Deck 9: Solutions
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Match between columns
Question
Question
Match between columns
Question
Question
Match between columns
Premises:
water
water
water
7% glucose
7% glucose
7% glucose
5% glucose
5% glucose
5% glucose
Responses:
hypotonic
hypertonic
isotonic
hypotonic
hypertonic
isotonic
hypotonic
hypertonic
isotonic
hypotonic
hypertonic
isotonic
hypotonic
hypertonic
isotonic
hypotonic
hypertonic
isotonic
hypotonic
hypertonic
isotonic
Question
Question
Question
Question
Question
Match between columns
Question
Question
Match between columns
Premises:
HF,a weak electrolyte
HF,a weak electrolyte
HF,a weak electrolyte
urea,a nonelectrolyte
urea,a nonelectrolyte
urea,a nonelectrolyte
NaCl,a strong electrolyte
NaCl,a strong electrolyte
NaCl,a strong electrolyte
Responses:
ions
molecules
both
ions
molecules
both
ions
molecules
both
ions
molecules
both
ions
molecules
both
ions
molecules
both
ions
molecules
both
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/81
Play
Full screen (f)
Deck 9: Solutions
1
A hydrogen bond is ________.
A)an attractive force between molecules where partially positive hydrogen atoms are attracted to partially negative atoms of F,O,or N
B)a covalent bond between H and O
C)an ionic bond between H and another atom
D)a bond that is stronger than a covalent bond
E)the polar O-H bond in water
A)an attractive force between molecules where partially positive hydrogen atoms are attracted to partially negative atoms of F,O,or N
B)a covalent bond between H and O
C)an ionic bond between H and another atom
D)a bond that is stronger than a covalent bond
E)the polar O-H bond in water
an attractive force between molecules where partially positive hydrogen atoms are attracted to partially negative atoms of F,O,or N
2
What is the concentration,in mass percent (m/m),of a solution prepared from 50.0 g NaCl and 150.0 g of water?
A)0.250% (m/m)
B)33.3% (m/m)
C)40.0% (m/m)
D)25.0% (m/m)
E)3.00% (m/m)
A)0.250% (m/m)
B)33.3% (m/m)
C)40.0% (m/m)
D)25.0% (m/m)
E)3.00% (m/m)
25.0% (m/m)
3
How many equivalents are in 0.40 mole of 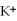 ?
?
A)0.40 Eq
B)0.80 Eq
C)0.20 Eq
D)2.0 Eq
E)1.0 Eq
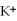 ?
?A)0.40 Eq
B)0.80 Eq
C)0.20 Eq
D)2.0 Eq
E)1.0 Eq
0.40 Eq
4
The solubility of KI is 50 g in 100 g of H2O at 20 °C.If 110 grams of KI are added to 200 grams of H2O,________.
A)all of the KI will dissolve
B)the solution will freeze
C)the solution will start boiling
D)a saturated solution will form
E)the solution will be unsaturated
A)all of the KI will dissolve
B)the solution will freeze
C)the solution will start boiling
D)a saturated solution will form
E)the solution will be unsaturated

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
5
An equivalent is ________.
A)the amount of ion that has a 1+ charge
B)the amount of ion that has a 1- charge
C)the amount of ion that carries 1 mole of electrical charge
D)1 mole of any ion
E)1 mole of an ionic compound
A)the amount of ion that has a 1+ charge
B)the amount of ion that has a 1- charge
C)the amount of ion that carries 1 mole of electrical charge
D)1 mole of any ion
E)1 mole of an ionic compound

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
6
In water,a substance that ionizes completely in solution is called a ________.
A)weak electrolyte
B)nonelectrolyte
C)semiconductor
D)nonconductor
E)strong electrolyte
A)weak electrolyte
B)nonelectrolyte
C)semiconductor
D)nonconductor
E)strong electrolyte

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
7
The mass percent concentration refers to ________.
A)grams of solute in 1 kg of solvent
B)grams of solute in 1 kg of solution
C)grams of solute in 100 g of solvent
D)grams of solute in 100 g of solution
E)grams of solvent in 100 g of solution
A)grams of solute in 1 kg of solvent
B)grams of solute in 1 kg of solution
C)grams of solute in 100 g of solvent
D)grams of solute in 100 g of solution
E)grams of solvent in 100 g of solution

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
8
In a solution,the solvent ________.
A)is a liquid.
B)can be a liquid or gas.
C)can be a solid,liquid,or gas.
D)is never a solid.
E)is the substance present in the smallest concentration.
A)is a liquid.
B)can be a liquid or gas.
C)can be a solid,liquid,or gas.
D)is never a solid.
E)is the substance present in the smallest concentration.

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
9
Oil does not dissolve in water because ________.
A)oil is polar
B)oil is nonpolar
C)water is nonpolar
D)water is saturated
E)oil is hydrated
A)oil is polar
B)oil is nonpolar
C)water is nonpolar
D)water is saturated
E)oil is hydrated

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
10
When some of the sugar added to iced tea remains undissolved at the bottom of the glass,the solution is ________.
A)dilute
B)polar
C)nonpolar
D)saturated
E)unsaturated
A)dilute
B)polar
C)nonpolar
D)saturated
E)unsaturated

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
11
A mixture is prepared by dissolving 2 g of KCl in 100 g of H2O.In this mixture, H2O is the ________.
A)solute
B)solvent
C)solution
D)solid
E)ionic compound
A)solute
B)solvent
C)solution
D)solid
E)ionic compound

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
12
A solution contains 43 mEq/L of of 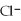 and 11 mEq/L of
and 11 mEq/L of 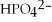 .If the only cation in the solution is
.If the only cation in the solution is 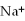 ,what is the
,what is the 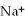 concentration in mEq/L?
concentration in mEq/L?
A)43 mEq/L
B)11 mEq/L
C)54 mEq/L
D)32 mEq/L
E)2.0 mEq/L
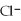 and 11 mEq/L of
and 11 mEq/L of 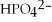 .If the only cation in the solution is
.If the only cation in the solution is 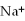 ,what is the
,what is the 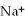 concentration in mEq/L?
concentration in mEq/L?A)43 mEq/L
B)11 mEq/L
C)54 mEq/L
D)32 mEq/L
E)2.0 mEq/L

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
13
When KCl dissolves in water ________.
A)the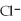 ions are attracted to dissolved
ions are attracted to dissolved 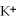 ions
ions
B)the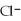 ions are attracted to the partial negative charge on the oxygen atom of the water molecule
ions are attracted to the partial negative charge on the oxygen atom of the water molecule
C)the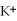 ions are attracted to
ions are attracted to 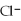 ions on the KCl crystal
ions on the KCl crystal
D)the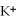 ions are attracted to the partial negative charge on the oxygen atom of the water molecule
ions are attracted to the partial negative charge on the oxygen atom of the water molecule
E)the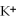 ions are attracted to the partial positive charge on the hydrogen atoms of the water molecule
ions are attracted to the partial positive charge on the hydrogen atoms of the water molecule
A)the
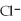 ions are attracted to dissolved
ions are attracted to dissolved 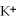 ions
ionsB)the
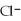 ions are attracted to the partial negative charge on the oxygen atom of the water molecule
ions are attracted to the partial negative charge on the oxygen atom of the water moleculeC)the
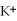 ions are attracted to
ions are attracted to 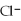 ions on the KCl crystal
ions on the KCl crystalD)the
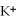 ions are attracted to the partial negative charge on the oxygen atom of the water molecule
ions are attracted to the partial negative charge on the oxygen atom of the water moleculeE)the
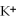 ions are attracted to the partial positive charge on the hydrogen atoms of the water molecule
ions are attracted to the partial positive charge on the hydrogen atoms of the water molecule
Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
14
Water is a polar solvent and hexane ( 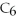
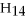 )is a nonpolar solvent.Which of the following correctly describes the solubility of the solute in the given solvent?
)is a nonpolar solvent.Which of the following correctly describes the solubility of the solute in the given solvent?
A)mineral oil,soluble in water
B)Ca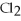 ,soluble in hexane
,soluble in hexane
C)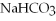 ,soluble in water
,soluble in water
D)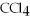 ,soluble in water
,soluble in water
E)octane,soluble in water
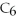
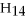 )is a nonpolar solvent.Which of the following correctly describes the solubility of the solute in the given solvent?
)is a nonpolar solvent.Which of the following correctly describes the solubility of the solute in the given solvent?A)mineral oil,soluble in water
B)Ca
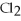 ,soluble in hexane
,soluble in hexaneC)
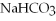 ,soluble in water
,soluble in waterD)
 ,soluble in water
,soluble in waterE)octane,soluble in water

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
15
An increase in the temperature of a solution usually ________.
A)increases the boiling point
B)increases the solubility of a gas in the solution
C)increases the solubility of a solid solute in the solution
D)decreases the solubility of a solid solute in the solution
E)decreases the solubility of a liquid solute in the solution
A)increases the boiling point
B)increases the solubility of a gas in the solution
C)increases the solubility of a solid solute in the solution
D)decreases the solubility of a solid solute in the solution
E)decreases the solubility of a liquid solute in the solution

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
16
According to Henry's law,the solubility of a gas in a liquid ________.
A)decreases as the gas pressure above the liquid increases
B)increases as the gas pressure above the liquid increases
C)remains the same as the temperature increases
D)depends on the liquid polarity
E)depends on the liquid density
A)decreases as the gas pressure above the liquid increases
B)increases as the gas pressure above the liquid increases
C)remains the same as the temperature increases
D)depends on the liquid polarity
E)depends on the liquid density

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
17
How many equivalents are in 0.60 mole of 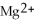 ?
?
A)0.60 Eq
B)0.30 Eq
C)1.2 Eq
D)2.0 Eq
E)1.0 Eq
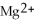 ?
?A)0.60 Eq
B)0.30 Eq
C)1.2 Eq
D)2.0 Eq
E)1.0 Eq

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
18
Which of the following molecules can form hydrogen bonds?
A)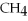
B)NaH
C)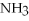
D)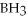
E)HI
A)
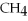
B)NaH
C)
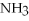
D)
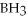
E)HI

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
19
The compound KOH is ________.
A)soluble,because all compounds containing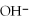 are soluble
are soluble
B)insoluble,because all compounds containing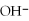 are in soluble
are in soluble
C)soluble,because all compounds containing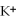 are soluble
are soluble
D)insoluble,because all compounds containing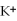 are insoluble
are insoluble
E)insoluble,because KOH is insoluble
A)soluble,because all compounds containing
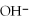 are soluble
are solubleB)insoluble,because all compounds containing
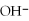 are in soluble
are in solubleC)soluble,because all compounds containing
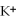 are soluble
are solubleD)insoluble,because all compounds containing
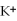 are insoluble
are insolubleE)insoluble,because KOH is insoluble

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
20
The O-H bond in water is polar because ________.
A)it is an ionic bond
B)oxygen is much more electronegative than hydrogen
C)oxygen occupies more space than hydrogen
D)hydrogen is much more electronegative than oxygen
E)it is a hydrogen bond
A)it is an ionic bond
B)oxygen is much more electronegative than hydrogen
C)oxygen occupies more space than hydrogen
D)hydrogen is much more electronegative than oxygen
E)it is a hydrogen bond

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
21
What is the concentration,mass/volume percent (m/v),of a solution prepared from 50.g NaCl and 2.5 L of water?
A)5.0% (m/v)
B)2.0% (m/v)
C)0.020% (m/v)
D)0.050% (m/v)
E)20.% (m/v)
A)5.0% (m/v)
B)2.0% (m/v)
C)0.020% (m/v)
D)0.050% (m/v)
E)20.% (m/v)

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
22
What is the molarity of a KCl solution made by diluting 75.0 mL of a 0.200 M solution to a final volume of 100.mL?
A)0.267 M
B)0.150 M
C)0.200 M
D)6.67 M
E)0.100 M
A)0.267 M
B)0.150 M
C)0.200 M
D)6.67 M
E)0.100 M

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
23
What volume (mL)of a 15% (m/v)NaOH solution contains 120 g NaOH?
A)18 mL
B)0.13 mL
C)13 mL
D)120 mL
E)8.0 ×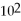 mL
mL
A)18 mL
B)0.13 mL
C)13 mL
D)120 mL
E)8.0 ×
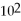 mL
mL
Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
24
How many grams of glucose are needed to prepare 400.mL of a 2.0%(m/v)glucose solution?
A)800.g
B)0.0050 g
C)8.0 g
D)2.0 g
E)200.g
A)800.g
B)0.0050 g
C)8.0 g
D)2.0 g
E)200.g

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
25
What is the new mass/volume percent (m/v)of a KOH solution that is prepared by diluting 110 mL of a 6.0% (m/v)KOH solution to 330 mL?
A)2.0% (m/v)
B)1.0% (m/v)
C)6.0% (m/v)
D)12% (m/v)
E)18% (m/v)
A)2.0% (m/v)
B)1.0% (m/v)
C)6.0% (m/v)
D)12% (m/v)
E)18% (m/v)

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
26
During the process of diluting a solution to a lower concentration,________.
A)the amount of solute does not change
B)the amount of solvent does not change
C)there is more solute in the concentrated solution
D)the volume of the solution does not change
E)water is removed from the concentrated solution
A)the amount of solute does not change
B)the amount of solvent does not change
C)there is more solute in the concentrated solution
D)the volume of the solution does not change
E)water is removed from the concentrated solution

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
27
How many moles of 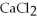 are in 250 mL of a 3.0 M of
are in 250 mL of a 3.0 M of 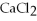 solution?
solution?
A)750 moles
B)1.3 moles
C)83 moles
D)0.75 mole
E)3.0 moles
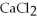 are in 250 mL of a 3.0 M of
are in 250 mL of a 3.0 M of 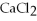 solution?
solution?A)750 moles
B)1.3 moles
C)83 moles
D)0.75 mole
E)3.0 moles

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
28
What volume of 2.5% (m/v)KOH can be prepared from 125 mL of a 5.0% (m/v)KOH solution?
A)0.0040 mL
B)63 mL
C)0.10 mL
D)125 mL
E)250 mL
A)0.0040 mL
B)63 mL
C)0.10 mL
D)125 mL
E)250 mL

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
29
What volume of 0.10 M NaOH can be prepared from 250.mL of 0.30 M NaOH?
A)0.075 L
B)0.25 L
C)0.75 L
D)0.083 L
E)750 L
A)0.075 L
B)0.25 L
C)0.75 L
D)0.083 L
E)750 L

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
30
The molarity (M)of a solution refers to ________.
A)moles of solute/L of solution
B)moles of solute/ L of solvent
C)moles of solute/100 mL of solution
D)grams of solute/100 mL of solution
E)grams of solute/L of solution
A)moles of solute/L of solution
B)moles of solute/ L of solvent
C)moles of solute/100 mL of solution
D)grams of solute/100 mL of solution
E)grams of solute/L of solution

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
31
What mass of KCl is in 350 mL of 0.24 M KCl?
A)0.84 g
B)1.1.g
C)84 g
D)18 g
E)6.3 g
A)0.84 g
B)1.1.g
C)84 g
D)18 g
E)6.3 g

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
32
What is the molarity of a solution which contains 58.5 g of sodium chloride dissolved in 0.500 L of solution?
A)0.500 M
B)1.00 M
C)1.50 M
D)2.00 M
E)4.00 M
A)0.500 M
B)1.00 M
C)1.50 M
D)2.00 M
E)4.00 M

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
33
How many milliliters of a 25% (m/v)NaOH solution would contain 75 g of NaOH?
A)25 mL
B)75 mL
C)33 mL
D)19 mL
E)3.0 ×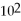 mL
mL
A)25 mL
B)75 mL
C)33 mL
D)19 mL
E)3.0 ×
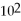 mL
mL
Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
34
A homogeneous mixture that does not settle out upon standing is ________.
A)an element
B)a colloid
C)a suspension
D)solid
E)hydrated
A)an element
B)a colloid
C)a suspension
D)solid
E)hydrated

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
35
What is the molarity of a solution that contains 17 g of 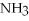 in 0.50 L of solution?
in 0.50 L of solution?
A)34 M
B)2.0 M
C)0.50 M
D)0.029 M
E)1.0 M
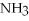 in 0.50 L of solution?
in 0.50 L of solution?A)34 M
B)2.0 M
C)0.50 M
D)0.029 M
E)1.0 M

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
36
What volume of a 1.5 M KOH solution is needed to provide 3.0 moles of KOH?
A)3.0 L
B)0.50 L
C)2.0 L
D)4.5 L
E)0.22 L
A)3.0 L
B)0.50 L
C)2.0 L
D)4.5 L
E)0.22 L

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
37
What is the molarity of a solution containing 5.0 moles of KCl in 2.0 L of solution?
A)2.5 M
B)1.0 M
C)5.0 M
D)10.M
E)2.0 M
A)2.5 M
B)1.0 M
C)5.0 M
D)10.M
E)2.0 M

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
38
In the process known as osmosis,________ moves through a semipermeable membrane into an area of ________ concentration.
A)solute,lower solute
B)solute,higher solute
C)solvent,lower solute
D)solvent,lower solvent
E)solvent,higher solvent
A)solute,lower solute
B)solute,higher solute
C)solvent,lower solute
D)solvent,lower solvent
E)solvent,higher solvent

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
39
Rubbing alcohol is 70.% (m/v)isopropyl alcohol by volume.How many mL of isopropyl alcohol are in a 1 pint (473 mL)container?
A)70.mL
B)0.15 mL
C)680 mL
D)470 mL
E)330 mL
A)70.mL
B)0.15 mL
C)680 mL
D)470 mL
E)330 mL

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
40
What volume of a 2.00 M KCl solution is required to prepare 500.mL of a 0.100 M KCl solution?
A)0.0400 mL
B)25.0 mL
C)2.00 mL
D)1.00 ×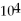 mL
mL
E)5.00 ×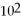 mL
mL
A)0.0400 mL
B)25.0 mL
C)2.00 mL
D)1.00 ×
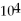 mL
mLE)5.00 ×
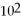 mL
mL
Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
41
A substance that completely ionizes in water is a ________ electrolyte.

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
42
Polar solutes are soluble in ________ solvents.

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
43
The process that occurs in this system is ________.
A)filtration
B)hydration
C)neutralization
D)dialysis
E)osmosis
A)filtration
B)hydration
C)neutralization
D)dialysis
E)osmosis

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
44
Acetic acid can be classified as a ________.
A)gas
B)solid
C)weak electrolyte
D)strong electrolyte
E)ionic compound
A)gas
B)solid
C)weak electrolyte
D)strong electrolyte
E)ionic compound

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
45
An aqueous mixture containing starch (a colloid),NaCl,glucose,and albumin (a colloid)is placed in a dialysis bag and immersed in distilled water.Which of the following correctly describes the location of the indicated substance after dialysis?
A)albumin inside
B)starch outside
C)albumin inside and outside
D)water inside only
E)starch inside and outside
A)albumin inside
B)starch outside
C)albumin inside and outside
D)water inside only
E)starch inside and outside

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
46
A red blood cell will undergo hemolysis in ________.
A)water
B)0.9% NaCl
C)5% glucose
D)5% NaCl
E)10% glucose
A)water
B)0.9% NaCl
C)5% glucose
D)5% NaCl
E)10% glucose

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
47
The process by which a semipermeable membrane allows water molecules,small molecules,and ions to pass through while retaining large particles is called ________.
A)osmotic pressure
B)dialysis
C)solvation
D)dilution
E)hydration
A)osmotic pressure
B)dialysis
C)solvation
D)dilution
E)hydration

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
48
The molarity of a solution of 5.0 g of KCl in 100.mL of solution is ________.
A)0.038 M
B)0.067 M
C)0.67 M
D)0.13 M
E)1.3 M
A)0.038 M
B)0.067 M
C)0.67 M
D)0.13 M
E)1.3 M

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
49
Which solution is isotonic to a red blood cell?
A)water
B)0.5% NaCl
C)2% glucose
D)0.9% NaCl
E)10% glucose
A)water
B)0.5% NaCl
C)2% glucose
D)0.9% NaCl
E)10% glucose

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
50
A solution that has an osmotic pressure less than that of red blood cells is called ________.
A)saturated
B)hypertonic
C)isotonic
D)hypotonic
E)unsaturated
A)saturated
B)hypertonic
C)isotonic
D)hypotonic
E)unsaturated

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
51
A solution with the same osmotic pressure as the blood is ________.
A)isotonic to the blood
B)hypotonic to the blood
C)hypertonic to the blood
D)nontonic to the blood
E)molar to the blood
A)isotonic to the blood
B)hypotonic to the blood
C)hypertonic to the blood
D)nontonic to the blood
E)molar to the blood

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
52
Which of the following also occurs in this system?
A)Water flows equally in both directions.
B)There is a net flow of water from the 4% starch solution into the 10% starch solution.
C)There is a net flow of water from the 10% starch solution into the 4% starch solution.
D)Water does not cross the membrane at all.
E)Starch moves out of the 10% starch solution into the 4% starch solution.
A)Water flows equally in both directions.
B)There is a net flow of water from the 4% starch solution into the 10% starch solution.
C)There is a net flow of water from the 10% starch solution into the 4% starch solution.
D)Water does not cross the membrane at all.
E)Starch moves out of the 10% starch solution into the 4% starch solution.

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
53
A red blood cell will undergo crenation in ________.
A)water
B)0.5% NaCl
C)3% glucose
D)5% glucose
E)7% NaCl
A)water
B)0.5% NaCl
C)3% glucose
D)5% glucose
E)7% NaCl

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
54
A substance that carries an electric current when dissolved in water is called a(n)________.

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
55
Which starch solution will decrease in volume as osmosis occurs?
A)4%
B)10%
C)Neither exerts osmotic pressure.
D)They exert equal osmotic pressures.
E)They exert opposite osmotic pressures.
A)4%
B)10%
C)Neither exerts osmotic pressure.
D)They exert equal osmotic pressures.
E)They exert opposite osmotic pressures.

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
56
A ________ will pass through a filter but not a semipermeable membrane.
A)solid
B)precipitate
C)solution
D)suspension
E)colloid
A)solid
B)precipitate
C)solution
D)suspension
E)colloid

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
57
Using a kidney machine to remove waste products from the blood is known as ________.
A)osmosis
B)osmolysis
C)autolysis
D)hemolysis
E)hemodialysis
A)osmosis
B)osmolysis
C)autolysis
D)hemolysis
E)hemodialysis

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
58
How many equivalents are in 0.036 mole of Ca2+?

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
59
A 10% starch solution is separated from a 2% starch solution by a semipermeable membrane.Starch is a colloid.Which of the following is correct?
A)The 10% solution has the higher osmotic pressure.
B)The 2% solution has the higher osmotic pressure.
C)Neither solution has osmotic pressure.
D)The solutions have the same osmotic pressure.
E)The solutions have opposite osmotic pressures.
A)The 10% solution has the higher osmotic pressure.
B)The 2% solution has the higher osmotic pressure.
C)Neither solution has osmotic pressure.
D)The solutions have the same osmotic pressure.
E)The solutions have opposite osmotic pressures.

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
60
A substance that produces only a small number of ions in solution is known as a ________ electrolyte.

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
61
________ can pass through filters but cannot pass through semipermeable membranes.

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
62
When MgO is added to water,the salt will be ________.

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
63
Match between columns

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
64
The number of moles of a compound dissolved in one liter of a solution is called the
________.
________.

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
65
Match between columns

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
66
When NH4Cl is added to water,the salt will be ________.

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
67
Match between columns
Premises:
water
water
water
7% glucose
7% glucose
7% glucose
5% glucose
5% glucose
5% glucose
Responses:
hypotonic
hypertonic
isotonic
hypotonic
hypertonic
isotonic
hypotonic
hypertonic
isotonic
hypotonic
hypertonic
isotonic
hypotonic
hypertonic
isotonic
hypotonic
hypertonic
isotonic
hypotonic
hypertonic
isotonic

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
68
If a red blood cell is placed in 1% glucose solution,the red blood cell will________.

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
69
When 50.mL of 10.% (m/v)NaCl is diluted to 500.mL,the NaCl concentration is _____%.

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
70
If 450.mL of 6.0 M KBr solution is diluted to 900.mL,the concentration of the KBr solution is _____M.

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
71
A solution which has 12 g of solute dissolved in 200.mL of solution has a m/v concentration of ________.

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
72
Match between columns

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
73
200.mL of a 12.0% (m/v)NaCl solution is diluted to 600.mL.The new concentration is ________ %.

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
74
Match between columns
Premises:
HF,a weak electrolyte
HF,a weak electrolyte
HF,a weak electrolyte
urea,a nonelectrolyte
urea,a nonelectrolyte
urea,a nonelectrolyte
NaCl,a strong electrolyte
NaCl,a strong electrolyte
NaCl,a strong electrolyte
Responses:
ions
molecules
both
ions
molecules
both
ions
molecules
both
ions
molecules
both
ions
molecules
both
ions
molecules
both
ions
molecules
both

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
75
Indicate whether each of the following compounds dissolves in water to give ions,molecules,or both.
A)molecules
B)both
C)ions
6)NaCl,a strong electrolyte
7)urea,a nonelectrolyte
8)HF,a weak electrolyte
9)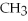
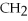 OH,a nonelectrolyte
OH,a nonelectrolyte
10)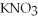 ,a soluble salt
,a soluble salt
11)glucose,a nonelectrolyte
12)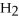
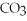 ,a weak electrolyte
,a weak electrolyte
A)molecules
B)both
C)ions
6)NaCl,a strong electrolyte
7)urea,a nonelectrolyte
8)HF,a weak electrolyte
9)
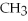
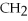 OH,a nonelectrolyte
OH,a nonelectrolyte10)
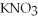 ,a soluble salt
,a soluble salt11)glucose,a nonelectrolyte
12)
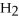
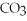 ,a weak electrolyte
,a weak electrolyte
Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
75
Which has a higher osmotic pressure 1.0 M sucrose or water?

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
76
When KCl is added to water,the salt will be ________.

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
76
Match the type of mixture with the appropriate characteristics.
A)solution
B)colloid
C)suspension
13)a mixture of sodium chloride in water
14)a mixture whose particles settle on standing
15)a homogeneous mixture in which suspended particles cannot pass through a semipermeable membrane
16)a mixture whose particles cannot be separated by filters or semipermeable membranes
17)a mixture whose particles can be separated by filters
A)solution
B)colloid
C)suspension
13)a mixture of sodium chloride in water
14)a mixture whose particles settle on standing
15)a homogeneous mixture in which suspended particles cannot pass through a semipermeable membrane
16)a mixture whose particles cannot be separated by filters or semipermeable membranes
17)a mixture whose particles can be separated by filters

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
77
A 2.0% (m/v)NaCl solution contains ________ g NaCl in 300.mL of solution.

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck
77
Compare the osmotic pressure of these solutions to the osmotic pressure of red blood cells.
A)hypertonic
B)hypotonic
C)isotonic
18)water
19)0.5% NaCl
20)0.9% glucose
21)7% glucose
22)5% NaCl
23)5% glucose
24)0.9% NaCl
A)hypertonic
B)hypotonic
C)isotonic
18)water
19)0.5% NaCl
20)0.9% glucose
21)7% glucose
22)5% NaCl
23)5% glucose
24)0.9% NaCl

Unlock Deck
Unlock for access to all 81 flashcards in this deck.
Unlock Deck
k this deck


