Deck 31: Atomic Physics
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/75
Play
Full screen (f)
Deck 31: Atomic Physics
1
According to one of the assumptions of the Bohr Model, the electron in a hydrogen atom moves in an elliptical orbit about the nucleus.
False
2
The maximum number of electron states with a principal quantum number n = 3 is 15.
False
3
The state of a hydrogen atom is defined to be a specific assignment of values for each of the four quantum numbers.
True
4
The Bohr model correctly predicts the energy for the ground state of the hydrogen atom.

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
5
Ernest Rutherford tested Thomson's model by directing a beam of beta particles, at a thin gold foil.

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
6
In the Bohr model of the hydrogen atom the state of the atom is characterized by a single quantum number n.

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
7
Bohr's model of the structure of an atom includes ideas from both classical and quantum physics.

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
8
What range of electromagnetic radiation is emitted when transitions from higher levels into n = 1 occur in a hydrogen atom?
A) Visible
B) Infrared
C) Microwave
D) X-Ray
E) Ultraviolet
A) Visible
B) Infrared
C) Microwave
D) X-Ray
E) Ultraviolet

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
9
In a hydrogen atom, the difference in the energy between adjacent orbit radii increases with the increasing value of n.

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
10
The energy for n = 4 and ℓ = 0 state is greater than the energy for n = 3 and ℓ = 2 state.

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
11
The energy for n = 4 and ℓ = 2 state is greater than the energy for n = 5 and ℓ = 0 state.

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
12
To which of the following values of n does the longest wavelength in the Balmer series correspond?
A) 3
B) 5
C) 1
D) 7
E) ∞ (very large)
A) 3
B) 5
C) 1
D) 7
E) ∞ (very large)

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
13
Hydrogen atoms can emit four lines with visible colors from red to violet. These four visible lines emitted by hydrogen atoms are produced by electrons
A) that start in the n = 2 level.
B) that end up in the n = 2 level.
C) that end up in the n = 3 level.
D) that end up in the ground state.
E) that start in the ground state.
A) that start in the n = 2 level.
B) that end up in the n = 2 level.
C) that end up in the n = 3 level.
D) that end up in the ground state.
E) that start in the ground state.

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
14
The constant term in the Balmer series is called the Rydberg constant.

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
15
The allowed values for the magnetic quantum number, mℓ, are from mℓ = -ℓ to +ℓ.

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
16
To which of the following values of n does the shortest wavelength in the Balmer series correspond?
A) 3
B) 5
C) 7
D) 1
E) ∞ (very large)
A) 3
B) 5
C) 7
D) 1
E) ∞ (very large)

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
17
The kinetic energy of the electron in the first Bohr orbit of hydrogen is 2.18 × 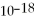 J.
J.
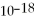 J.
J.
Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
18
The electron was discovered in 1897 by the English physicist J. J. Thomson.

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
19
The electron in a doubly ionized lithium atom experiences a weaker attractive force than the single electron in a hydrogen atom.

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
20
The Balmer series does not lie in the visible region of the electromagnetic spectrum.

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
21
In principle, a hologram is a result of which of the following properties of light?
A) Transmission
B) Interference
C) Refraction
D) Reflection
E) Dispersion
A) Transmission
B) Interference
C) Refraction
D) Reflection
E) Dispersion

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
22
What is the energy of a photon that, when it is absorbed by a hydrogen atom, excites an electron from the ground state of the hydrogen atom to the n = 2 state?
A) 10.2 eV
B) 1.63 × 10-18 J
C) 1.63 × 10-18 Nm
D) All of the answers are correct.
E) None of the answers is correct.
A) 10.2 eV
B) 1.63 × 10-18 J
C) 1.63 × 10-18 Nm
D) All of the answers are correct.
E) None of the answers is correct.

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
23
Which one of the following is the correct electronic configuration for carbon?
A) 1s2 2s2 2p2
B) 1s1 2p1
C) 1s1 2s2 2p1
D) 1s1 2s1 2p1
E) 1s2 2s2 2p4
A) 1s2 2s2 2p2
B) 1s1 2p1
C) 1s1 2s2 2p1
D) 1s1 2s1 2p1
E) 1s2 2s2 2p4

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
24
According to Pauli's exclusion principle, how many electrons in an atom may have a particular set of quantum numbers?
A) 1
B) 3
C) 2
D) 4
E) 5
A) 1
B) 3
C) 2
D) 4
E) 5

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
25
The value of a wavelength in the Balmer series is 410.2 nm. What is the value of n?
A) 4
B) 5
C) 6
D) 7
E) 9
A) 4
B) 5
C) 6
D) 7
E) 9

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
26
What is the wavelength in the Balmer series corresponding to a value of n = 15?
A) 277.1 nm
B) 371.1 nm
C) 188.6 nm
D) 656 nm
E) 754.2 nm
A) 277.1 nm
B) 371.1 nm
C) 188.6 nm
D) 656 nm
E) 754.2 nm

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
27
The orbital angular momentum quantum number can take which of the following values for any given value of the principal quantum number, n?
A) ℓ = 0, 1, 2, . . .
B) ℓ = 0, 1, 2, . . . , n
C) ℓ = 0, 1, 2, . . . , (n - 1)
D) ℓ = 1, 2, 3, 4, . . .
E) ℓ = 1, 2, 3, 4, . . ., (n + 1)
A) ℓ = 0, 1, 2, . . .
B) ℓ = 0, 1, 2, . . . , n
C) ℓ = 0, 1, 2, . . . , (n - 1)
D) ℓ = 1, 2, 3, 4, . . .
E) ℓ = 1, 2, 3, 4, . . ., (n + 1)

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
28
What type of spectrum is produced when a high energy electron beam is incident on a metal target producing X-rays?
A) line spectrum
B) emission spectrum
C) absorption spectrum
D) continuous spectrum
E) line spectrum superimposed on a continuous spectrum
A) line spectrum
B) emission spectrum
C) absorption spectrum
D) continuous spectrum
E) line spectrum superimposed on a continuous spectrum

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
29
Bohr's atomic theory predicted energy levels for hydrogen of En = -(2 π2 k2 e4 m)/(hn)2. If instead we place 1 electron in "orbit" about a bare nucleus of atomic number Z, how do you expect Z to be incorporated into the En formula and why?

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
30
According to the quantum mechanical model of the hydrogen atom, if the orbital angular momentum quantum number is ℓ, there will be how many permitted magnetic quantum numbers?
A) ℓ/2
B) 2ℓ
C) 2ℓ + 1
D) 2ℓ - 1
E) 3ℓ
A) ℓ/2
B) 2ℓ
C) 2ℓ + 1
D) 2ℓ - 1
E) 3ℓ

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
31
According to the quantum mechanical model of the hydrogen atom, if the principal quantum number is n, how many different orbital angular momentum quantum number(s) are permitted?
A) n/2
B) n
C) 2n
D) 3n
E) 4n
A) n/2
B) n
C) 2n
D) 3n
E) 4n

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
32
Which one of the following properties of the beam of light applies to a laser beam?
A) It is coherent.
B) It is incoherent.
C) It is diverging.
D) It is converging.
E) None of the other answers given is correct.
A) It is coherent.
B) It is incoherent.
C) It is diverging.
D) It is converging.
E) None of the other answers given is correct.

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
33
The emission of light of lower frequency after illumination by a higher frequency is referred to as
A) absorption.
B) stimulated emission.
C) coherence.
D) phosphorescence.
E) fluorescence.
A) absorption.
B) stimulated emission.
C) coherence.
D) phosphorescence.
E) fluorescence.

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
34
Which one of the following is the correct electronic configuration for the sodium atom?
A) 1s1 2s2 3p6 2s2
B) 1s2 2s1 3p6 2s2
C) 1s1 2s2 2p6
D) 1s2 2s2 2p6 3s2
E) 1 s2 2 s2 2 p6 3 s1
A) 1s1 2s2 3p6 2s2
B) 1s2 2s1 3p6 2s2
C) 1s1 2s2 2p6
D) 1s2 2s2 2p6 3s2
E) 1 s2 2 s2 2 p6 3 s1

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
35
Which one of the following is true for the wavelength of the photon emitted when an electron in a hydrogen atom jumps from the ni = 70 state to the nf = 2 state as compared to the wavelength of the photon emitted when the electron jumps from the ni = 4 state to the nf = 2 state?
A) They are equal.
B) It is shorter.
C) It is longer.
D) The wavelength of the photon is not related to the change in state.
A) They are equal.
B) It is shorter.
C) It is longer.
D) The wavelength of the photon is not related to the change in state.

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
36
Which of the following values are associated with the electron spin quantum number, ms?
A) ±1/2
B) 0
C) ±1
D) ±2
E) ±3
A) ±1/2
B) 0
C) ±1
D) ±2
E) ±3

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
37
The value of a wavelength in the Balmer series is 372.1 nm. What is the value of n?
A) 6
B) 3
C) 9
D) 10
E) 14
A) 6
B) 3
C) 9
D) 10
E) 14

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
38
If a hydrogen atom originally in a state with principal quantum number n is excited to state n' = 2n, then
A) its radius and binding energy will double.
B) its radius will quadruple and the binding energy will double.
C) its radius will double and the binding energy will quadruple.
D) its radius will quadruple and the binding energy will be reduced by a factor of four.
E) its radius and binding energy will quadruple.
A) its radius and binding energy will double.
B) its radius will quadruple and the binding energy will double.
C) its radius will double and the binding energy will quadruple.
D) its radius will quadruple and the binding energy will be reduced by a factor of four.
E) its radius and binding energy will quadruple.

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
39
Consider the Bohr Theory Hydrogen atom.
(a) How much energy is needed to cause a transition of an electron from the 2 nd excited state to the 3rd excited state?
(b) What wavelength photon just has enough energy to initiate the transition?
(a) How much energy is needed to cause a transition of an electron from the 2 nd excited state to the 3rd excited state?
(b) What wavelength photon just has enough energy to initiate the transition?

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
40
Consider the Bohr Theory Hydrogen atom in the 2 nd excited state.
(a) Determine the binding energy (eV) of the electron.
(b) What is the radius of the electron orbit?
(c) How far is it from the next higher excited orbit?
(a) Determine the binding energy (eV) of the electron.
(b) What is the radius of the electron orbit?
(c) How far is it from the next higher excited orbit?

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
41
What is the wavelength of the emitted photon if an electron in the hydrogen atom makes a transition from n = 2 state to the ground state?
A) 121.5 nm
B) 203.1 nm
C) 243.0 nm
D) 388.8 nm
E) 411.1 nm
A) 121.5 nm
B) 203.1 nm
C) 243.0 nm
D) 388.8 nm
E) 411.1 nm

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
42
What is the shortest wavelength of the Lyman series?
A) 91.16 nm
B) 45.60 nm
C) 121.5 nm
D) 204.1 nm
E) 365 nm
A) 91.16 nm
B) 45.60 nm
C) 121.5 nm
D) 204.1 nm
E) 365 nm

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
43
An electron in the hydrogen atom has a wavelength of 1.99 × 10-9 m. To what state of the hydrogen atom does this electron belong?
A) n = 1
B) n = 3
C) n = 4
D) n = 6
E) n = 8
A) n = 1
B) n = 3
C) n = 4
D) n = 6
E) n = 8

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
44
What is the longest wavelength in the Lyman Series?
A) 45.60 nm
B) 91.20 nm
C) 121.5 nm
D) 240.1 nm
E) 365 nm
A) 45.60 nm
B) 91.20 nm
C) 121.5 nm
D) 240.1 nm
E) 365 nm

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
45
In a hydrogen atom, the electron makes a transition from the n = 8 to the n = 3 state. What is the wavelength of the emitted photon?
A) 9.54 × 10-7 m
B) 1.13 × 10-6 m
C) 3.12 × 10-7 m
D) 4.52 × 10-6 m
E) 6.34 × 10-7 m
A) 9.54 × 10-7 m
B) 1.13 × 10-6 m
C) 3.12 × 10-7 m
D) 4.52 × 10-6 m
E) 6.34 × 10-7 m

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
46
What value of n corresponds to a wavelength of 91.7 nm in the Lyman series?
A) 1
B) 3
C) 5
D) 9
E) 13
A) 1
B) 3
C) 5
D) 9
E) 13

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
47
What is the kinetic energy of an electron in the n = 2 Bohr orbit of the hydrogen atom?
A) 2.71 × 10-19 J
B) 3.20 × 10-19 J
C) 5.45 × 10-19 J
D) 6.10 × 10-19 J
E) 2.16 × 10-18 J
A) 2.71 × 10-19 J
B) 3.20 × 10-19 J
C) 5.45 × 10-19 J
D) 6.10 × 10-19 J
E) 2.16 × 10-18 J

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
48
What is the wavelength of the photon emitted when an electron in a hydrogen atom which is in the initial state ni = 8 jumps to the final state nf = 2?
A) 205.1 nm
B) 104.0 nm
C) 388.8 nm
D) 486.2 nm
E) 610.0 nm
A) 205.1 nm
B) 104.0 nm
C) 388.8 nm
D) 486.2 nm
E) 610.0 nm

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
49
What is the longest wavelength in the Paschen series?
A) 2.01 nm
B) 2.01 μm
C) 365 nm
D) 1.88 nm
E) 1.88 μm
A) 2.01 nm
B) 2.01 μm
C) 365 nm
D) 1.88 nm
E) 1.88 μm

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
50
What is the shortest wavelength in the Balmer series?
A) 328 nm
B) 365 nm
C) 456 nm
D) 656 nm
E) 820 nm
A) 328 nm
B) 365 nm
C) 456 nm
D) 656 nm
E) 820 nm

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
51
What is the speed of an electron in the n = 4 Bohr orbit of the hydrogen atom?
A) 5.47 × 105 m/s
B) 310 × 105 m/s
C) 1.39 × 105 m/s
D) 1.78 × 105 m/s
E) 2.18 × 106 m/s
A) 5.47 × 105 m/s
B) 310 × 105 m/s
C) 1.39 × 105 m/s
D) 1.78 × 105 m/s
E) 2.18 × 106 m/s

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
52
What is the longest wavelength in the Balmer series?
A) 240 nm
B) 328 nm
C) 365 nm
D) 656 nm
E) 820 nm
A) 240 nm
B) 328 nm
C) 365 nm
D) 656 nm
E) 820 nm

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
53
What is the radius of the smallest Bohr orbit?
A) 2.14 × 10-9 m
B) 1.12 × 10-9 m
C) 1.56 × 10-11 m
D) 3.17 × 10-11 m
E) 5.29 × 10-11 m
A) 2.14 × 10-9 m
B) 1.12 × 10-9 m
C) 1.56 × 10-11 m
D) 3.17 × 10-11 m
E) 5.29 × 10-11 m

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
54
What is the wavelength of the photon emitted when an electron in a hydrogen atom which is in the initial state ni = 4 jumps to the final state nf = 2?
A) 243.1 nm
B) 556.2 nm
C) 486.2 nm
D) 312.2 nm
E) 609.1 nm
A) 243.1 nm
B) 556.2 nm
C) 486.2 nm
D) 312.2 nm
E) 609.1 nm

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
55
What frequency must a photon have if it is to raise an electron in a hydrogen atom from the n = 2 to the n = 4 state?
A) 3.06 × 1014 Hz
B) 6.16 × 1014 Hz
C) 4.11 × 1014 Hz
D) 5.20 × 1014 Hz
E) 9.24 × 1014 Hz
A) 3.06 × 1014 Hz
B) 6.16 × 1014 Hz
C) 4.11 × 1014 Hz
D) 5.20 × 1014 Hz
E) 9.24 × 1014 Hz

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
56
What is the wavelength associated with the electron in the n = 2 state of hydrogen?
A) 3.32 × 10-10 m
B) 2.03 × 10-10 m
C) 6.66 × 10-10 m
D) 4.63 × 10-10 m
E) 8.17 × 10-10 m
A) 3.32 × 10-10 m
B) 2.03 × 10-10 m
C) 6.66 × 10-10 m
D) 4.63 × 10-10 m
E) 8.17 × 10-10 m

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
57
What is the wavelength of the emitted photon if an electron in the hydrogen atom makes a transition from the ni = 2 to the nf = 1 state?
A) 205.2 nm
B) 87.5 nm
C) 91.2 nm
D) 121.5 nm
E) 402.1 nm
A) 205.2 nm
B) 87.5 nm
C) 91.2 nm
D) 121.5 nm
E) 402.1 nm

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
58
The kinetic energy of an electron in a Bohr orbit of the hydrogen atom is 8.57 × 10-20 J. What is the radius of the orbit?
A) 1.35 × 10-9 m
B) 2.01 × 10-9 m
C) 2.64 × 10-9 m
D) 4.12 × 10-9 m
E) 5.29 × 10-11 m
A) 1.35 × 10-9 m
B) 2.01 × 10-9 m
C) 2.64 × 10-9 m
D) 4.12 × 10-9 m
E) 5.29 × 10-11 m

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
59
What is the shortest wavelength in the Paschen series?
A) 410.2 nm
B) 410.2 μm
C) 365 nm
D) 820.4 nm
E) 820.4 μm
A) 410.2 nm
B) 410.2 μm
C) 365 nm
D) 820.4 nm
E) 820.4 μm

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
60
What value of n corresponds to a wavelength of 922.7 nm in the Paschen series?
A) 3
B) 5
C) 7
D) 9
E) 15
A) 3
B) 5
C) 7
D) 9
E) 15

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
61
A hydrogen atom is in the 6h state. Determine the principal quantum number.
A) 0
B) 3
C) 5
D) 6
E) 7
A) 0
B) 3
C) 5
D) 6
E) 7

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
62
How many values of the magnetic quantum number, mℓ, correspond to a value of 4 for the orbital quantum number, ℓ?
A) 3
B) 5
C) 8
D) 9
E) 7
A) 3
B) 5
C) 8
D) 9
E) 7

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
63
An electron in the hydrogen atom has a wavelength of 2.67 nm. To what state of the hydrogen atom does this electron belong?
A) n = 2
B) n = 5
C) n = 8
D) n = 11
E) n = 14
A) n = 2
B) n = 5
C) n = 8
D) n = 11
E) n = 14

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
64
For the ground state of the hydrogen atom, which of the following represents the correct value of the magnetic quantum number?
A) -1
B) 0
C) 1
D) 2
E) -2
A) -1
B) 0
C) 1
D) 2
E) -2

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
65
What is the wavelength associated with the electron in the n = 6 state of hydrogen?
A) 2.99 × 10-9 m
B) 3.11 × 10-9 m
C) 4.08 × 10-9 m
D) 3.99 × 10-9 m
E) 1.99 × 10-9 m
A) 2.99 × 10-9 m
B) 3.11 × 10-9 m
C) 4.08 × 10-9 m
D) 3.99 × 10-9 m
E) 1.99 × 10-9 m

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
66
A hydrogen atom is in the 6h state. Which of the following could be an orbital quantum number?
A) 5
B) 6
C) 7
D) 8
E) 9
A) 5
B) 6
C) 7
D) 8
E) 9

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
67
The ground state of the Li++ ion is at (Li has atomic number 3)
A) -40.8 eV.
B) -122 eV.
C) Li++ does not have a ground state.
D) -61 eV.
E) -1.51 eV.
A) -40.8 eV.
B) -122 eV.
C) Li++ does not have a ground state.
D) -61 eV.
E) -1.51 eV.

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
68
A hydrogen atom is in the 6h state. Which one of the following is not a magnetic quantum number?
A) 0
B) 1
C) 2
D) 4
E) 6
A) 0
B) 1
C) 2
D) 4
E) 6

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
69
A hydrogen atom is in the 6h state. How many electrons are allowed in this state?
A) 22
B) 18
C) 14
D) 10
E) 6
A) 22
B) 18
C) 14
D) 10
E) 6

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
70
How many values of the magnetic quantum number, mℓ, correspond to a value of 2 for the orbital quantum number, ℓ?
A) 1
B) 2
C) 3
D) 4
E) 5
A) 1
B) 2
C) 3
D) 4
E) 5

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
71
A certain laser radiates with a wavelength of 2488 nm. What is the energy difference, in electron volts, between the two energy levels involved in producing this light?
A) 0.50 eV
B) 0.65 eV
C) 0.45 eV
D) 0.60 eV
E) 0.55 eV
A) 0.50 eV
B) 0.65 eV
C) 0.45 eV
D) 0.60 eV
E) 0.55 eV

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
72
What is the minimum energy an incoming electron must have to knock a K shell electron out of an atom of molybdenum (Z = 42)?
A) 10.1 × 103 eV
B) 11.3 × 103 eV
C) 22.9 × 103 eV
D) 34.4 × 103 eV
E) 44.0 × 103 eV
A) 10.1 × 103 eV
B) 11.3 × 103 eV
C) 22.9 × 103 eV
D) 34.4 × 103 eV
E) 44.0 × 103 eV

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
73
For the ground state of the hydrogen atom, which of the following represents the correct value of the orbital angular momentum quantum number?
A) -1
B) 1
C) 0
D) 2
E) -2
A) -1
B) 1
C) 0
D) 2
E) -2

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
74
How many values can the magnetic quantum number have in a hydrogen atom in which the electron has the value of the orbital angular momentum quantum number equal to 8?
A) 9
B) 15
C) 5
D) 8
E) 17
A) 9
B) 15
C) 5
D) 8
E) 17

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
75
How many unique quantum states correspond to the lowest possible energy level of an electron in the hydrogen atom?
A) 3
B) 0
C) 1
D) 4
E) 2
A) 3
B) 0
C) 1
D) 4
E) 2

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck


