Deck 30: Quantum Physics
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/100
Play
Full screen (f)
Deck 30: Quantum Physics
1
The Compton effect justifies that light acts as photons.
True
2
In quantum tunneling, electrons and other quantum particles can tunnel through a region of space that would be forbidden to them if they were classical particles.
True
3
A photon is a particle with positive charge.
False
4
The minimum amount of energy necessary to eject an electron from a metal surface is referred as Planck's constant.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
5
An ideal blackbody absorbs all of the light that is incident on it.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
6
In the spectrum of white light which one of the following colors corresponds to the highest temperature?
A) Yellow
B) Violet
C) Green
D) Red
E) Orange
A) Yellow
B) Violet
C) Green
D) Red
E) Orange

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
7
The de Broglie wavelength is inversely proportional to the momentum of the particle.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
8
The momentum of a photon is inversely proportional to its total energy.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
9
Which one of the following is true for the surface temperature of a bluish-white star in a constellation as compared to the red star in the same constellation?
A) It is greater.
B) It is less.
C) It is the same.
A) It is greater.
B) It is less.
C) It is the same.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
10
The distribution of energy in the blackbody radiation depends upon the material from which the blackbody is constructed.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
11
A blackbody is a perfect emitter of the radiation it generates.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
12
Unlike other particles, photons have a finite momentum even though they have no mass.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
13
The rest mass of a photon is zero kg.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
14
If a beam of X-rays is scattered from protons instead of electrons, the change in the wavelength of this X-ray beam for a given angle of scattering decreases.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
15
Electrons come in discrete units of well defined mass and charge, but they also have wave properties.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
16
Which one of the following is the correct expression for the energy of the photon?
A) E = h/f
B) E = hλ/c
C) E = hc/λ
D) E = λc/h
E) E = hλ
A) E = h/f
B) E = hλ/c
C) E = hc/λ
D) E = λc/h
E) E = hλ

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
17
The energy of an ultraviolet photon is more than the energy of an infrared photon.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
18
The wavelength of a photon decreases when it is scattered from an electron.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
19
In the scattering of a photon striking an electron at rest, the energy of the incident photon is equal to the energy of scattered photon minus the final kinetic energy of the electron.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
20
A zero value for the Planck's constant would mean that the laws of classical physics would apply to quantum physics.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
21
The frequency of a light beam is doubled. Which one of the following is correct for the momentum of the photons in that beam of light?
A) It stays the same.
B) It is halved.
C) It is doubled.
D) It is reduced by one-fourth
E) It is quadrupled.
A) It stays the same.
B) It is halved.
C) It is doubled.
D) It is reduced by one-fourth
E) It is quadrupled.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
22
The wavelength of a light beam is doubled. Which one of the following is correct for the momentum of photons for that light beam?
A) It is halved.
B) It stays the same.
C) It is doubled.
D) It is reduced by one-fourth
E) It is quadrupled.
A) It is halved.
B) It stays the same.
C) It is doubled.
D) It is reduced by one-fourth
E) It is quadrupled.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
23
What happens to the frequency of the scattered X-rays beam as the scattering angle in the Compton effect decreases?
A) It stays the same.
B) It decreases.
C) It increases.
D) Not enough information
A) It stays the same.
B) It decreases.
C) It increases.
D) Not enough information

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
24
Light of a given wavelength is used to illuminate the surface of a metal, however, no photoelectrons are emitted. In order to cause electrons to be ejected from the surface of this metal you should
A) use light of a longer wavelength.
B) use light of a shorter wavelength.
C) use light of the same wavelength but increase its intensity.
D) use light of the same wavelength but decrease its intensity.
A) use light of a longer wavelength.
B) use light of a shorter wavelength.
C) use light of the same wavelength but increase its intensity.
D) use light of the same wavelength but decrease its intensity.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
25
When you decrease, by a factor of two, the wavelength of the light causing photoelectrons to be emitted from a metal surface, the kinetic energy of the photoelectrons will
A) decrease by a factor of 2.
B) double
C) more than double.
D) increase by a factor of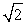 .
.
E) decrease by a factor of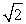 .
.
A) decrease by a factor of 2.
B) double
C) more than double.
D) increase by a factor of
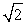 .
.E) decrease by a factor of
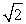 .
.
Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
26
Which one of the following is the correct expression of the cutoff frequency of the metal in terms of its work function?
A) fo = Wo/h
B) fo = Wo/c
C) fo = c/Wo
D) fo = h/Wo
E) None of the other choices is correct.
A) fo = Wo/h
B) fo = Wo/c
C) fo = c/Wo
D) fo = h/Wo
E) None of the other choices is correct.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
27
If the wavelength of a photon is doubled, what happens to its energy?
A) It is halved.
B) It stays the same.
C) It is doubled.
D) It is quadrupled.
E) It is reduced by one-fourth.
A) It is halved.
B) It stays the same.
C) It is doubled.
D) It is quadrupled.
E) It is reduced by one-fourth.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
28
You perform Compton scattering experiments with X-rays of a fixed wavelength. In one experiment you scatter the X-rays from free electrons. In the second experiment you scatter the X-rays from protons instead. For X-rays scattered under a given angle, the wavelength change
A) will be larger for scattering from protons.
B) will be the same for both cases.
C) will be larger for scattering from electrons.
D) depends on the actual wavelength of the X-rays.
A) will be larger for scattering from protons.
B) will be the same for both cases.
C) will be larger for scattering from electrons.
D) depends on the actual wavelength of the X-rays.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
29
Protons are being accelerated in a particle accelerator. When the speed of the protons is doubled, their de Broglie wavelength will
A) increase by a factor of 4.
B) increase by a factor of 2.
C) decrease by a factor of 2.
D) increase by a factor of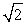 .
.
E) decrease by a factor of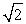 .
.
A) increase by a factor of 4.
B) increase by a factor of 2.
C) decrease by a factor of 2.
D) increase by a factor of
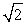 .
.E) decrease by a factor of
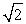 .
.
Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
30
Which one of the following equations is correct for the maximum kinetic energy of photoelectrons ejected from a metal?
A) Kmax = hc - Wo
B) Kmax = hλ - Wo
C) Kmax = hf - Wo
D) Kmax = hλ/c - Wo
E) None of the other choices is correct.
A) Kmax = hc - Wo
B) Kmax = hλ - Wo
C) Kmax = hf - Wo
D) Kmax = hλ/c - Wo
E) None of the other choices is correct.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
31
Which one of the following is the correct expression for the Heisenberg uncertainty principle?
A) ΔpyΔy ≥ h/2π
B) ΔpyΔy ≤ h/2π
C) Δpy/Δy ≤ h/2π
D) Δy/Δpy ≥ h/2π
E) None of the other answers is correct.
A) ΔpyΔy ≥ h/2π
B) ΔpyΔy ≤ h/2π
C) Δpy/Δy ≤ h/2π
D) Δy/Δpy ≥ h/2π
E) None of the other answers is correct.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
32
Protons are being accelerated in a particle accelerator. When the energy of the protons is doubled, their de Broglie wavelength will
A) increase by a factor of 4.
B) increase by a factor of 2.
C) decrease by a factor of 2.
D) increase by a factor of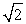 .
.
E) decrease by a factor of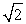 .
.
A) increase by a factor of 4.
B) increase by a factor of 2.
C) decrease by a factor of 2.
D) increase by a factor of
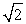 .
.E) decrease by a factor of
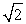 .
.
Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
33
Our bodies, at 37.°C, emit radiation which peaks at what wavelength?

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
34
In the spectrum of white light which one of the following colors corresponds to the lowest temperature?
A) Yellow
B) Blue
C) Red
D) Green
E) Orange
A) Yellow
B) Blue
C) Red
D) Green
E) Orange

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
35
Which of the following is the correct equation for the momentum of a photon?
A) p = (hc)/λ
B) p = λ/c
C) p = (hc)/f
D) p = (hf)/c
E) p = (hλ)/c
A) p = (hc)/λ
B) p = λ/c
C) p = (hc)/f
D) p = (hf)/c
E) p = (hλ)/c

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
36
If the position of an electron is measured very precisely there is an uncertainty in measuring its
A) kinetic energy.
B) momentum.
C) potential energy.
D) thermal energy.
E) None of the other answers is correct.
A) kinetic energy.
B) momentum.
C) potential energy.
D) thermal energy.
E) None of the other answers is correct.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
37
What scattering angle in the Compton effect produces the minimum change in frequency?
A) 45°
B) 90°
C) 0°
D) 180°
E) 270°
A) 45°
B) 90°
C) 0°
D) 180°
E) 270°

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
38
A proton and an electron are both accelerated to the same final kinetic energy. If λp is the de Broglie wavelength of the proton and λe is the de Broglie wavelength of the electron, then
A) λp > λe.
B) λp = λe.
C) λp < λe.
D) Not enough data to answer this question.
A) λp > λe.
B) λp = λe.
C) λp < λe.
D) Not enough data to answer this question.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
39
If the frequency of a photon is halved, what happens to its energy?
A) It is doubled.
B) It is halved.
C) It is tripled.
D) It is quadrupled.
E) It is reduced by one-fourth.
A) It is doubled.
B) It is halved.
C) It is tripled.
D) It is quadrupled.
E) It is reduced by one-fourth.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
40
A proton and an electron are both accelerated to the same final speed. If λp is the de Broglie wavelength of the proton and λe is the de Broglie wavelength of the electron, then
A) λp > λe.
B) λp = λe.
C) λp < λe.
D) Not enough data to answer this question.
A) λp > λe.
B) λp = λe.
C) λp < λe.
D) Not enough data to answer this question.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
41
What is the energy, in eV, of a photon of wavelength 550. nm?

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
42
X-rays with a wavelength of 0.00100 nm are scattered by free electrons at 130°. What is the kinetic energy of each recoil electron?

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
43
If the threshold wavelength to dislodge electrons from a metal is 373. nm, what is the work function?

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
44
What would be the deBroglie wavelength for 1 gram moving at "escape speed" 25,000. mph?

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
45
A small gas laser of the type used in classrooms may radiate light at a power level of 2.0 mW. If the wavelength of the emitted light is 642. nm, how many photons are emitted per second?

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
46
Atoms in crystals are typically separated by distances of 0.10 nm. What KE must an electron have, in eV, in order to have a wavelength of 0.10 nm?

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
47
How "slow" must a 200. gram ball move to have a deBroglie wavelength of 1.0 mm?

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
48
If in Compton scattering of X-rays from a metal, the wavelength increases by 5% at an angle of 60°, what was the original wavelength before scattering?

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
49
The cosmic background radiation permeating the universe has the spectrum of a 2.7 K thermal radiator. What is the peak wavelength?

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
50
At what rate are photons emitted by a 50. W sodium vapor lamp?
(Assume that the lamp's light is monochromatic and of wavelength 589. nm.)
(Assume that the lamp's light is monochromatic and of wavelength 589. nm.)

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
51
Mathilda collects photoelectric data in her physics laboratory. She measures a stopping potential of 5.82 volts for radiation of wavelength 100. nm, and 17.99 volts stops 50.0 nm.
(a) What is the work function for the material in eV?
(b) From her data one determines Planck's constant to be what?
(a) What is the work function for the material in eV?
(b) From her data one determines Planck's constant to be what?

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
52
A metallic surface is illuminated with light of wavelength 400. nm. If the work function for this metal is 2.4 eV, calculate the kinetic energy of the ejected electrons, in electron-volts.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
53
Through what potential must an electron be accelerated to observe maximum diffraction at 70.0° to the surface of a crystal with 0.334 nm between surface planes?

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
54
What primary wavelength and corresponding photon energy is emitted by a body at:
(a) 400.°C?
(b) 800.°C?
(c) 1200.°C?
(a) 400.°C?
(b) 800.°C?
(c) 1200.°C?

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
55
What is the wavelength of a photon of energy 2.00 eV?

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
56
A metallic surface has a work function of 2.50 eV. What is the longest wavelength that will eject electrons from the surface of this metal?

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
57
A 140. KeV photon strikes an electron and scatters through an angle of 120.°
(a) What is the wavelength of the photon before scattering?
(b) What is the photon wavelength after scattering?
(a) What is the wavelength of the photon before scattering?
(b) What is the photon wavelength after scattering?

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
58
FIGURE 30-1 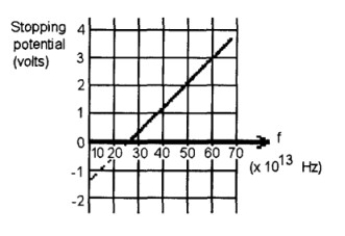
The graph shown in Figure 30-1 is a plot based on student data from their testing of a
photoelectric material.
(a) Determine the cutoff frequency.
(b) Determine the work function.

The graph shown in Figure 30-1 is a plot based on student data from their testing of a
photoelectric material.
(a) Determine the cutoff frequency.
(b) Determine the work function.

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
59
The human eye can just detect green light of wavelength 500. nm, which arrives at the retina at the rate of 2 × 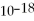 W. How many photons arrive each second?
W. How many photons arrive each second?
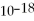 W. How many photons arrive each second?
W. How many photons arrive each second?
Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
60
What are the shortest wavelength X-rays produced by a 50,000-V X-ray tube?

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
61
A proton, moving freely, has a wavelength of 0.600 pm.
(a) What is its momentum?
(b) What is its speed?
(c) Through what potential difference might it have been accelerated through, from rest, to reach this speed?
(a) What is its momentum?
(b) What is its speed?
(c) Through what potential difference might it have been accelerated through, from rest, to reach this speed?

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
62
What is the energy of a photon with a frequency of 6.60 × 1015 Hz?
A) 27.3 eV
B) 12.3 eV
C) 18.1 eV
D) 19.6 eV
E) 6.60 eV
A) 27.3 eV
B) 12.3 eV
C) 18.1 eV
D) 19.6 eV
E) 6.60 eV

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
63
The surface temperature of the star is 6000 K. What is the wavelength associated with the light emitted by this star?
A) 850 nm
B) 907 nm
C) 311 nm
D) 492 nm
E) 502 nm
A) 850 nm
B) 907 nm
C) 311 nm
D) 492 nm
E) 502 nm

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
64
A photon has energy of 4.20 eV. To what wavelength does this energy correspond?
A) 321 nm
B) 103 nm
C) 296 nm
D) 412 nm
E) 420 nm
A) 321 nm
B) 103 nm
C) 296 nm
D) 412 nm
E) 420 nm

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
65
An electron determined to be at y = 2.000 ± 0.0010 cm has what minimum uncertainty in velocity?

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
66
A photon has an energy of 6.60 eV. To what wavelength does this energy correspond?
A) 100 nm
B) 118 nm
C) 151 nm
D) 173 nm
E) 189 nm
A) 100 nm
B) 118 nm
C) 151 nm
D) 173 nm
E) 189 nm

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
67
An electron is moving with speed 21. m/s.
(a) What is its deBroglie wavelength?
(b) What Diffraction Grating spacing between slits would give a 1st order maximum at 30.0°?
(a) What is its deBroglie wavelength?
(b) What Diffraction Grating spacing between slits would give a 1st order maximum at 30.0°?

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
68
What is the cutoff frequency for a metal surface that has a work function of 5.42 eV?
A) 1.31 × 1015 Hz
B) 2.01 × 1015 Hz
C) 3.01 × 1015 Hz
D) 5.02 × 1015 Hz
E) 6.04 × 1015 Hz
A) 1.31 × 1015 Hz
B) 2.01 × 1015 Hz
C) 3.01 × 1015 Hz
D) 5.02 × 1015 Hz
E) 6.04 × 1015 Hz

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
69
Suppose that the speed of an electron traveling 1.0 km/s is known to an accuracy of 1 part in 105 (i.e., within 0.01%). What is the greatest possible accuracy within which we can determine the position of this electron?

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
70
A 2.00 kg mass is attached to a spring with a spring constant of 48.0 N/m. If the maximum speed of the mass in this system is 1.14 m/s, what is the energy of the quanta of energy in this system?
A) 2.23 × 10-34 J
B) 6.63 J
C) 6.63 × 10-34 J
D) 5.17 J
E) 5.17 × 10-34 J
A) 2.23 × 10-34 J
B) 6.63 J
C) 6.63 × 10-34 J
D) 5.17 J
E) 5.17 × 10-34 J

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
71
What is the surface temperature of a star, given that its radiation peak occurs at a frequency of 1.06 × 1015 Hz?
A) 17,000 K
B) 14,500 K
C) 19,000 K
D) 20,400 K
E) 18,000 K
A) 17,000 K
B) 14,500 K
C) 19,000 K
D) 20,400 K
E) 18,000 K

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
72
An electron is trapped in a box of width 0.10 nm. What is the wavelength of the photon emitted when an electron makes a transition from the n = 3 to the n = 1 state?

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
73
A 60-W bulb is operating at an efficiency of 6.20%. What is the number of photons per second given off by the bulb assuming the wavelength of light to be 580 nm?
A) 5.42 × 1018 photons/s
B) 10.8 × 1018 photons/s
C) 16.2 × 1018 photons/s
D) 21.7 × 1018 photons/s
E) 25.3 × 1018 photons/s
A) 5.42 × 1018 photons/s
B) 10.8 × 1018 photons/s
C) 16.2 × 1018 photons/s
D) 21.7 × 1018 photons/s
E) 25.3 × 1018 photons/s

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
74
An electron is known to be confined to a region of width 0.10 nm. What is an approximate expression for the least kinetic energy it could have, expressed in eV?

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
75
The excited state of a certain atom is 3.2 eV ± 0.21 eV.
(a) What is the average lifetime of this state?
(b) If the excited energy doubled (6.4 eV ± 0.21 eV) how would the lifetime be affected?
(a) What is the average lifetime of this state?
(b) If the excited energy doubled (6.4 eV ± 0.21 eV) how would the lifetime be affected?

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
76
Which one of the following is correct for the energy of a 620 nm beam of light?
A) 1.60 × 10-19 eV
B) 2.10 × 10-19 J
C) 3.21 × 10-19 J
D) 1.60 × 10-18 eV
E) 3.21 × 10-18 eV
A) 1.60 × 10-19 eV
B) 2.10 × 10-19 J
C) 3.21 × 10-19 J
D) 1.60 × 10-18 eV
E) 3.21 × 10-18 eV

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
77
The work function of a certain metal is 1.90 eV. What is the longest wavelength of light that can cause photoelectron emission from this metal?
A) 231 nm
B) 14.0 nm
C) 62.4 nm
D) 344 nm
E) 653 nm
A) 231 nm
B) 14.0 nm
C) 62.4 nm
D) 344 nm
E) 653 nm

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
78
The radius of a typical nucleus is about 5.0 × 10-15 m. Assuming this to be the uncertainty in the position of a proton in the nucleus, estimate the uncertainty in the proton's energy (in eV).

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
79
What is the number of photons per second given off by a light bulb with a power of 100 Watts operating at an efficiency of 4.80%? Assume the wavelength of light to be 600 nm.
A) 6.34 × 1018 photons/s
B) 5.42 × 1018 photons/s
C) 3.11 × 1018 photons/s
D) 25.3 × 1018 photons/s
E) 14.5 × 1018 photons/s
A) 6.34 × 1018 photons/s
B) 5.42 × 1018 photons/s
C) 3.11 × 1018 photons/s
D) 25.3 × 1018 photons/s
E) 14.5 × 1018 photons/s

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck
80
A crystal diffracted a beam of electrons. The crystal spacing was 0.18 nm and the maximum scattering occurred at 80.° relative to the surface normal. The electrons were directed normally onto the crystal.
(a) What is the wavelength of the electrons?
(b) What potential difference accelerated the electrons?
(a) What is the wavelength of the electrons?
(b) What potential difference accelerated the electrons?

Unlock Deck
Unlock for access to all 100 flashcards in this deck.
Unlock Deck
k this deck


