Deck 8: Gases
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/73
Play
Full screen (f)
Deck 8: Gases
1
A 5.00-L tank contains helium gas at 1.50 atm.What is the pressure of the gas in mmHg?
A)1.50 mmHg
B)507 mmHg
C)760 mmHg
D)1140 mmHg
E)3800 mmHg
A)1.50 mmHg
B)507 mmHg
C)760 mmHg
D)1140 mmHg
E)3800 mmHg
1140 mmHg
2
The unit of 1 atmosphere used to describe the pressure of a gas is equal to ________.
A)1 mmHg
B)100 mmHg
C)200 mmHg
D)600 mmHg
E)760 mmHg
A)1 mmHg
B)100 mmHg
C)200 mmHg
D)600 mmHg
E)760 mmHg
760 mmHg
3
The force of gas particles against the walls of a container is called ________.
A)pressure
B)volume
C)temperature
D)quantity of gas
E)density
A)pressure
B)volume
C)temperature
D)quantity of gas
E)density
pressure
4
Which measurement describes the pressure of a gas?
A)315 K
B)1.2 g/L
C)2.5 L
D)725 mmHg
E)0.45 moles
A)315 K
B)1.2 g/L
C)2.5 L
D)725 mmHg
E)0.45 moles

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
5
In the kinetic molecular theory of gas behavior,particles of a gas tend to move ________ and collisions between them are ________.
A)rapidly,rare
B)slowly,rare
C)rapidly,elastic
D)slowly,elastic
E)slowly,unusual
A)rapidly,rare
B)slowly,rare
C)rapidly,elastic
D)slowly,elastic
E)slowly,unusual

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
6
In response to Boyle's law,the pressure of a gas increases as the volume decreases because ________.
A)the gas particles get bigger
B)the kinetic energy of the gas particles increases
C)the temperature of the gas increases
D)the gas particles strike the walls of the container with more force
E)the gas particles strike the walls of the container more often
A)the gas particles get bigger
B)the kinetic energy of the gas particles increases
C)the temperature of the gas increases
D)the gas particles strike the walls of the container with more force
E)the gas particles strike the walls of the container more often

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
7
According to the kinetic theory of gases,a gas can be compressed much more than a liquid or solid because ________.
A)a gas is composed of very small particles
B)the particles of a gas are very far apart
C)gas particles move rapidly
D)gas particles do not attract or repel one another
E)gas particles move faster when the temperature increases
A)a gas is composed of very small particles
B)the particles of a gas are very far apart
C)gas particles move rapidly
D)gas particles do not attract or repel one another
E)gas particles move faster when the temperature increases

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
8
The volume of a gas with a pressure of 1.2 atm increases from 1.0 L to 4.0 L.What is the final pressure of the gas,assuming constant temperature?
A)1.2 atm
B)0.30 atm
C)3.3 atm
D)4.8 atm
E)1.0 atm
A)1.2 atm
B)0.30 atm
C)3.3 atm
D)4.8 atm
E)1.0 atm

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
9
Complete the following statement: In Charles's law,the volume of a gas ________ when the ________ decreases.
A)increases,temperature
B)increases,quantity of gas
C)increases,pressure
D)decreases,temperature
E)decreases,pressure
A)increases,temperature
B)increases,quantity of gas
C)increases,pressure
D)decreases,temperature
E)decreases,pressure

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
10
The pressure of 5.0 L of gas increases from 1.50 atm to 1240 mmHg.What is the final volume of the gas,assuming constant temperature?
A)4100 L
B)5.0 L
C)0.0060 L
D)5.4 L
E)4.6 L
A)4100 L
B)5.0 L
C)0.0060 L
D)5.4 L
E)4.6 L

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
11
A balloon is filled with helium gas.For the following question(s),select the letter of the balloon diagram that corresponds to the given change in conditions. 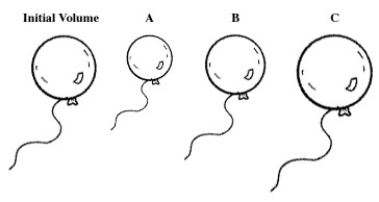
The balloon is put into a chamber where the pressure is less than the atmospheric pressure.
A)A
B)B
C)C
D)A and B
E)B and C

The balloon is put into a chamber where the pressure is less than the atmospheric pressure.
A)A
B)B
C)C
D)A and B
E)B and C

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
12
At constant temperature,a sample of helium at 760.torr in a closed container was compressed from 5.00 L to 3.00 L.What was the new pressure exerted by the helium on its container?
A)800.torr
B)2280 torr
C)15.0 torr
D)3800 torr
E)1270 torr
A)800.torr
B)2280 torr
C)15.0 torr
D)3800 torr
E)1270 torr

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
13
A balloon is filled with helium gas.For the following question(s),select the letter of the balloon diagram that corresponds to the given change in conditions. 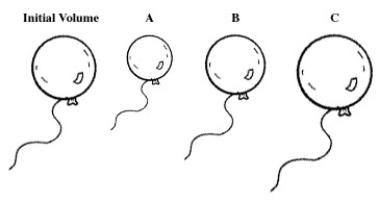
The temperature is changed from 50 °C to -150 °C at constant pressure.
A)A
B)B
C)C
D)A and B
E)B and C

The temperature is changed from 50 °C to -150 °C at constant pressure.
A)A
B)B
C)C
D)A and B
E)B and C

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
14
A gas sample in a closed,expandable container of initial volume 5.00 L was allowed to warm from 25 °C to 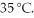 What was its new volume?
What was its new volume?
A)4.84 L
B)5.17 L
C)7.00 L
D)3.57 L
E)4380 L
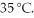 What was its new volume?
What was its new volume?A)4.84 L
B)5.17 L
C)7.00 L
D)3.57 L
E)4380 L

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
15
The gas with an initial volume of 24.0 L at a pressure of 565 torr is compressed until the volume is 16.0 L.What is the final pressure of the gas,assuming the temperature does not change?
A)377 torr
B)760 torr
C)848 torr
D)500.torr
E)465 torr
A)377 torr
B)760 torr
C)848 torr
D)500.torr
E)465 torr

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
16
According to the kinetic theory of gases,particles of a gas ________.
A)are very large particles
B)are very far apart
C)lose their valence electrons
D)move slowly
E)decrease kinetic energy as temperature increases
A)are very large particles
B)are very far apart
C)lose their valence electrons
D)move slowly
E)decrease kinetic energy as temperature increases

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
17
In the kinetic molecular theory of gas behavior,the assumption is made that gas molecules ________.
A)move rapidly in random directions
B)are attracted to each other by strong forces
C)are close together in their container
D)move with a kinetic energy equal to their centigrade temperature
E)occasionally come to rest
A)move rapidly in random directions
B)are attracted to each other by strong forces
C)are close together in their container
D)move with a kinetic energy equal to their centigrade temperature
E)occasionally come to rest

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
18
Which of the following correctly describes the process of inspiration (air entering the lungs)?
A)The lungs expand,causing their internal pressure to decrease.
B)The lungs expand,causing their internal pressure to increase.
C)The lungs contract,causing their internal pressure to decrease.
D)The lungs contract,causing their internal pressure to increase.
E)There is no change in the internal pressure in the lungs.
A)The lungs expand,causing their internal pressure to decrease.
B)The lungs expand,causing their internal pressure to increase.
C)The lungs contract,causing their internal pressure to decrease.
D)The lungs contract,causing their internal pressure to increase.
E)There is no change in the internal pressure in the lungs.

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
19
Which of the following is not part of the kinetic theory of gases?
A)A gas is composed of very small particles.
B)There is very little empty space in a gas.
C)Gas particles move rapidly.
D)Gas particles do not attract or repel one another.
E)Gas particles move faster when the temperature increases.
A)A gas is composed of very small particles.
B)There is very little empty space in a gas.
C)Gas particles move rapidly.
D)Gas particles do not attract or repel one another.
E)Gas particles move faster when the temperature increases.

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
20
What temperature scale is used in gas law calculations?
A)Fahrenheit
B)Celsius
C)Kelvin
D)either Celsius or Fahrenheit
E)either Celsius or Kelvin
A)Fahrenheit
B)Celsius
C)Kelvin
D)either Celsius or Fahrenheit
E)either Celsius or Kelvin

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
21
A temperature of 125 °C is the same as ________.
A)-148 K
B)398 K
C)257 K
D)530.K
E)273 K
A)-148 K
B)398 K
C)257 K
D)530.K
E)273 K

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
22
How many moles of neon occupy a volume of 14.3 L at STP?
A)36.7 moles
B)32.0 moles
C)6.45 moles
D)0.638 moles
E)1.57 moles
A)36.7 moles
B)32.0 moles
C)6.45 moles
D)0.638 moles
E)1.57 moles

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
23
The temperature of a 500.mL sample of gas increases from 150.K to 350.K.What is the final volume of the sample of gas,if the pressure in the container is kept constant?
A)210 mL
B)1170 mL
C)0.00950 mL
D)0.00474 mL
E)110 mL
A)210 mL
B)1170 mL
C)0.00950 mL
D)0.00474 mL
E)110 mL

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
24
At STP,temperature and pressure have the values of ________.
A)0 K and 1 atm
B)273 K and 1 mmHg
C)273 K and 760 mmHg
D)0 K and 760 mmHg
E)760 K and 273 atm
A)0 K and 1 atm
B)273 K and 1 mmHg
C)273 K and 760 mmHg
D)0 K and 760 mmHg
E)760 K and 273 atm

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
25
In Gay-Lussac's law,the pressure of a gas increases due to an increase in temperature because ________.
A)the molecules strike the walls of the container less often
B)the molecules strike the walls of the container more often
C)the molecules get bigger
D)there is a decrease in the volume of the container
E)there is an increase in the number of gas particles
A)the molecules strike the walls of the container less often
B)the molecules strike the walls of the container more often
C)the molecules get bigger
D)there is a decrease in the volume of the container
E)there is an increase in the number of gas particles

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
26
The total pressure in a mixture of gases is equal to the partial pressure(s)of ________.
A)the gas with the greatest number of moles
B)the gas with the smallest number of moles
C)the gas with the highest molecular weight
D)the gas that occupies the largest volume
E)all the gases added together
A)the gas with the greatest number of moles
B)the gas with the smallest number of moles
C)the gas with the highest molecular weight
D)the gas that occupies the largest volume
E)all the gases added together

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
27
A cyclopropane-oxygen mixture is used as an anesthetic.If the partial pressure of cyclopropane in the mixture is 330 mmHg and the partial pressure of the oxygen is 1.0 atm,what is the total pressure of the mixture in torr?
A)330 torr
B)430 torr
C)760 torr
D)1.4 torr
E)1100 torr
A)330 torr
B)430 torr
C)760 torr
D)1.4 torr
E)1100 torr

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
28
Which of the following correctly describes the partial pressures of gases in the body?
A)high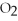 ,low
,low 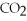 ,oxygenated blood
,oxygenated blood
B)high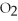 ,low
,low 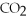 ,deoxygenated blood
,deoxygenated blood
C)high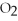 ,high
,high 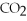 ,oxygenated blood
,oxygenated blood
D)high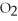 ,high
,high 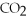 ,tissue
,tissue
E)low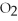 ,low
,low 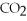 ,deoxygenated blood
,deoxygenated blood
A)high
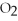 ,low
,low 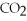 ,oxygenated blood
,oxygenated bloodB)high
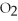 ,low
,low 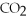 ,deoxygenated blood
,deoxygenated bloodC)high
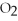 ,high
,high 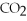 ,oxygenated blood
,oxygenated bloodD)high
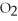 ,high
,high 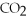 ,tissue
,tissueE)low
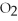 ,low
,low 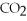 ,deoxygenated blood
,deoxygenated blood
Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
29
A gas sample containing 12 g of 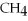 has a volume of 14 L.What will the volume be if 12 g of He is added? (Temperature and pressure do not change.)
has a volume of 14 L.What will the volume be if 12 g of He is added? (Temperature and pressure do not change.)
A)22.4.L
B)53 L
C)56 L
D)70 L
E)3.5 L
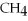 has a volume of 14 L.What will the volume be if 12 g of He is added? (Temperature and pressure do not change.)
has a volume of 14 L.What will the volume be if 12 g of He is added? (Temperature and pressure do not change.)A)22.4.L
B)53 L
C)56 L
D)70 L
E)3.5 L

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
30
A sample of nitrogen gas had a volume of 500.mL,a pressure in its closed container of 740 torr,and a temperature of 25 °C.What was the new volume of the gas when the temperature was changed to 50 °C and the new pressure was 760 torr?
A)530 mL
B)450 mL
C)970 mL
D)240 mL
E)400 mL
A)530 mL
B)450 mL
C)970 mL
D)240 mL
E)400 mL

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
31
At 570.mmHg and 25 °C,a gas sample has a volume of 2270 mL.What is the final pressure (in mmHg)at a volume of 1250 mL and a temperature of 175 °C?
A)1560 mmHg
B)210 mmHg
C)7000 mmHg
D)690 mmHg
E)470 mmHg
A)1560 mmHg
B)210 mmHg
C)7000 mmHg
D)690 mmHg
E)470 mmHg

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
32
A gas sample contains 4.0 g of 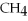 and 2.0 g of He.What is the volume of the sample at STP?
and 2.0 g of He.What is the volume of the sample at STP?
A)130 L
B)11 L
C)17 L
D)30.L
E)5.6 L
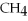 and 2.0 g of He.What is the volume of the sample at STP?
and 2.0 g of He.What is the volume of the sample at STP?A)130 L
B)11 L
C)17 L
D)30.L
E)5.6 L

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
33
A gas at 5.00 atm pressure was stored in a tank during the winter at 5.0 °C.During the summer,the temperature in the storage area reached 40.0 °C.What was the pressure in the gas tank in the summer?
A)0.625 atm
B)4.44 atm
C)5.63 atm
D)40.0 atm
E)69.5 atm
A)0.625 atm
B)4.44 atm
C)5.63 atm
D)40.0 atm
E)69.5 atm

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
34
A tank contains helium gas at 490 mmHg,nitrogen gas at 0.75 atm,and neon at 520 torr.What is the total pressure in atm?
A)2.1 atm
B)0.55 atm
C)1.0 ×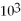 atm
atm
D)1.5 atm
E)1600 atm
A)2.1 atm
B)0.55 atm
C)1.0 ×
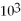 atm
atmD)1.5 atm
E)1600 atm

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
35
A gas contained in a steel tank has a pressure of 1.5 atm at a temperature of 320 K.What will be the gas pressure when the temperature changes to 450 K?
A)1.5 atm
B)0.94 atm
C)0.47 atm
D)2.1 atm
E)1.1 atm
A)1.5 atm
B)0.94 atm
C)0.47 atm
D)2.1 atm
E)1.1 atm

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
36
According to Avogadro's law,________.
A)the volume of a gas is inversely related to the number of moles at constant temperature and pressure
B)the volume of a gas is inversely related to the number of moles at standard temperature and pressure
C)the volume of a gas depends only on the temperature and pressure
D)the volume of a gas depends only on the number of moles in the sample
E)the volume of a gas is directly related to the number of moles at constant temperature and pressure
A)the volume of a gas is inversely related to the number of moles at constant temperature and pressure
B)the volume of a gas is inversely related to the number of moles at standard temperature and pressure
C)the volume of a gas depends only on the temperature and pressure
D)the volume of a gas depends only on the number of moles in the sample
E)the volume of a gas is directly related to the number of moles at constant temperature and pressure

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
37
A sample of argon at 300.°C and 50.0 atm pressure is cooled in the same container to a temperature of 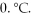 What is the new pressure?
What is the new pressure?
A)105 atm
B)45.5 atm
C)54.9 atm
D)23.8 atm
E)42.7 atm
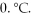 What is the new pressure?
What is the new pressure?A)105 atm
B)45.5 atm
C)54.9 atm
D)23.8 atm
E)42.7 atm

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
38
At STP conditions,11 g of 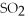 have a volume of ________.
have a volume of ________.
A)250 L
B)3.8 L
C)22 L
D)0.0076 L
E)130 L
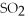 have a volume of ________.
have a volume of ________.A)250 L
B)3.8 L
C)22 L
D)0.0076 L
E)130 L

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
39
A diver exhales a bubble with a volume of 250 mL at a pressure of 2.4 atm and a temperature of 15 °C.What is the volume of the bubble when it reaches the surface where the pressure is 1.0 atm and the temperature is 27 °C?
A)580 mL
B)630 mL
C)100 mL
D)110 mL
E)1100 mL
A)580 mL
B)630 mL
C)100 mL
D)110 mL
E)1100 mL

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
40
1 mole of a gas occupies 22.4 L at ________.
A)0 °C and 0.50 atm
B)0 °C and 760 mmHg
C)100 °C and 1 atm
D)100 °C and 10 atm
E)0 K and 1 atm
A)0 °C and 0.50 atm
B)0 °C and 760 mmHg
C)100 °C and 1 atm
D)100 °C and 10 atm
E)0 K and 1 atm

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
41
A temperature of 273 °C is equivalent to ________ K.

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
42
A gas has a volume of 46.0 L when the temperature is 400.K.When the temperature changes to 500.K,what is the new volume,if there is no change in pressure or amount of gas?

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
43
Which of the following is not a potential use for a hyperbaric chamber?
A)treatment for burns and infections
B)counteracting carbon monoxide poisoning
C)increasing the rate at which a broken bone heals
D)treating a diver with the bends
E)treating some cancers
A)treatment for burns and infections
B)counteracting carbon monoxide poisoning
C)increasing the rate at which a broken bone heals
D)treating a diver with the bends
E)treating some cancers

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
44
A gas has a volume of 12.0 L when the pressure is 840.torr.When the pressure changes to 600.torr,what is the new volume,if there is no change in temperature or amount of gas?

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
45
According to Boyle's Law,when the volume increases,the pressure ________.

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
46
One atmosphere is the same pressure as ________ mmHg.

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
47
What is the volume,in L,of 36.0 g of CH4 at STP?

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
48
The pressure exerted by a gas on its container is directly proportional to ________.
A)the volume of the container
B)the mass of the individual gas molecules
C)the Celsius temperature of the gas
D)the number of molecules of gas in the sample
E)the Fahrenheit temperature of the gas
A)the volume of the container
B)the mass of the individual gas molecules
C)the Celsius temperature of the gas
D)the number of molecules of gas in the sample
E)the Fahrenheit temperature of the gas

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
49
STP is ________ mmHg and ________K.

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
50
A barometer is usually filled with ________.

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
51
At STP,what is the volume of 4.50 moles of nitrogen gas?
A)167 L
B)3420 L
C)101 L
D)60.7 L
E)1230 L
A)167 L
B)3420 L
C)101 L
D)60.7 L
E)1230 L

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
52
A gas has a volume of 36.0 L and a pressure of 750.torr when the temperature is 10.°C.What is the pressure if the volume changes to 15.0 L and the temperature changes to 78 °C,if the amount of gas stays the same?

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
53
At STP,what is the mass 13.0 L of methane,C 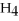 ?
?
A)0.580 g
B)16.0 g
C)9.31 g
D)27.6 g
E)22.4 g
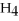 ?
?A)0.580 g
B)16.0 g
C)9.31 g
D)27.6 g
E)22.4 g

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
54
A gas has a volume of 45.0 L and a pressure of 540.mmHg when the temperature is 27 °C.What is the volume when the pressure changes to 800.mmHg with a temperature of -65 °C,if there is no change in the amount of gas?

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
55
Nitrogen makes up about ________ percent of the atmosphere.

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
56
A gas has a pressure of 540.torr when the temperature is 127 °C.When the temperature changes to 27 °C,what is the new pressure,if there is no change in volume or amount of gas?

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
57
At STP,what is the volume of 1.0 mole of carbon dioxide?
A)1.00 L
B)44.0 L
C)273 L
D)22.4 L
E)12.2 L
A)1.00 L
B)44.0 L
C)273 L
D)22.4 L
E)12.2 L

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
58
At STP,how many moles of helium would occupy 1.00 L?
A)2.24 moles
B)224 moles
C)22.4 moles
D)0.446 moles
E)0.0446 moles
A)2.24 moles
B)224 moles
C)22.4 moles
D)0.446 moles
E)0.0446 moles

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
59
A gas has a pressure of 700.mmHg when the temperature is 107 °C.At what temperature (°C)will the pressure be 600.mmHg,if there is no change in volume or amount of gas?

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
60
The pressure unit 1 mmHg is the same pressure unit as the pressure unit ________.

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
61
The kinetic energy of a gas sample is directly proportional to the Kelvin temperature of the gas.

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
62
During inspiration,we use 100% of the oxygen in the air we breathe.

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
63
The volume of 1 mole of any gas at STP is 22.4 L.

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
64
The air we breathe is about 21% oxygen.

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
65
What is the total pressure (in atm)of a gas mixture,which has CH4 600.torr and He 450.mmHg?

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
66
The total pressure of a mixture of CO2 and O2 is 780 mmHg.If the pressure of the CO2 is 560.torr,what is the pressure of O2?

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
67
STP stands for 0 °C and 760 mmHg.

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
68
The pressure increases as the volume increases,if temperature does not change.

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
69
At 0 K,all molecular motion stops.

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
70
The speed of gas molecules is related to the volume of the container.

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
71
Gas law calculations require the use of the Kelvin temperature scale.

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
72
The pressure exerted by a gas on its container is inversely related to its Kelvin temperature.

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck
73
In deoxygenated blood,the partial pressure of carbon dioxide is greater than the partial pressure of oxygen left.

Unlock Deck
Unlock for access to all 73 flashcards in this deck.
Unlock Deck
k this deck


