Deck 2: Chemical Composition of the Body
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/103
Play
Full screen (f)
Deck 2: Chemical Composition of the Body
1
When an atom gains one or more electrons, it
A)becomes positively charged.
B)has no change in its charge.
C)is called an anion.
D)is called a cation.
A)becomes positively charged.
B)has no change in its charge.
C)is called an anion.
D)is called a cation.
C
2
An atom with 5 protons, 5 neutrons, and 6 electrons would have a net charge of
A)-1.
B)-2.
C)+1.
D)+2.
A)-1.
B)-2.
C)+1.
D)+2.
A
3
Most of the water found in the body is in the
A)blood.
B)intracellular fluid compartment.
C)extracellular fluid compartment.
D)blood and extracellular fluid compartment.
A)blood.
B)intracellular fluid compartment.
C)extracellular fluid compartment.
D)blood and extracellular fluid compartment.
B
4
The atomic nucleus does not contain _____________, which are negatively charged subatomic particles.
A)protons
B)electrons
C)neutrons
A)protons
B)electrons
C)neutrons

Unlock Deck
Unlock for access to all 103 flashcards in this deck.
Unlock Deck
k this deck
5
The _______________ electrons are the outermost electrons of an atom.
A)kernel
B)valence
C)atomic
D)anion
A)kernel
B)valence
C)atomic
D)anion

Unlock Deck
Unlock for access to all 103 flashcards in this deck.
Unlock Deck
k this deck
6
The __________ is the physical space which an electron occupies in an atom.
A)nucleus
B)orbital
C)energy level
D)Both orbital and energy level are correct.
A)nucleus
B)orbital
C)energy level
D)Both orbital and energy level are correct.

Unlock Deck
Unlock for access to all 103 flashcards in this deck.
Unlock Deck
k this deck
7
The term "chemical element" refers to the most common isotope of that element.

Unlock Deck
Unlock for access to all 103 flashcards in this deck.
Unlock Deck
k this deck
8
Isotopes have the same ___________ number, but a different __________ number.
A)mass, atomic
B)neutron, mass
C)atomic, mass
D)atomic, proton
A)mass, atomic
B)neutron, mass
C)atomic, mass
D)atomic, proton

Unlock Deck
Unlock for access to all 103 flashcards in this deck.
Unlock Deck
k this deck
9
_______________ bonds are formed when atoms share electrons unequally.
A)Nonpolar covalent
B)Ionic
C)Polar covalent
D)van der Waals
A)Nonpolar covalent
B)Ionic
C)Polar covalent
D)van der Waals

Unlock Deck
Unlock for access to all 103 flashcards in this deck.
Unlock Deck
k this deck
10
Negatively charged ions will migrate toward the anode in an electrical field.

Unlock Deck
Unlock for access to all 103 flashcards in this deck.
Unlock Deck
k this deck
11
Neutrons are uncharged particles found in the nucleus of an atom.

Unlock Deck
Unlock for access to all 103 flashcards in this deck.
Unlock Deck
k this deck
12
Hydrogen bonds form between the partially charged atoms of two polar molecules, such as the slightly negatively charged hydrogen atom of one water molecule and the slightly positively charged oxygen atom of another.

Unlock Deck
Unlock for access to all 103 flashcards in this deck.
Unlock Deck
k this deck
13
Which of the following is NOT true of isotopes of a given atom?
A)have the same number of neutrons
B)have the same number of protons
C)have different atomic masses
D)All of these choices are correct.
A)have the same number of neutrons
B)have the same number of protons
C)have different atomic masses
D)All of these choices are correct.

Unlock Deck
Unlock for access to all 103 flashcards in this deck.
Unlock Deck
k this deck
14
An element with 5 protons, 5 neutrons, and 5 electrons would have an atomic number of 15.

Unlock Deck
Unlock for access to all 103 flashcards in this deck.
Unlock Deck
k this deck
15
Molecules with polar covalent bonds are hydrophilic.

Unlock Deck
Unlock for access to all 103 flashcards in this deck.
Unlock Deck
k this deck
16
Water makes up _____ of the total body weight of an average adult.
A)50-60%
B)55-65%
C)60-70%
D)65-75%
A)50-60%
B)55-65%
C)60-70%
D)65-75%

Unlock Deck
Unlock for access to all 103 flashcards in this deck.
Unlock Deck
k this deck
17
Which of the following subatomic particles have negligible mass?
A)electrons
B)neutrons
C)protons
D)Both neutrons and protons.
A)electrons
B)neutrons
C)protons
D)Both neutrons and protons.

Unlock Deck
Unlock for access to all 103 flashcards in this deck.
Unlock Deck
k this deck
18
Atoms sharing a pair of electrons form covalent bonds.

Unlock Deck
Unlock for access to all 103 flashcards in this deck.
Unlock Deck
k this deck
19
When an atom loses one or more electrons, it
A)becomes positively charged.
B)becomes negatively charged.
C)is called an anion.
D)has no change in its charge.
A)becomes positively charged.
B)becomes negatively charged.
C)is called an anion.
D)has no change in its charge.

Unlock Deck
Unlock for access to all 103 flashcards in this deck.
Unlock Deck
k this deck
20
An element with 11 neutrons, 11 protons, and 11 electrons would have an atomic mass of __.
A)11
B)33
C)22
D)cannot be determined
A)11
B)33
C)22
D)cannot be determined

Unlock Deck
Unlock for access to all 103 flashcards in this deck.
Unlock Deck
k this deck
21
A solution of a pH above 7 is called _____________.
A)acidic
B)neutral
C)basic
A)acidic
B)neutral
C)basic

Unlock Deck
Unlock for access to all 103 flashcards in this deck.
Unlock Deck
k this deck
22
The pH of a solution is directly proportional to the hydrogen ion concentration of the solution.

Unlock Deck
Unlock for access to all 103 flashcards in this deck.
Unlock Deck
k this deck
23
Surface tension between water molecules occurs because adjacent water molecules form _______ bonds with each other.
A)nonpolar covalent
B)polar covalent
C)hydrogen
D)ionic
A)nonpolar covalent
B)polar covalent
C)hydrogen
D)ionic

Unlock Deck
Unlock for access to all 103 flashcards in this deck.
Unlock Deck
k this deck
24
Molecules that contain carbon and hydrogen atoms are
A)ionic.
B)inorganic.
C)organic.
D)carbonic.
A)ionic.
B)inorganic.
C)organic.
D)carbonic.

Unlock Deck
Unlock for access to all 103 flashcards in this deck.
Unlock Deck
k this deck
25
Regarding acids and bases,
A)acids will increase the pH of a solution.
B)bases will decrease the pH of a solution.
C)acids will accept hydrogen ions in a solution.
D)bases will accept hydrogen ions in a solution.
A)acids will increase the pH of a solution.
B)bases will decrease the pH of a solution.
C)acids will accept hydrogen ions in a solution.
D)bases will accept hydrogen ions in a solution.

Unlock Deck
Unlock for access to all 103 flashcards in this deck.
Unlock Deck
k this deck
26
Hydration spheres can be formed by compounds which contain _______________ bonds.
A)nonpolar covalent
B)polar covalent
C)ionic
D)either polar covalent or ionic
A)nonpolar covalent
B)polar covalent
C)ionic
D)either polar covalent or ionic

Unlock Deck
Unlock for access to all 103 flashcards in this deck.
Unlock Deck
k this deck
27
Bases will _______________ protons in a solution.
A)accept
B)donate
C)ignore
D)repel
A)accept
B)donate
C)ignore
D)repel

Unlock Deck
Unlock for access to all 103 flashcards in this deck.
Unlock Deck
k this deck
28
The type of bond found in sodium chloride is
A)an ionic bond.
B)a polar covalent bond.
C)a hydrogen bond.
D)a nonpolar covalent bond.
A)an ionic bond.
B)a polar covalent bond.
C)a hydrogen bond.
D)a nonpolar covalent bond.

Unlock Deck
Unlock for access to all 103 flashcards in this deck.
Unlock Deck
k this deck
29
Bonds that are formed between oxygen and hydrogen atoms within water molecules are called
A)hydrogen bonds.
B)ionic bonds.
C)nonpolar covalent bonds.
D)polar covalent bonds.
A)hydrogen bonds.
B)ionic bonds.
C)nonpolar covalent bonds.
D)polar covalent bonds.

Unlock Deck
Unlock for access to all 103 flashcards in this deck.
Unlock Deck
k this deck
30
Which of the following would be most easily broken?
A)a hydrogen bond
B)a nonpolar covalent bond
C)an ionic bond
D)a polar covalent bond
A)a hydrogen bond
B)a nonpolar covalent bond
C)an ionic bond
D)a polar covalent bond

Unlock Deck
Unlock for access to all 103 flashcards in this deck.
Unlock Deck
k this deck
31
The primary buffer in the blood is the _______________ buffer.
A)hydronium
B)ammonia
C)phosphate
D)bicarbonate
A)hydronium
B)ammonia
C)phosphate
D)bicarbonate

Unlock Deck
Unlock for access to all 103 flashcards in this deck.
Unlock Deck
k this deck
32
In an acidic solution,
A)the OH- ion concentration is greater than the H+ ion concentration.
B)the OH- ion concentration is less than the H+ ion concentration.
C)the H+ ion concentration is equal to the OH- ion concentration.
D)the H+ ion concentration is less than the OH- ion concentration only if the solution is buffered.
A)the OH- ion concentration is greater than the H+ ion concentration.
B)the OH- ion concentration is less than the H+ ion concentration.
C)the H+ ion concentration is equal to the OH- ion concentration.
D)the H+ ion concentration is less than the OH- ion concentration only if the solution is buffered.

Unlock Deck
Unlock for access to all 103 flashcards in this deck.
Unlock Deck
k this deck
33
Organic acids contain carbonyl groups.

Unlock Deck
Unlock for access to all 103 flashcards in this deck.
Unlock Deck
k this deck
34
Hydrophobic molecules would contain _______________ bonds.
A)nonpolar covalent
B)polar covalent
C)hydrogen
D)ionic
A)nonpolar covalent
B)polar covalent
C)hydrogen
D)ionic

Unlock Deck
Unlock for access to all 103 flashcards in this deck.
Unlock Deck
k this deck
35
The pH of a solution increases as the _______________ ion concentration decreases.
A)hydrogen
B)hydroxide
C)bicarbonate
D)sodium
A)hydrogen
B)hydroxide
C)bicarbonate
D)sodium

Unlock Deck
Unlock for access to all 103 flashcards in this deck.
Unlock Deck
k this deck
36
Water molecules form _______________ ions when they associate with a hydrogen ion.
A)hydroxide
B)bicarbonate
C)hydronium
D)water
A)hydroxide
B)bicarbonate
C)hydronium
D)water

Unlock Deck
Unlock for access to all 103 flashcards in this deck.
Unlock Deck
k this deck
37
A blood pH of 7.6 is
A)indicative of acidosis.
B)indicative of alkalosis.
C)in the normal physiological range.
D)indicates effective buffering by the bicarbonate/carbonic acid system.
A)indicative of acidosis.
B)indicative of alkalosis.
C)in the normal physiological range.
D)indicates effective buffering by the bicarbonate/carbonic acid system.

Unlock Deck
Unlock for access to all 103 flashcards in this deck.
Unlock Deck
k this deck
38
Acids release hydrogen ions into solutions.

Unlock Deck
Unlock for access to all 103 flashcards in this deck.
Unlock Deck
k this deck
39
Ammonia usually
A)acts as a base.
B)acts as an acid.
C)acts as a buffer.
D)ionizes to form a hydroxyl ion.
A)acts as a base.
B)acts as an acid.
C)acts as a buffer.
D)ionizes to form a hydroxyl ion.

Unlock Deck
Unlock for access to all 103 flashcards in this deck.
Unlock Deck
k this deck
40
As the pH of the blood decreases, the amount of hydrogen ions in the blood would decrease.

Unlock Deck
Unlock for access to all 103 flashcards in this deck.
Unlock Deck
k this deck
41
Carbohydrate molecules have a ratio of twice as many oxygen atoms to carbon atoms.

Unlock Deck
Unlock for access to all 103 flashcards in this deck.
Unlock Deck
k this deck
42
Ketones contain a(n) _______________ group within the carbon chain.
A)hydroxyl
B)carbonyl
C)carboxyl
D)aromatic
A)hydroxyl
B)carbonyl
C)carboxyl
D)aromatic

Unlock Deck
Unlock for access to all 103 flashcards in this deck.
Unlock Deck
k this deck
43
Fats and carbohydrates are the primary energy stores in the body.

Unlock Deck
Unlock for access to all 103 flashcards in this deck.
Unlock Deck
k this deck
44
Fructose is a ketone.

Unlock Deck
Unlock for access to all 103 flashcards in this deck.
Unlock Deck
k this deck
45
Glycogen
A)is more highly branched than plant starch.
B)is a glycoprotein found in the liver.
C)is a glycolipid found in skeletal muscles.
D)is composed of alternating glucose and galactose molecules.
A)is more highly branched than plant starch.
B)is a glycoprotein found in the liver.
C)is a glycolipid found in skeletal muscles.
D)is composed of alternating glucose and galactose molecules.

Unlock Deck
Unlock for access to all 103 flashcards in this deck.
Unlock Deck
k this deck
46
An ionized organic acid is designated with the suffix - ate.

Unlock Deck
Unlock for access to all 103 flashcards in this deck.
Unlock Deck
k this deck
47
_________ are molecules with the same ratio of atoms but different arrangements of atoms.
A)Isotopes
B)Structural isomers
C)Stereoisomers
D)Radioactive isotopes
A)Isotopes
B)Structural isomers
C)Stereoisomers
D)Radioactive isotopes

Unlock Deck
Unlock for access to all 103 flashcards in this deck.
Unlock Deck
k this deck
48
Covalent bonds are formed between monosaccharides through dehydration synthesis.

Unlock Deck
Unlock for access to all 103 flashcards in this deck.
Unlock Deck
k this deck
49
The ionized form of the organic lactic acid is lactate.

Unlock Deck
Unlock for access to all 103 flashcards in this deck.
Unlock Deck
k this deck
50
An example of an aromatic substance is
A)hexane.
B)cyclohexane.
C)fructose.
D)benzene.
A)hexane.
B)cyclohexane.
C)fructose.
D)benzene.

Unlock Deck
Unlock for access to all 103 flashcards in this deck.
Unlock Deck
k this deck
51
Glucose, galactose, and fructose can be considered structural isomers of each other.

Unlock Deck
Unlock for access to all 103 flashcards in this deck.
Unlock Deck
k this deck
52
Which reaction represents a dehydration synthesis reaction? 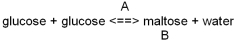
A)Reaction A
B)Reaction B
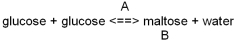
A)Reaction A
B)Reaction B

Unlock Deck
Unlock for access to all 103 flashcards in this deck.
Unlock Deck
k this deck
53
How many single bonds can a carbon atom form if it is double-bonded to an oxygen atom?
A)1
B)2
C)3
D)4
A)1
B)2
C)3
D)4

Unlock Deck
Unlock for access to all 103 flashcards in this deck.
Unlock Deck
k this deck
54
Only L-stereoisomers are absorbed by the digestive tract and used to synthesize organic molecules.

Unlock Deck
Unlock for access to all 103 flashcards in this deck.
Unlock Deck
k this deck
55
Glucose is stored as a polysaccharide to prevent osmosis of water into the cells.

Unlock Deck
Unlock for access to all 103 flashcards in this deck.
Unlock Deck
k this deck
56
The addition of water with the proper enzymes to a molecule is called
A)dehydration synthesis.
B)condensation.
C)hydrolysis.
D)combustion.
A)dehydration synthesis.
B)condensation.
C)hydrolysis.
D)combustion.

Unlock Deck
Unlock for access to all 103 flashcards in this deck.
Unlock Deck
k this deck
57
An example of a monosaccharide is
A)maltose.
B)sucrose.
C)glucose.
D)glycogen.
A)maltose.
B)sucrose.
C)glucose.
D)glycogen.

Unlock Deck
Unlock for access to all 103 flashcards in this deck.
Unlock Deck
k this deck
58
A six-sided organic molecule with alternating double bonds is termed a(n)
A)aromatic compound.
B)ketone.
C)alcohol.
D)organic acid.
A)aromatic compound.
B)ketone.
C)alcohol.
D)organic acid.

Unlock Deck
Unlock for access to all 103 flashcards in this deck.
Unlock Deck
k this deck
59
Sucrose is a disaccharide that is composed of _______________ and _____________.
A)glucose, glucose
B)glucose, galactose
C)glucose, fructose
D)fructose, galactose
A)glucose, glucose
B)glucose, galactose
C)glucose, fructose
D)fructose, galactose

Unlock Deck
Unlock for access to all 103 flashcards in this deck.
Unlock Deck
k this deck
60
Organic acids will contain
A)a carboxyl group.
B)a carbonyl group.
C)an amino group.
D)a hydroxyl group.
A)a carboxyl group.
B)a carbonyl group.
C)an amino group.
D)a hydroxyl group.

Unlock Deck
Unlock for access to all 103 flashcards in this deck.
Unlock Deck
k this deck
61
Unsaturated fatty acids contain more hydrogen atoms than saturated fatty acids of the same length.

Unlock Deck
Unlock for access to all 103 flashcards in this deck.
Unlock Deck
k this deck
62
________________ are liver synthesized derivatives of free fatty acids that can be used as an immediate source of energy by many organs.
A)Glycerols
B)Ketone bodies
C)Steroids
D)Cholesterols
A)Glycerols
B)Ketone bodies
C)Steroids
D)Cholesterols

Unlock Deck
Unlock for access to all 103 flashcards in this deck.
Unlock Deck
k this deck
63
Which of the following is NOT true of steroids?
A)They have three 6-carbon rings joined to one 5-carbon ring.
B)They contain a variety of functional groups.
C)They are derived from palmitate.
D)They differ in the position of the double covalent bonds between the carbon atoms in the rings.
A)They have three 6-carbon rings joined to one 5-carbon ring.
B)They contain a variety of functional groups.
C)They are derived from palmitate.
D)They differ in the position of the double covalent bonds between the carbon atoms in the rings.

Unlock Deck
Unlock for access to all 103 flashcards in this deck.
Unlock Deck
k this deck
64
Unsaturated fatty acids
A)contain one or more double bonds.
B)are usually liquid at room temperature.
C)contain a maximal number of hydrogen atoms.
D)Both contain one or more double bonds and are usually liquid at room temperature are correct.
A)contain one or more double bonds.
B)are usually liquid at room temperature.
C)contain a maximal number of hydrogen atoms.
D)Both contain one or more double bonds and are usually liquid at room temperature are correct.

Unlock Deck
Unlock for access to all 103 flashcards in this deck.
Unlock Deck
k this deck
65
Which of the following is NOT a type of lipid?
A)prostaglandins
B)triglycerides
C)cholesterol
D)glycogen
A)prostaglandins
B)triglycerides
C)cholesterol
D)glycogen

Unlock Deck
Unlock for access to all 103 flashcards in this deck.
Unlock Deck
k this deck
66
Phospholipids
A)are glycolipids originally isolated from the prostate gland.
B)are major components of the cell membrane.
C)have a polar head and a nonpolar tail.
D)Both are major components of the cell membrane and have a polar head and a nonpolar tail are correct.
A)are glycolipids originally isolated from the prostate gland.
B)are major components of the cell membrane.
C)have a polar head and a nonpolar tail.
D)Both are major components of the cell membrane and have a polar head and a nonpolar tail are correct.

Unlock Deck
Unlock for access to all 103 flashcards in this deck.
Unlock Deck
k this deck
67
Steroids are derived from cholesterol.

Unlock Deck
Unlock for access to all 103 flashcards in this deck.
Unlock Deck
k this deck
68
All amino acids contain carboxyl and amino groups.

Unlock Deck
Unlock for access to all 103 flashcards in this deck.
Unlock Deck
k this deck
69
Rapid, uncontrolled hydrolysis of body fats can result in ketoacidosis.

Unlock Deck
Unlock for access to all 103 flashcards in this deck.
Unlock Deck
k this deck
70
This group of organic compounds acts as surfactants:
A)carbohydrates
B)phospholipids
C)nucleic acids
D)prostaglandins
A)carbohydrates
B)phospholipids
C)nucleic acids
D)prostaglandins

Unlock Deck
Unlock for access to all 103 flashcards in this deck.
Unlock Deck
k this deck
71
In order to maintain proper health, total dietary fat intake should not exceed _______________ of total dietary energy intake.
A)10%
B)20%
C)30%
D)40%
A)10%
B)20%
C)30%
D)40%

Unlock Deck
Unlock for access to all 103 flashcards in this deck.
Unlock Deck
k this deck
72
Lipids containing glycerol would include _______________ and _____________.
A)triglycerides, steroids
B)prostaglandins, phospholipids
C)triglycerides, phospholipids
D)steroids, prostaglandins
A)triglycerides, steroids
B)prostaglandins, phospholipids
C)triglycerides, phospholipids
D)steroids, prostaglandins

Unlock Deck
Unlock for access to all 103 flashcards in this deck.
Unlock Deck
k this deck
73
Corticosteroids are a type of lipid commonly found in cell membranes.

Unlock Deck
Unlock for access to all 103 flashcards in this deck.
Unlock Deck
k this deck
74
Ketosis
A)occurs when stored fats are rapidly degraded by the body.
B)stimulates an increased blood pH.
C)may lead to alkalosis.
D)occurs as the concentration of ketones in the urine decreases.
A)occurs when stored fats are rapidly degraded by the body.
B)stimulates an increased blood pH.
C)may lead to alkalosis.
D)occurs as the concentration of ketones in the urine decreases.

Unlock Deck
Unlock for access to all 103 flashcards in this deck.
Unlock Deck
k this deck
75
Which of the following is NOT a derivative of cholesterol?
A)corticosteroids
B)vitamin D3
C)aldosterone
D)lecithin
A)corticosteroids
B)vitamin D3
C)aldosterone
D)lecithin

Unlock Deck
Unlock for access to all 103 flashcards in this deck.
Unlock Deck
k this deck
76
In the formation of triglycerides,
A)hydroxyl and carbonyl groups interact.
B)amino and carbonyl groups interact.
C)carboxyl and amino groups interact.
D)carboxyl and hydroxyl groups interact.
A)hydroxyl and carbonyl groups interact.
B)amino and carbonyl groups interact.
C)carboxyl and amino groups interact.
D)carboxyl and hydroxyl groups interact.

Unlock Deck
Unlock for access to all 103 flashcards in this deck.
Unlock Deck
k this deck
77
Which of the following polysaccharides cannot be digested by animals themselves?
A)glycogen
B)cellulose
C)starch
D)All of these can be digested by animals themselves.
A)glycogen
B)cellulose
C)starch
D)All of these can be digested by animals themselves.

Unlock Deck
Unlock for access to all 103 flashcards in this deck.
Unlock Deck
k this deck
78
_____________ are fatty acids with a cyclic hydrocarbon group.
A)Triglycerides
B)Prostaglandins
C)Proteins
D)Carbohydrates
A)Triglycerides
B)Prostaglandins
C)Proteins
D)Carbohydrates

Unlock Deck
Unlock for access to all 103 flashcards in this deck.
Unlock Deck
k this deck
79
Which of the following is NOT a disaccharide?
A)fructose
B)sucrose
C)maltose
D)lactose
A)fructose
B)sucrose
C)maltose
D)lactose

Unlock Deck
Unlock for access to all 103 flashcards in this deck.
Unlock Deck
k this deck
80
Which of the following describes a trans-fat?
A)Has carbon-carbon single bonds.
B)Has carbon-carbon double bonds with hydrogens on opposite sides of the bonds.
C)Has carbon-carbon double bonds with hydrogens on the same side of the bonds.
D)The fatty acids form a bent chain.
A)Has carbon-carbon single bonds.
B)Has carbon-carbon double bonds with hydrogens on opposite sides of the bonds.
C)Has carbon-carbon double bonds with hydrogens on the same side of the bonds.
D)The fatty acids form a bent chain.

Unlock Deck
Unlock for access to all 103 flashcards in this deck.
Unlock Deck
k this deck


