Deck 3: Matter and Energy
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/110
Play
Full screen (f)
Deck 3: Matter and Energy
1
Which one of the following properties describes a liquid?
A)has its own shape
B)particles are close together and move randomly
C)particles move very rapidly
D)fills the entire volume of the container
E)There is essentially no interaction between the particles.
A)has its own shape
B)particles are close together and move randomly
C)particles move very rapidly
D)fills the entire volume of the container
E)There is essentially no interaction between the particles.
particles are close together and move randomly
2
Which of the following is a chemical change?
A)cutting a rope
B)bending a steel rod
C)making a snowman
D)burning sugar
E)melting gold
A)cutting a rope
B)bending a steel rod
C)making a snowman
D)burning sugar
E)melting gold
burning sugar
3
Gold in a ring is a(n)________.
A)compound
B)heterogeneous mixture
C)element
D)homogeneous mixture
E)none of the above
A)compound
B)heterogeneous mixture
C)element
D)homogeneous mixture
E)none of the above
element
4
Which of the following is a physical change?
A)iron rusts
B)ice melts
C)sugar caramelizes
D)food digests
E)natural gas burns
A)iron rusts
B)ice melts
C)sugar caramelizes
D)food digests
E)natural gas burns

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
5
Which of the following is not an element?
A)tin
B)water
C)gold
D)silver
E)carbon
A)tin
B)water
C)gold
D)silver
E)carbon

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
6
Which of the following is a physical property of both liquids and gases?
A)has its own shape
B)has a definite volume
C)has strong interactions between its particles
D)has randomly arranged particles
E)has large spaces between molecules
A)has its own shape
B)has a definite volume
C)has strong interactions between its particles
D)has randomly arranged particles
E)has large spaces between molecules

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
7
Which of the following would not be a physical change?
A)freezing water to make ice cubes
B)tearing a piece of aluminum foil
C)boiling water for soup
D)burning gasoline in a lawnmower
E)melting gold to make jewelry
A)freezing water to make ice cubes
B)tearing a piece of aluminum foil
C)boiling water for soup
D)burning gasoline in a lawnmower
E)melting gold to make jewelry

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
8
If the temperature is 20.°C,what is the corresponding temperature on the Fahrenheit scale?
A)-22 °F
B)68 °F
C)43 °F
D)239 °F
E)94 °F
A)-22 °F
B)68 °F
C)43 °F
D)239 °F
E)94 °F

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
9
Which of the following is an example of a physical change?
A)grinding coffee beans
B)baking a cake
C)converting water to hydrogen and oxygen
D)digesting a cheeseburger
E)burning coal
A)grinding coffee beans
B)baking a cake
C)converting water to hydrogen and oxygen
D)digesting a cheeseburger
E)burning coal

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
10
Which of the following is a property of a solid?
A)It takes the shape of the container.
B)It fills the volume of the container.
C)The particles move at a rapid rate.
D)The interactions between its particles are very weak.
E)The particles have fixed positions and are very close together.
A)It takes the shape of the container.
B)It fills the volume of the container.
C)The particles move at a rapid rate.
D)The interactions between its particles are very weak.
E)The particles have fixed positions and are very close together.

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
11
Which of the following is a physical change?
A)baking a cake
B)solid dry ice changing to a gas
C)fermenting grapes to produce wine
D)digesting a meal
E)a tomato ripening
A)baking a cake
B)solid dry ice changing to a gas
C)fermenting grapes to produce wine
D)digesting a meal
E)a tomato ripening

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
12
Which of the following is a heterogeneous mixture?
A)noodle soup
B)water
C)sugar
D)tea
E)carbon
A)noodle soup
B)water
C)sugar
D)tea
E)carbon

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
13
In a gas,the distance between the particles is ________.
A)very close relative to the size of the molecules
B)close relative to the size of the molecules
C)fixed relative to the size of the molecules
D)small relative to the size of the molecules
E)very large relative to the size of the molecules
A)very close relative to the size of the molecules
B)close relative to the size of the molecules
C)fixed relative to the size of the molecules
D)small relative to the size of the molecules
E)very large relative to the size of the molecules

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
14
Helium is a(n)________.
A)compound
B)heterogeneous mixture
C)element
D)homogeneous mixture
E)electron
A)compound
B)heterogeneous mixture
C)element
D)homogeneous mixture
E)electron

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
15
Which of the following is an element?
A)tin
B)water
C)salt
D)sugar
E)iced tea
A)tin
B)water
C)salt
D)sugar
E)iced tea

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
16
Which of the following is a chemical change?
A)burning natural gas
B)melting ice
C)hammering gold into foil
D)cutting a tomato
E)cutting paper
A)burning natural gas
B)melting ice
C)hammering gold into foil
D)cutting a tomato
E)cutting paper

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
17
Coins in a piggy bank are an example of a(n)________.
A)compound
B)heterogeneous mixture
C)element
D)homogeneous mixture
E)none of the above
A)compound
B)heterogeneous mixture
C)element
D)homogeneous mixture
E)none of the above

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
18
Which of the following is a homogeneous mixture?
A)noodle soup
B)water
C)sugar
D)tea
E)carbon
A)noodle soup
B)water
C)sugar
D)tea
E)carbon

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
19
Which of the following is not a characteristic of the solid state?
A)has a definite shape
B)has a definite volume
C)particles are close together
D)particles are moving very fast
E)particles are in fixed positions
A)has a definite shape
B)has a definite volume
C)particles are close together
D)particles are moving very fast
E)particles are in fixed positions

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
20
In which of the following would the particles move most rapidly?
A)ice at -20 °C
B)water at 20 °C
C)steam at 110 °C
D)boiling water
E)ice at 0 °C
A)ice at -20 °C
B)water at 20 °C
C)steam at 110 °C
D)boiling water
E)ice at 0 °C

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
21
A patient has a temperature of 38.5 °C.What is the temperature in degrees Fahrenheit?
A)70.5 °F
B)311 °F
C)126.9 °F
D)101.3 °F
E)11.7 °F
A)70.5 °F
B)311 °F
C)126.9 °F
D)101.3 °F
E)11.7 °F

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
22
The energy associated with the motion of particles in a substance is called ________.
A)temperature
B)electrical energy
C)heat
D)chemical energy
E)potential energy
A)temperature
B)electrical energy
C)heat
D)chemical energy
E)potential energy

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
23
A potato contains 20 g of carbohydrate.If carbohydrate has a caloric value of 4 kcal/g,how many kcal are obtained from the carbohydrate in the potato?
A)5 kcal
B)20 kcal
C)40 kcal
D)60 kcal
E)80 kcal
A)5 kcal
B)20 kcal
C)40 kcal
D)60 kcal
E)80 kcal

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
24
Absolute zero is ________.
A)the freezing point of water using the Celsius scale
B)the boiling point of liquid nitrogen
C)the temperature on the Kelvin scale corresponding to 32 °F
D)the coldest temperature possible
E)the freezing point of liquid nitrogen
A)the freezing point of water using the Celsius scale
B)the boiling point of liquid nitrogen
C)the temperature on the Kelvin scale corresponding to 32 °F
D)the coldest temperature possible
E)the freezing point of liquid nitrogen

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
25
On a hot day,the thermometer read 95 °F.What is the temperature in degrees Celsius?
A)77 °C
B)113 °C
C)35 °C
D)63 °C
E)178 °C
A)77 °C
B)113 °C
C)35 °C
D)63 °C
E)178 °C

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
26
A slice of pizza contains 28 g of carbohydrate,13 g of protein and fat.If the pizza contains 280 kcal,how many grams of fat are present?(The caloric values are: 4 kcal/g for carbohydrate,9 kcal/g for fat,and 4 kcal/g for protein.Give the answer to 2 significant figures.)
A)28 g
B)13 g
C)10.g
D)55 g
E)250 g
A)28 g
B)13 g
C)10.g
D)55 g
E)250 g

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
27
A temperature of 41 °F is the same as ________.
A)5 °C
B)310 °C
C)-9 °C
D)16 °C
E)42 °C
A)5 °C
B)310 °C
C)-9 °C
D)16 °C
E)42 °C

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
28
650.J is the same amount of energy as ________.
A)155 cal
B)2720 cal
C)650.cal
D)1550 cal
E)2.72 cal
A)155 cal
B)2720 cal
C)650.cal
D)1550 cal
E)2.72 cal

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
29
An example of kinetic energy is ________.
A)a coiled spring
B)running water
C)a tree
D)natural gas
E)food
A)a coiled spring
B)running water
C)a tree
D)natural gas
E)food

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
30
The energy of motion is referred to as ________.
A)work
B)freezing
C)specific heat
D)potential energy
E)kinetic energy
A)work
B)freezing
C)specific heat
D)potential energy
E)kinetic energy

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
31
If the temperature is -55 °F,what is the corresponding temperature on the Kelvin scale?
A)225 K
B)218 K
C)55 K
D)273 K
E)328 K
A)225 K
B)218 K
C)55 K
D)273 K
E)328 K

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
32
Which of the following is an example of potential energy?
A)chewing food
B)water stored in a reservoir
C)burning wood
D)a fan blade turning
E)riding an exercise bike
A)chewing food
B)water stored in a reservoir
C)burning wood
D)a fan blade turning
E)riding an exercise bike

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
33
The temperature of liquid nitrogen is -196 °C.What is the corresponding reading on the Kelvin scale?
A)77 K
B)-127 K
C)-91 K
D)48 K
E)146 K
A)77 K
B)-127 K
C)-91 K
D)48 K
E)146 K

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
34
A cheeseburger from a fast food restaurant contains 19 g of fat,20 g of carbohydrate,and 28 g of protein.How many kcal of energy does the cheeseburger contain? (The caloric values are: 4 kcal/g for carbohydrate,9 kcal/g for fat,and 4 kcal/g for protein.)Give the answer to 2 significant figures.
A)70.kcal
B)360 kcal
C)17 kcal
D)630 kcal
E)280 kcal
A)70.kcal
B)360 kcal
C)17 kcal
D)630 kcal
E)280 kcal

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
35
One cup of kidney beans contains 15 g of protein,1 g of fat,and 42 g of carbohydrate.How many kilocalories,to two significant figures,does this sample contain? (The caloric values are: 4 kcal/g for carbohydrate,9 kcal/g for fat,and 4 kcal/g for protein.)
A)60.kcal
B)88 kcal
C)230 kcal
D)240 kcal
E)520 kcal
A)60.kcal
B)88 kcal
C)230 kcal
D)240 kcal
E)520 kcal

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
36
Climate change is believed to result from all of the following except ________.
A)burning of fossil fuels
B)increasing levels of carbon dioxide in the atmosphere
C)deforestation
D)movement of the earth closer to the sun
E)carbon dioxide trapping the heat produced by the sun
A)burning of fossil fuels
B)increasing levels of carbon dioxide in the atmosphere
C)deforestation
D)movement of the earth closer to the sun
E)carbon dioxide trapping the heat produced by the sun

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
37
3.25 kcal is the same amount of energy as ________.
A)3.25 J
B)0.777 J
C)777 J
D)13600 J
E)13.6 J
A)3.25 J
B)0.777 J
C)777 J
D)13600 J
E)13.6 J

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
38
The energy stored in molecules is ________.
A)specific heat
B)kinetic energy
C)potential energy
D)work
E)a calorie
A)specific heat
B)kinetic energy
C)potential energy
D)work
E)a calorie

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
39
The phrase "ability to do work" is a definition of ________.
A)specific heat
B)energy
C)calorie
D)heating
E)cooling
A)specific heat
B)energy
C)calorie
D)heating
E)cooling

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
40
A serving of fish contains 50 g protein and 4 g of fat.If protein has a caloric value of 4 kcal/g and fat has 9 kcal/g,how many kcal are in the serving? Give the answer to 2 significant figures.
A)240 kcal
B)54 kcal
C)470 kcal
D)220 kcal
E)490 kcal
A)240 kcal
B)54 kcal
C)470 kcal
D)220 kcal
E)490 kcal

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
41
The heat of vaporization for water is 540 cal/g.How many kilocalories are needed to change 22 g of liquid water to steam at 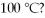
A)540 kcal
B)12 kcal
C)12000 kcal
D)25 kcal
E)1.8 kcal
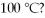
A)540 kcal
B)12 kcal
C)12000 kcal
D)25 kcal
E)1.8 kcal

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
42
The specific heat of a substance is the amount of heat needed to ________.
A)change 1 g of the substance from the solid to the liquid state
B)raise the temperature of 1 g of the substance by 1 °C
C)change 1 g of the substance from the liquid to the solid state
D)convert 1 g of a liquid to gas
E)convert 1 g of a solid to a gas
A)change 1 g of the substance from the solid to the liquid state
B)raise the temperature of 1 g of the substance by 1 °C
C)change 1 g of the substance from the liquid to the solid state
D)convert 1 g of a liquid to gas
E)convert 1 g of a solid to a gas

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
43
Which of the following does not involve a change of state?
A)melting ice
B)freezing water
C)vaporization of alcohol
D)sublimation of dry ice
E)pouring water into a vacuum-insulated bottle
A)melting ice
B)freezing water
C)vaporization of alcohol
D)sublimation of dry ice
E)pouring water into a vacuum-insulated bottle

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
44
How many calories are required to raise the temperature of a 35.0 g sample of iron from 25 °C to 35 °C? Iron has a specific heat of 0.108 cal/g °C.
A)38 cal
B)1.1 cal
C)3.8 cal
D)93 cal
E)130 cal
A)38 cal
B)1.1 cal
C)3.8 cal
D)93 cal
E)130 cal

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
45
If the heat of vaporization for water is 540 cal/g,how many kilocalories are released when 5.00 g of steam is converted to liquid at 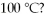
A)540 kcal
B)5.0 kcal
C)110 kcal
D)2.7 kcal
E)5.4 kcal
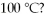
A)540 kcal
B)5.0 kcal
C)110 kcal
D)2.7 kcal
E)5.4 kcal

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
46
A kilocalorie of heat is required to raise the temperature of ________.
A)1 g of water from 14 °C to 15 °C
B)1 g of water by 10 °C
C)10 g of water by 10 °C
D)100 g of water by 10 °C
E)100 g of water by 100 °C
A)1 g of water from 14 °C to 15 °C
B)1 g of water by 10 °C
C)10 g of water by 10 °C
D)100 g of water by 10 °C
E)100 g of water by 100 °C

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
47
A burn from steam at 100 °C is expected to be more severe than a burn from boiling water at 100 °C because ________.
A)the steam is hotter than the boiling water
B)there is more steam than water
C)the steam will give off a large amount of heat as it condenses
D)you are more likely to come into contact with the steam than with the boiling water
E)All of these answers are correct.
A)the steam is hotter than the boiling water
B)there is more steam than water
C)the steam will give off a large amount of heat as it condenses
D)you are more likely to come into contact with the steam than with the boiling water
E)All of these answers are correct.

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
48
How many calories are required to raise the temperature of a 150.g sample of gold from 25 °C to 175 °C? The specific heat of gold is 0.0308 cal/g °C.
A)4.62 cal
B)116 cal
C)22500 cal
D)693 cal
E)130.cal
A)4.62 cal
B)116 cal
C)22500 cal
D)693 cal
E)130.cal

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
49
The formation of a gas resulting from the escape of high-energy particles from the surface of a liquid is known as ________.
A)evaporation
B)deposition
C)boiling
D)melting
E)sublimation
A)evaporation
B)deposition
C)boiling
D)melting
E)sublimation

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
50
If the heat of fusion for water is 80.cal/g,how many calories are needed to melt 45.0 g of ice at 0 °C?
A)3.6 cal
B)3.6 ×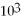 cal
cal
C)1.8 cal
D)80.cal
E)0.56 cal
A)3.6 cal
B)3.6 ×
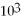 cal
calC)1.8 cal
D)80.cal
E)0.56 cal

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
51
The dietary calorie(Cal)is equal to ________.
A)1000 kcal
B)1000 cal
C)100 cal
D)10 cal
E)1 cal
A)1000 kcal
B)1000 cal
C)100 cal
D)10 cal
E)1 cal

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
52
The specific heat of copper is 0.0920 cal/g °C,and the specific heat of silver is 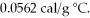 If 100 cal of heat is added to one g of each metal at 25 °C,what is the expected result?
If 100 cal of heat is added to one g of each metal at 25 °C,what is the expected result?
A)The copper will reach a higher temperature.
B)The silver will reach a higher temperature.
C)The two samples will reach the same temperature.
D)The copper will reach a temperature lower than 25 °C.
E)The silver will soften.
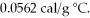 If 100 cal of heat is added to one g of each metal at 25 °C,what is the expected result?
If 100 cal of heat is added to one g of each metal at 25 °C,what is the expected result?A)The copper will reach a higher temperature.
B)The silver will reach a higher temperature.
C)The two samples will reach the same temperature.
D)The copper will reach a temperature lower than 25 °C.
E)The silver will soften.

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
53
Raising the temperature of 10.0 g of water from 10.0 °C to 20.0 °C requires 100.0 cal of energy,while raising the temperature of 10.0 g of aluminum from 10.0 °C to 20.0 °C requires 22.0 cal.More calories are required to heat the water because ________.
A)water is a liquid and aluminum is a solid at 10.0 °C
B)ten grams of water occupies a larger volume than 10.0 g of aluminum
C)water has a greater potential energy than aluminum
D)water has a larger specific heat than aluminum
E)10.0 °C is closer to the melting point of water than to the melting point of aluminum
A)water is a liquid and aluminum is a solid at 10.0 °C
B)ten grams of water occupies a larger volume than 10.0 g of aluminum
C)water has a greater potential energy than aluminum
D)water has a larger specific heat than aluminum
E)10.0 °C is closer to the melting point of water than to the melting point of aluminum

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
54
Which of the following quantities is not required to calculate the amount of heat energy required to heat water from 25 °C to 55 °C?
A)the mass of the water sample
B)the initial temperature
C)the final temperature
D)the specific heat of water
E)the heat of vaporization for water
A)the mass of the water sample
B)the initial temperature
C)the final temperature
D)the specific heat of water
E)the heat of vaporization for water

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
55
The number of calories needed to raise the temperature of 32 g of water from 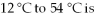 ________.
________.
A)380 cal
B)1.3 cal
C)1300 cal
D)1700 cal
E)0.76 cal
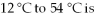 ________.
________.A)380 cal
B)1.3 cal
C)1300 cal
D)1700 cal
E)0.76 cal

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
56
How many calories are required to increase the temperature of 13 g of ethanol from 11 °C to 23 °C? The specific heat of ethanol is 0.59 cal/g °C.
A)83 cal
B)0.63 cal
C)92 cal
D)0.54 cal
E)170 cal
A)83 cal
B)0.63 cal
C)92 cal
D)0.54 cal
E)170 cal

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
57
The heat of fusion for water is 80.cal/g.How many calories of heat are released when 20.0 g of water at 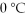 is frozen to ice?
is frozen to ice?
A)620 cal
B)1600 cal
C)2000 cal
D)2200 cal
E)0 cal
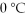 is frozen to ice?
is frozen to ice?A)620 cal
B)1600 cal
C)2000 cal
D)2200 cal
E)0 cal

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
58
When a solid is converted directly to a gas,the change of state is called ________.
A)freezing
B)melting
C)boiling
D)condensation
E)sublimation
A)freezing
B)melting
C)boiling
D)condensation
E)sublimation

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
59
The physical state(s)present when a substance is melting is (are)________.
A)solid
B)liquid
C)gas
D)solid + liquid
E)liquid + gas
A)solid
B)liquid
C)gas
D)solid + liquid
E)liquid + gas

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
60
The heat of fusion for water is 80.cal/g.How many calories of heat are needed to melt a 35 g ice cube that has a temperature of 0 °C?
A)2300 cal
B)1600 cal
C)2800 cal
D)540 cal
E)0 cal
A)2300 cal
B)1600 cal
C)2800 cal
D)540 cal
E)0 cal

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
61
Bromine 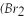 )has a freezing point of -7 °C,and a boiling point of 60 °C.
)has a freezing point of -7 °C,and a boiling point of 60 °C.
Indicate the state present or the change of state occurring at each temperature.
60 °C
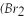 )has a freezing point of -7 °C,and a boiling point of 60 °C.
)has a freezing point of -7 °C,and a boiling point of 60 °C.Indicate the state present or the change of state occurring at each temperature.
60 °C

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
62
A temperature of 50.°F is equivalent to ________°C.

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
63
Air is an example of a ________ mixture.

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
64
A heating curve illustrates ________.
A)what a substance looks like as it is heated
B)what happens to the particles of a substance as it is heated
C)what happens to the heat applied as the temperature is increased
D)the changes in the temperature and physical state of a substance as it is heated
E)the chemical changes that occur as the substance is heated
A)what a substance looks like as it is heated
B)what happens to the particles of a substance as it is heated
C)what happens to the heat applied as the temperature is increased
D)the changes in the temperature and physical state of a substance as it is heated
E)the chemical changes that occur as the substance is heated

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
65
A bungee jumper just before she leaps,has ________ energy.

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
66
The ________ state of matter has a constant shape and volume.

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
67
The heat of fusion is the amount of heat necessary to change one gram of a substance from the solid to the ________ state at its melting point.

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
68
Bromine 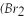 )has a freezing point of -7 °C,and a boiling point of 60 °C.
)has a freezing point of -7 °C,and a boiling point of 60 °C.
Indicate the state present or the change of state occurring at each temperature.
30 °C
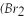 )has a freezing point of -7 °C,and a boiling point of 60 °C.
)has a freezing point of -7 °C,and a boiling point of 60 °C.Indicate the state present or the change of state occurring at each temperature.
30 °C

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
69
Which of the following does not represent a step on the heating curve of water?
A)The temperature of steam cannot exceed 100 °C.
B)The temperature of ice remains at 0 °C as it melts.
C)The temperature of liquid water increases linearly as it is heated.
D)The temperature of liquid water remains at 100 °C as it boils.
E)Both liquid water and ice are present at 0 °C.
A)The temperature of steam cannot exceed 100 °C.
B)The temperature of ice remains at 0 °C as it melts.
C)The temperature of liquid water increases linearly as it is heated.
D)The temperature of liquid water remains at 100 °C as it boils.
E)Both liquid water and ice are present at 0 °C.

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
70
Liquid water changing to ice is an example of a ________ change.

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
71
Iron rusting is an example of a ________ change.

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
72
A food sample contains 6 g of carbohydrate and 4 g of protein.How many kcal from carbohydrate and protein are in the sample?

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
73
The change of state from solid to gas is termed ________.

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
74
When a liquid boils,the process by which the molecules leave its surface is called ________.

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
75
A car driving on the freeway has ________ energy.

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
76
A 12-g sample of cooking oil,which is a fat,contains ________ kcal.

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
77
The amount of heat necessary for one gram of a substance to change from the solid state to the liquid state at its melting point is the ________.

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
78
The specific heat of water (in J/g °C )is ________.

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
79
On a heating curve a plateau corresponds to ________.
A)a change in temperature of a liquid
B)a change in temperature of a solid
C)a change in temperature of a gas
D)a change of state
E)the solid being broken into smaller pieces
A)a change in temperature of a liquid
B)a change in temperature of a solid
C)a change in temperature of a gas
D)a change of state
E)the solid being broken into smaller pieces

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck
80
A temperature of 20°C is equivalent to ________ K.

Unlock Deck
Unlock for access to all 110 flashcards in this deck.
Unlock Deck
k this deck


