by mass of sulfur in coal?
A)0.413%
B)1.68%
C)3.11%
D)42.8%
A)0.413%
B)1.68%
C)3.11%
D)42.8%

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
17
Coal switching refers to the practice of
A)converting oil or natural gas power plants to coal because coal is cheaper.
B)pulverizing coal in order to make it burn more efficiently.
C)converting coal power plants to oil or natural gas to reduce SOx emissions.
D)using coal with a lower sulfur content to reduce SOx emissions.
A)converting oil or natural gas power plants to coal because coal is cheaper.
B)pulverizing coal in order to make it burn more efficiently.
C)converting coal power plants to oil or natural gas to reduce SOx emissions.
D)using coal with a lower sulfur content to reduce SOx emissions.

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
18
The hydronium ion concentration in a solution with pH 10 is __________ than the hydronium ion concentration in a solution with pH 13.
A)1,000 times less
B)3 times greater
C)1,000 times greater
D)100 times less
A)1,000 times less
B)3 times greater
C)1,000 times greater
D)100 times less

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
19
Predict the products of this reaction: KOH + HNO3 →
A)KNO3 + H2O
B)KNO2 + H2O2
C)KH + HNO4
D)KO4 + H2N
A)KNO3 + H2O
B)KNO2 + H2O2
C)KH + HNO4
D)KO4 + H2N

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
20
Calculate the amount of sulfur dioxide produced annually by a power plant that burns 7.5 million metric tons (t)of coal over a year.Assume that the coal is 2.4% sulfur by mass.1 metric ton (t)= 1000 kg.
A)3.6 × 108 kg
B)3.6 × 108 t
C)1.8 × 1011 kg
D)7.5 × 1012 g
A)3.6 × 108 kg
B)3.6 × 108 t
C)1.8 × 1011 kg
D)7.5 × 1012 g

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
21
Calculate the percentage decease in NOx emissions after clean coal technology was installed at the Milliken power station. 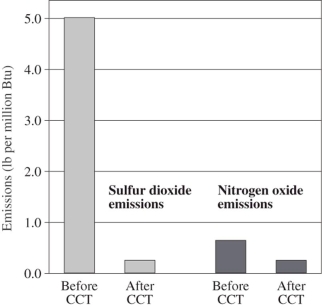
A)0.39%
B)39%
C)61%
D)156%

A)0.39%
B)39%
C)61%
D)156%

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
22
Carbon dioxide is the acid anhydride of which compound?
A)CH2O3
B)CO
C)CH2O
D)CHO2
A)CH2O3
B)CO
C)CH2O
D)CHO2

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
23
Lakes surrounded by ______ have very little acid-neutralizing capacity.
I.marble
II.granite
III.limestone
A)I only
B)II only
C)I and III only
D)II and III only
I.marble
II.granite
III.limestone
A)I only
B)II only
C)I and III only
D)II and III only

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
24
What is the current expert consensus concerning the role of acid rain on the health of forests?
A)Acid rain is indisputably responsible for the declining health of many forests.
B)Acid rain is not responsible for any of the decline observed in many forests.
C)Acid rain weakens trees and the surrounding soil,leaving them susceptible to disease and insects.
D)There is not sufficient evidence that acid rain has caused an appreciable decline in the health of any forests.
A)Acid rain is indisputably responsible for the declining health of many forests.
B)Acid rain is not responsible for any of the decline observed in many forests.
C)Acid rain weakens trees and the surrounding soil,leaving them susceptible to disease and insects.
D)There is not sufficient evidence that acid rain has caused an appreciable decline in the health of any forests.

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
25
The primary product of the combustion of sulfur is:
A)H2S
B)H2SO4
C)H2SO3
D)SO2
A)H2S
B)H2SO4
C)H2SO3
D)SO2

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
26
Sulfur dioxide,the primary product of sulfur combustion,further reacts with oxygen as shown.
2 SO2 + O2 → 2 SO3
What is the final step in the formation of the acidic aerosol?
A)Reaction of sulfur trioxide with nitrogen oxide.
B)Reaction of sulfur trioxide with water.
C)Reaction of sulfur trioxide with sodium hydroxide.
D)Reaction of sulfur trioxide with nitrogen gas.
2 SO2 + O2 → 2 SO3
What is the final step in the formation of the acidic aerosol?
A)Reaction of sulfur trioxide with nitrogen oxide.
B)Reaction of sulfur trioxide with water.
C)Reaction of sulfur trioxide with sodium hydroxide.
D)Reaction of sulfur trioxide with nitrogen gas.

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
27
Why did the industrial-scale production of ammonia from nitrogen and hydrogen present a difficult challenge?
A)Ammonia is explosive.
B)Nitrogen is difficult to purify.
C)Nitrogen is unreactive.
D)Hydrogen is unreactive.
A)Ammonia is explosive.
B)Nitrogen is difficult to purify.
C)Nitrogen is unreactive.
D)Hydrogen is unreactive.

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
28
One of the functions of an automobile catalytic converter contributes to the battle against acid rain.Which reaction represents this function as carried out by a catalytic converter?
A)HNO2 + NaOH → H2O + NaNO2
B)NO2 + H2O → HO+ + HNO2¯
C)2 NO → N2 + O2
D)HNO3 + NaOH → H2O + NaNO3
A)HNO2 + NaOH → H2O + NaNO2
B)NO2 + H2O → HO+ + HNO2¯
C)2 NO → N2 + O2
D)HNO3 + NaOH → H2O + NaNO3

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
29
Bases produce which ions in aqueous solution?
A)OH¯
B)NO2¯
C)Cl¯
D)SO3¯
A)OH¯
B)NO2¯
C)Cl¯
D)SO3¯

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
30
For what purpose was the Haber-Bosch process developed?
A)reduction of SOx in coal burning power plant emissions
B)reduction of NOx in vehicle emissions
C)nitrification of soil
D)production of ammonia
A)reduction of SOx in coal burning power plant emissions
B)reduction of NOx in vehicle emissions
C)nitrification of soil
D)production of ammonia

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
31
Which nitrogen-containing substance is generally unreactive?
A)NO
B)NaNO2
C)N2
D)NH3
A)NO
B)NaNO2
C)N2
D)NH3

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
32
Why have efforts to reduce sulfur oxide emissions met with greater success than those directed at nitrogen oxide emissions reductions?
A)Sulfur oxides have higher molar masses than nitrogen oxides.
B)Sulfur oxide emissions come from a limited number of point sources.
C)Nitrogen oxides are only produced at power plants.
D)Nitrogen reacts more readily with oxygen than sulfur.
A)Sulfur oxides have higher molar masses than nitrogen oxides.
B)Sulfur oxide emissions come from a limited number of point sources.
C)Nitrogen oxides are only produced at power plants.
D)Nitrogen reacts more readily with oxygen than sulfur.

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
33
Which solution is acidic?
A)MOH¯ = 0.0065 M
B)MH+ = 1.27 × 10¯9 M
C)MH+ = 5.79 × 10¯10 M
D)MOH¯ = 1.77 × 10¯10 M
A)MOH¯ = 0.0065 M
B)MH+ = 1.27 × 10¯9 M
C)MH+ = 5.79 × 10¯10 M
D)MOH¯ = 1.77 × 10¯10 M

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
34
Most of the sulfur in coal originates from
A)decaying vegetation in swamps and peat bogs.
B)sulfate ions (SO42-)naturally present in sea water.
C)sulfur deposits located adjacent to coal deposits.
D)the natural sulfur content of the plants making up the coal.
A)decaying vegetation in swamps and peat bogs.
B)sulfate ions (SO42-)naturally present in sea water.
C)sulfur deposits located adjacent to coal deposits.
D)the natural sulfur content of the plants making up the coal.

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
35
Cars and trucks produce most of the NOx emissions and they also suffer the deleterious effects of acid rain.Which negative effects of acid rain affect cars and trucks?
I.decreased gas mileage
II.damage to the paint and finish
III.increased susceptibility to rust
IV.reduced radio reception
A)I and II only
B)I and IV only
C)II and III only
D)III and IV only
I.decreased gas mileage
II.damage to the paint and finish
III.increased susceptibility to rust
IV.reduced radio reception
A)I and II only
B)I and IV only
C)II and III only
D)III and IV only

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
36
The acid neutralizing capacity of a lake or stream most often derives from the presence of _______ in the surrounding soil or rock.
A)CaCO3
B)HNO3
C)NaCl
D)Sr(OH)2
A)CaCO3
B)HNO3
C)NaCl
D)Sr(OH)2

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
37
Soil nitrification refers to which process?
A)conversion of NO to NO2
B)conversion of nitrogen oxides to nitric acid
C)conversion of N2 to NO2¯
D)conversion of NH4+to NO3¯
A)conversion of NO to NO2
B)conversion of nitrogen oxides to nitric acid
C)conversion of N2 to NO2¯
D)conversion of NH4+to NO3¯

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
38
Which statement(s)concerning SOx and NOx emissions are true?
I.Automobiles produce NOx but very little SOx.
II.Power plants produce only SOx.
III.Diesel powered vehicles produce only SOx.
IV.The high concentration of nitrogen compounds in gasoline accounts for the formation of NOx by automobiles and trucks.
A)I only
B)I and II only
C)II and IV only
D)I,II,III,and IV
I.Automobiles produce NOx but very little SOx.
II.Power plants produce only SOx.
III.Diesel powered vehicles produce only SOx.
IV.The high concentration of nitrogen compounds in gasoline accounts for the formation of NOx by automobiles and trucks.
A)I only
B)I and II only
C)II and IV only
D)I,II,III,and IV

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
39
Which reaction represents an acid base neutralization reaction?
A)HNO2 + H2O → H3O+ + NO2¯
B)NaOH + H2O → H3O+ + NaO¯
C)Ba(OH)2 + 2LiCl → BaCl2 + 2LiOH
D)Be(OH)2 + H2SO4 → BeSO4 + 2H2O
A)HNO2 + H2O → H3O+ + NO2¯
B)NaOH + H2O → H3O+ + NaO¯
C)Ba(OH)2 + 2LiCl → BaCl2 + 2LiOH
D)Be(OH)2 + H2SO4 → BeSO4 + 2H2O

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
40
The acidity of rain was first studied in
A)the mid-1750s.
B)the mid-1850s.
C)1900.
D)the mid-1950s.
A)the mid-1750s.
B)the mid-1850s.
C)1900.
D)the mid-1950s.

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
41
Predict the products of the chemical equation: 3 LiOH + H3PO4 →
A)3 LiH + (OH)3PO4
B)3 H + 3 O2 + H3Li3
C)Li3PO4 + 3 H2O
D)Li3P + 2 H2O + H3O5
A)3 LiH + (OH)3PO4
B)3 H + 3 O2 + H3Li3
C)Li3PO4 + 3 H2O
D)Li3P + 2 H2O + H3O5

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
42
An acidic fog in Pasadena was found to have a pH of 2.50.Which expression represents this pH measurement?
A)MOH¯ = 3.2 × 10¯12 M
B)MOH¯ = 3.2 × 10¯9 M
C)MH+ = 9.7 × 10¯3 M
D)MH+ = 2.5 M
A)MOH¯ = 3.2 × 10¯12 M
B)MOH¯ = 3.2 × 10¯9 M
C)MH+ = 9.7 × 10¯3 M
D)MH+ = 2.5 M

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
43
The amounts of SO2 and NOx emissions from anthropogenic sources are ______ those from natural sources.
A)considerably greater than
B)roughly equivalent to
C)somewhat less than
D)considerably less than
A)considerably greater than
B)roughly equivalent to
C)somewhat less than
D)considerably less than

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
44
Which reaction accounts for the fact that the pH of rain is naturally slightly acidic?
A)CO2 + H2O → H+ + HCO3¯
B)SO3 + H2O → 2 H+ + SO42¯
C)Ca2+ + CO2 + H2O → CaCO3 + 2 H+
D)NO2 + H2O → 2 H+ + NO3¯
A)CO2 + H2O → H+ + HCO3¯
B)SO3 + H2O → 2 H+ + SO42¯
C)Ca2+ + CO2 + H2O → CaCO3 + 2 H+
D)NO2 + H2O → 2 H+ + NO3¯

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
45
Most aquatic life in lakes cannot survive in water with a pH less than
A)9.
B)8.
C)6.
D)5.
A)9.
B)8.
C)6.
D)5.

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
46
What is the source of the nitrogen in the NOx emitted from fossil fuel combustion?
A)the atmosphere
B)coal
C)natural gas
D)oil
A)the atmosphere
B)coal
C)natural gas
D)oil

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
47
Scrubbers installed on coal fired power plants remove acidic emissions by the following reaction:
CaCO3 + H2SO4 → H2O + CaSO4 + CO2
If a plant burns 2.4 million metric tons of coal (1 t = 1000 kg)containing 1.3% sulfur,how much limestone (CaCO3)will be consumed neutralizing the resulting acid? Assume that all of the sulfur is converted to sulfuric acid and that the acid is completely converted to calcium sulfate.
A)2.4 × 109 kg
B)1.3 × 108 kg
C)3.1 × 107 kg
D)9.7 × 107 kg
CaCO3 + H2SO4 → H2O + CaSO4 + CO2
If a plant burns 2.4 million metric tons of coal (1 t = 1000 kg)containing 1.3% sulfur,how much limestone (CaCO3)will be consumed neutralizing the resulting acid? Assume that all of the sulfur is converted to sulfuric acid and that the acid is completely converted to calcium sulfate.
A)2.4 × 109 kg
B)1.3 × 108 kg
C)3.1 × 107 kg
D)9.7 × 107 kg

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
48
A substance that is hygroscopic
A)absorbs and retains moisture from the atmosphere.
B)releases water to the atmosphere.
C)purifies water by removing nitrate and sulfate ions.
D)prevents atmospheric gases from forming aerosols.
A)absorbs and retains moisture from the atmosphere.
B)releases water to the atmosphere.
C)purifies water by removing nitrate and sulfate ions.
D)prevents atmospheric gases from forming aerosols.

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
49
Which reactive nitrogen compound is produced using the Haber-Bosch process?
A)NaNO3
B)KNO3
C)NH3
D)NO2
A)NaNO3
B)KNO3
C)NH3
D)NO2

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
50
The production of nickel and copper metals from their ores involves sulfur dioxide emissions because
A)the ores of these elements are composed primarily of NiSO2 and CuSO2.
B)the ores of these elements are composed primarily of NiS and CuS.
C)to release the metals from their ores,the ores are heated with sulfur-containing coal.
D)the release of metals from their ores involves the reaction of SO3 with the ore.
A)the ores of these elements are composed primarily of NiSO2 and CuSO2.
B)the ores of these elements are composed primarily of NiS and CuS.
C)to release the metals from their ores,the ores are heated with sulfur-containing coal.
D)the release of metals from their ores involves the reaction of SO3 with the ore.

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
51
Which reaction illustrates how acid rain causes the decomposition of marble monuments?
A)Ca(OH)2 + H2+ → Ca2+ + 2 H2O
B)CaCO3 + 2 H+ → Ca2+ + CO2 + H2O
C)4 Fe2+ + O2 + 4 H2O → 2 Fe2O3 + 8 H+
D)Ca(OH)2 + 2 H2O → CaO22¯ + 2 H3O+
A)Ca(OH)2 + H2+ → Ca2+ + 2 H2O
B)CaCO3 + 2 H+ → Ca2+ + CO2 + H2O
C)4 Fe2+ + O2 + 4 H2O → 2 Fe2O3 + 8 H+
D)Ca(OH)2 + 2 H2O → CaO22¯ + 2 H3O+

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
52
Nationally,the greatest amounts of NOx in the atmosphere are produced by which of these?
I.transporation sources (motor vehicles,aircraft,trains)
II.combustion of fuel in electrical utility plants and industry releases
III.nitrification in farm fields
A)I and II only
B)I and III only
C)II and III only
D)I,II,and III
I.transporation sources (motor vehicles,aircraft,trains)
II.combustion of fuel in electrical utility plants and industry releases
III.nitrification in farm fields
A)I and II only
B)I and III only
C)II and III only
D)I,II,and III

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
53
Iron,an important component of the steel used in the construction of buildings,bridges,and railroads,combines with oxygen to form Fe2O3,which we recognize as rust.At room temperature,iron
A)combines readily with atmospheric oxygen to form Fe2O3.
B)requires the presence of a galvanizing agent and oxygen before it can rust.
C)gains valence electrons from atmospheric oxygen,forming Fe2O3.
D)requires the presence of hydrogen ions and oxygen before it can form Fe2O3.
A)combines readily with atmospheric oxygen to form Fe2O3.
B)requires the presence of a galvanizing agent and oxygen before it can rust.
C)gains valence electrons from atmospheric oxygen,forming Fe2O3.
D)requires the presence of hydrogen ions and oxygen before it can form Fe2O3.

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
54
Bases are substances that increase the hydroxide ion concentration in aqueous solution.Why does ammonia (NH3),which does not contain a hydroxide group,act as a base?
A)Ammonia acts as a base only in the presence of hydroxide ion-containing compounds.
B)Ammonia molecules remove protons from water molecules,forming hydroxide ions.
C)Ammonia molecules donate protons to water molecules,forming hydroxide ions.
D)Ammonia acts as a base only in the presence of very strong acids.
A)Ammonia acts as a base only in the presence of hydroxide ion-containing compounds.
B)Ammonia molecules remove protons from water molecules,forming hydroxide ions.
C)Ammonia molecules donate protons to water molecules,forming hydroxide ions.
D)Ammonia acts as a base only in the presence of very strong acids.

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
55
If acids are compounds that release protons (H+),how is it that SOx and NOx cause acid rain?
A)They react with hydrogen gas in the atmosphere to produce acids.
B)There is not sufficient evidence to indicate that these compounds actually do cause acid rain.
C)They react with water to form acids.
D)They react with ammonia to form acids.
A)They react with hydrogen gas in the atmosphere to produce acids.
B)There is not sufficient evidence to indicate that these compounds actually do cause acid rain.
C)They react with water to form acids.
D)They react with ammonia to form acids.

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
56
What is the concentration of hydroxide ions in an aqueous solution containing [H+] = 1 x 10-5 M?
A)1 x 10-5 M
B)1 x 10-7 M
C)1 x 10-9 M
D)1 x 10-14 M
A)1 x 10-5 M
B)1 x 10-7 M
C)1 x 10-9 M
D)1 x 10-14 M

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
57
In the early 1990s,glass manufacturers developed a green chemistry solution to reducing NO emissions and energy consumption.This involved
A)reducing the temperature required to melt the glass.
B)using liquid rather than solid starting materials to make the glass.
C)substituting 100% oxygen for air in the large furnaces used to melt and reheat the glass.
D)melting the starting materials in the absence of air (in a vacuum).
A)reducing the temperature required to melt the glass.
B)using liquid rather than solid starting materials to make the glass.
C)substituting 100% oxygen for air in the large furnaces used to melt and reheat the glass.
D)melting the starting materials in the absence of air (in a vacuum).

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
58
Which of the following is a weak base?
A)HCl
B)NaOH
C)NH3
D)LiOH
A)HCl
B)NaOH
C)NH3
D)LiOH

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
59
The pH of rain water falling through an unpolluted atmosphere is closest to:
A)4.7
B)5.4
C)7.0
D)8.7
A)4.7
B)5.4
C)7.0
D)8.7

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
60
In addition to fossil fuel combustion,what other facet of modern industrial society contributes to the formation of acid rain?
A)the use of chlorofluorocarbons
B)deforestation
C)fertilizer production
D)the use of river water in cooling towers
A)the use of chlorofluorocarbons
B)deforestation
C)fertilizer production
D)the use of river water in cooling towers

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
61
Which of the following countries is the largest consumer of coal (by total mass)?
A)China
B)United States
C)India
D)Indonesia
A)China
B)United States
C)India
D)Indonesia

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
62
Why are mountaintops more susceptible than grassy plains to acid deposition?
A)They are made of softer rock.
B)They contact clouds with more concentrated acid droplets.
C)They are often buried under snow.
D)There is less oxygen at high altitude.
A)They are made of softer rock.
B)They contact clouds with more concentrated acid droplets.
C)They are often buried under snow.
D)There is less oxygen at high altitude.

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
63
Every increase of one pH unit indicates
A)an increase in acidity.
B)10 times more hydrogen ions in solution.
C)10 times less hydrogen ions in solution.
D)none of the above.
A)an increase in acidity.
B)10 times more hydrogen ions in solution.
C)10 times less hydrogen ions in solution.
D)none of the above.

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
64
Which of the following compounds contributes to "dry" acid deposition?
A)Calcium carbonate
B)Ammonium nitrate
C)Sodium hydroxide
D)All of the above
A)Calcium carbonate
B)Ammonium nitrate
C)Sodium hydroxide
D)All of the above

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
65
Which of the following is not carried out by bacteria in soil?
A)Atmospheric nitrogen is fixed in soils.
B)Ammonium is converted into nitrites.
C)Nitrites are converted to nitrates.
D)All of these are carried out by bacteria.
A)Atmospheric nitrogen is fixed in soils.
B)Ammonium is converted into nitrites.
C)Nitrites are converted to nitrates.
D)All of these are carried out by bacteria.

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
66
Scientists predict that shells of some ocean creatures will start to dissolve after how many more years of ocean acidification?
A)40 years
B)150 years
C)300 years
D)85 years
A)40 years
B)150 years
C)300 years
D)85 years

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
67
The ocean has an acidic pH due to carbon dioxide emissions.

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
68
Why are automobiles a contributor to acid rain when there is no sulfur in gasoline to make SOx?
A)This is incorrect because there is sulfur in gasoline.
B)Catalytic converters contain sulfur compounds.
C)Hydrocarbons contribute to acid rain,also.
D)Nitrogen from the atmosphere combines with oxygen in the hot engine to make NOx.
A)This is incorrect because there is sulfur in gasoline.
B)Catalytic converters contain sulfur compounds.
C)Hydrocarbons contribute to acid rain,also.
D)Nitrogen from the atmosphere combines with oxygen in the hot engine to make NOx.

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck


