Deck 3: Patterns in Nature: Minerals
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/60
Play
Full screen (f)
Deck 3: Patterns in Nature: Minerals
1
Minerals in geodes (as seen below)form spectacular euhedral crystals because 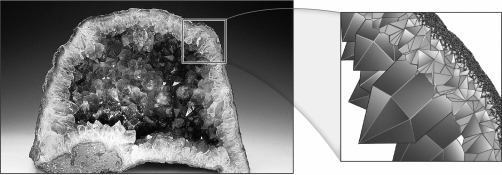
A) all of the elements incorporated in the crystals are in plentiful supply.
B) the crystals have abundant room to grow in their hollow surroundings.
C) minerals within geodes are always framework silicates.
D) minerals within geodes always contain iron.

A) all of the elements incorporated in the crystals are in plentiful supply.
B) the crystals have abundant room to grow in their hollow surroundings.
C) minerals within geodes are always framework silicates.
D) minerals within geodes always contain iron.
B
2
When two different minerals have the same chemical formula but different crystal structures,they are said to be
A) polymorphs.
B) polyliths.
C) monoliths.
D) pseudomorphs.
A) polymorphs.
B) polyliths.
C) monoliths.
D) pseudomorphs.
A
3
Which of the following minerals is more commonly known as rock salt?
A) gypsum
B) feldspar
C) halite
D) quartz
A) gypsum
B) feldspar
C) halite
D) quartz
C
4
When a solution becomes oversaturated,new solid particles are said to _______
A) precipitate from the solution.
B) dissolve into the solution.
C) react with the solution and produce heat.
D) rapidly expand,causing an explosion.
A) precipitate from the solution.
B) dissolve into the solution.
C) react with the solution and produce heat.
D) rapidly expand,causing an explosion.

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
5
Minerals are all naturally occurring solid substances with a definable chemical composition.They must also possess
A) the ability to be synthesized in the laboratory as well as be found in nature.
B) metallic elements,such as iron,calcium,or magnesium.
C) metallic luster.
D) a fixed crystalline structure (spatial arrangement of atoms and ions).
A) the ability to be synthesized in the laboratory as well as be found in nature.
B) metallic elements,such as iron,calcium,or magnesium.
C) metallic luster.
D) a fixed crystalline structure (spatial arrangement of atoms and ions).

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
6
Which of the following is a mineral?
A) petroleum (oil),which is a liquid
B) cubic zirconia,which is a synthetic diamond substitute
C) ice,which is water in the solid state
D) obsidian,a type of volcanic glass
A) petroleum (oil),which is a liquid
B) cubic zirconia,which is a synthetic diamond substitute
C) ice,which is water in the solid state
D) obsidian,a type of volcanic glass

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
7
What is solid-state diffusion?
A) cooling of a liquid and turning it into a solid
B) movement of atoms through a solid to form a new mineral
C) bonding of dissolved ions into a solid crystal
D) growth of a mineral within or adjacent to a living organism
A) cooling of a liquid and turning it into a solid
B) movement of atoms through a solid to form a new mineral
C) bonding of dissolved ions into a solid crystal
D) growth of a mineral within or adjacent to a living organism

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
8
Of the ~4,000 known minerals,the vast majority ___________
A) are common.
B) are rare.
C) form only near volcanoes.
D) are characterized as gems.
A) are common.
B) are rare.
C) form only near volcanoes.
D) are characterized as gems.

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
9
Which of the following are good conductors due to the ability of the electrons in the atoms to move around freely?
A) metals
B) sulfides
C) silicates
D) carbonates
A) metals
B) sulfides
C) silicates
D) carbonates

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
10
We distinguish between a mineral and a naturally formed glass (such as obsidian)because
A) glass is not produced by geologic processes.
B) glass is organic.
C) glass does not have a fixed crystal structure.
D) glass can be made synthetically as well as occur naturally.
A) glass is not produced by geologic processes.
B) glass is organic.
C) glass does not have a fixed crystal structure.
D) glass can be made synthetically as well as occur naturally.

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
11
Crystals that exhibit well-formed faces are referred to as
A) pristine.
B) anhedral.
C) subhedral.
D) euhedral.
A) pristine.
B) anhedral.
C) subhedral.
D) euhedral.

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
12
Which tool is used to detect the pattern of atoms in a crystal?
A) magnetic resonance imaging (MRI)
B) X-ray diffraction
C) thermal ionization mass spectrometry (TIMS)
D) cathodized axial tomography (CAT)
A) magnetic resonance imaging (MRI)
B) X-ray diffraction
C) thermal ionization mass spectrometry (TIMS)
D) cathodized axial tomography (CAT)

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
13
Fewer than 50 minerals are ___________
A) found on the Earth.
B) used for industrial purposes.
C) found in the crust.
D) commonly found in rocks.
A) found on the Earth.
B) used for industrial purposes.
C) found in the crust.
D) commonly found in rocks.

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
14
Diamond and graphite are both polymorphs of
A) silicon.
B) iron.
C) magnesium.
D) carbon.
A) silicon.
B) iron.
C) magnesium.
D) carbon.

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
15
The atomic mass of an element approximately equals the number of
A) electrons.
B) protons plus neutrons.
C) neutrons.
D) protons.
A) electrons.
B) protons plus neutrons.
C) neutrons.
D) protons.

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
16
The atomic number of an element corresponds to the __________
A) number of electrons.
B) number of protons.
C) number of neutrons.
D) total weight of one atom.
A) number of electrons.
B) number of protons.
C) number of neutrons.
D) total weight of one atom.

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
17
Which of the following describes the process of mineral dissolution?
A) heating to a temperature where bonds between atoms break
B) reacting minerals with reagents to form new minerals
C) immersing in water such that atoms or ions separate from crystal faces
D) breaking apart bonds to release chemical energy for microbes
A) heating to a temperature where bonds between atoms break
B) reacting minerals with reagents to form new minerals
C) immersing in water such that atoms or ions separate from crystal faces
D) breaking apart bonds to release chemical energy for microbes

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
18
Where is the most recently formed portion of any crystal always found?
A) deep within the interior
B) on the outer edges
C) on whichever side is currently facing upward
D) There is no consistent pattern for crystal formation.
A) deep within the interior
B) on the outer edges
C) on whichever side is currently facing upward
D) There is no consistent pattern for crystal formation.

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
19
The angles between adjacent crystal faces of the same type of mineral _______ 
A) are always the same.
B) vary widely among different specimens of the mineral.
C) depend on the size of the crystal.
D) are 90° for every type of mineral.

A) are always the same.
B) vary widely among different specimens of the mineral.
C) depend on the size of the crystal.
D) are 90° for every type of mineral.

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
20
Which of the following choices lists atomic particles ordered from smallest to largest in size?
A) atom,nucleus,proton,electron
B) electron,proton,nucleus,atom
C) proton,electron,nucleus,atom
D) atom,electron,nucleus,proton
A) atom,nucleus,proton,electron
B) electron,proton,nucleus,atom
C) proton,electron,nucleus,atom
D) atom,electron,nucleus,proton

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
21
The shape of single crystals or aggregates of many well-formed crystals is known as:
A) streak
B) habit
C) luster
D) cleavage
A) streak
B) habit
C) luster
D) cleavage

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
22
Using the Mohs hardness scale below,which of the following statements about the actual hardness of minerals is false? 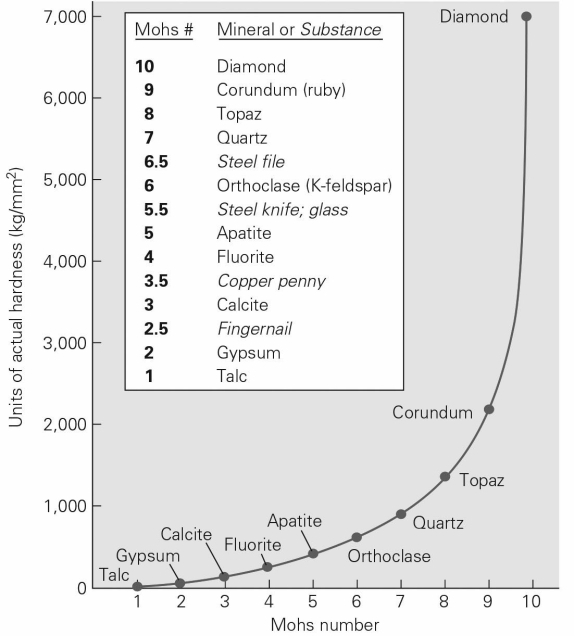
A) Your fingernail can scratch a sample of gypsum.
B) Diamonds are twice as hard as apatite.
C) Diamonds are more than seven times as hard as quartz.
D) Quartz will scratch anything made of steel.

A) Your fingernail can scratch a sample of gypsum.
B) Diamonds are twice as hard as apatite.
C) Diamonds are more than seven times as hard as quartz.
D) Quartz will scratch anything made of steel.

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
23
A mineral property defined as the density of the mineral sample divided by the density of water (1.0 g/cm3)is
A) mass.
B) specific gravity.
C) luster.
D) streak.
A) mass.
B) specific gravity.
C) luster.
D) streak.

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
24
Minerals are grouped into mineral classes primarily on the basis of
A) chemistry,specifically the cations within the chemical formula.
B) chemistry,specifically the anions within the chemical formula.
C) hardness; hard,soft,and medium are the three primary classes.
D) the number of cleavage directions present.
A) chemistry,specifically the cations within the chemical formula.
B) chemistry,specifically the anions within the chemical formula.
C) hardness; hard,soft,and medium are the three primary classes.
D) the number of cleavage directions present.

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
25
The mineral class that makes up more than 95% of the continental crust is termed the
A) silicates.
B) carbonates.
C) halides.
D) oxides.
A) silicates.
B) carbonates.
C) halides.
D) oxides.

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
26
If a mineral lacks cleavage,it will
A) break along planar surfaces with specific orientations.
B) break along irregular or conchoidal fractures.
C) not break apart unless melted.
D) not break apart unless dissolved.
A) break along planar surfaces with specific orientations.
B) break along irregular or conchoidal fractures.
C) not break apart unless melted.
D) not break apart unless dissolved.

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
27
Crystal habit,such as needle-like,platy,or cubic,depends on _________
A) the color of the mineral's streak.
B) the class the mineral belongs to.
C) the wavelength of light reflected off the mineral surface.
D) the mineral's growth rate in different directions.
A) the color of the mineral's streak.
B) the class the mineral belongs to.
C) the wavelength of light reflected off the mineral surface.
D) the mineral's growth rate in different directions.

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
28
Silicate minerals are subdivided into six groups based on the way silica tetrahedra are
A) bonded to anions.
B) bonded to iron atoms.
C) arranged and bonded.
D) arranged and charged.
A) bonded to anions.
B) bonded to iron atoms.
C) arranged and bonded.
D) arranged and charged.

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
29
Which of the following common minerals is softest?
A) quartz
B) calcite
C) talc
D) fluorite
A) quartz
B) calcite
C) talc
D) fluorite

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
30
SiO44-,S2-,and CO32- are all examples of
A) organic compounds.
B) silicate minerals.
C) anions.
D) cations.
A) organic compounds.
B) silicate minerals.
C) anions.
D) cations.

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
31
The way a mineral scatters light is a diagnostic property termed
A) color.
B) reflectivity.
C) luster.
D) streak.
A) color.
B) reflectivity.
C) luster.
D) streak.

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
32
The image below shows a mineral with hydrochloric acid applied to it.Based on the reaction and presence of gas bubbles,which of the following minerals is the most likely to be _______? 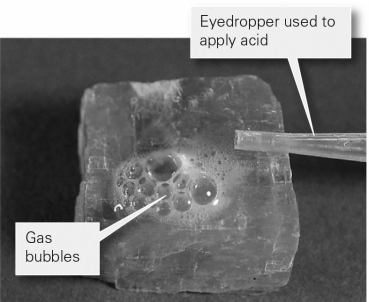
A) quartz
B) halite
C) calcite
D) fluorite

A) quartz
B) halite
C) calcite
D) fluorite

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
33
The tendency for minerals to break along distinct planar surfaces that have a specific orientation in relation to the crystal structure is called
A) fracture.
B) cleavage.
C) specific gravity.
D) hardness.
A) fracture.
B) cleavage.
C) specific gravity.
D) hardness.

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
34
On the Mohs hardness scale,quartz has a hardness of 7 and calcite has a hardness of 3.This means that _______.
A) calcite can scratch quartz
B) quartz is four times harder than calcite
C) calcite is four times harder than quartz
D) quartz can scratch calcite
A) calcite can scratch quartz
B) quartz is four times harder than calcite
C) calcite is four times harder than quartz
D) quartz can scratch calcite

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
35
Which of the following common minerals is hardest?
A) quartz
B) calcite
C) talc
D) fluorite
A) quartz
B) calcite
C) talc
D) fluorite

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
36
The image below shows a mineral specimen of mica.How many planes of cleavage does mica possess? 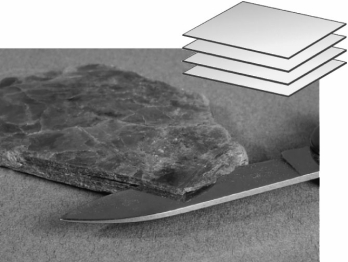
A) one
B) two
C) three
D) many

A) one
B) two
C) three
D) many

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
37
______ is a common mineral that can come in different colors,such as clear,milky,rose,and amethyst.
A) Pyrite
B) Halite
C) Quartz
D) Talc
A) Pyrite
B) Halite
C) Quartz
D) Talc

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
38
Hardness refers to a mineral's ability to resist
A) breaking.
B) being scratched.
C) chemically reacting with other substances.
D) weathering.
A) breaking.
B) being scratched.
C) chemically reacting with other substances.
D) weathering.

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
39
The color of a mineral in powdered form is termed
A) color.
B) specific gravity.
C) luster.
D) streak.
A) color.
B) specific gravity.
C) luster.
D) streak.

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
40
Glass and quartz crystals exhibit a smoothly curving,clamshell-shaped fracture pattern termed
A) glassy fracture.
B) conchoidal fracture.
C) one-directional cleavage.
D) obtuse fracture.
A) glassy fracture.
B) conchoidal fracture.
C) one-directional cleavage.
D) obtuse fracture.

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
41
Which common gemstone results from biomineralization?
A) diamond
B) garnet
C) pearl
D) sapphire
A) diamond
B) garnet
C) pearl
D) sapphire

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
42
What are the ways a mineral can crystallize in nature? List and explain each in some detail.

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
43
Diamond is a polymorph of graphite.What is a polymorph,and explain how graphite is different from a diamond.

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
44
Gemstones are often found in pegmatites,which are igneous rocks that are
A) exceptionally mafic.
B) extrusive,forming from lava.
C) exceptionally coarse-grained.
D) exceptionally fine-grained.
A) exceptionally mafic.
B) extrusive,forming from lava.
C) exceptionally coarse-grained.
D) exceptionally fine-grained.

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
45
The gems sapphire and ruby are two varieties of the mineral corundum (Al2O3).Considering the Mohs hardness of corundum,which mineral listed below would be able to scratch the gems sapphire and ruby?
A) quartz
B) feldspar
C) topaz
D) diamond
A) quartz
B) feldspar
C) topaz
D) diamond

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
46
How are diamonds formed?

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
47
What type of silicate is the mineral quartz?
A) chain silicate
B) framework silicate
C) sheet silicate
D) ring silicate.
A) chain silicate
B) framework silicate
C) sheet silicate
D) ring silicate.

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
48
In silicate minerals,the SiO4 tetrahedron can be linked together by sharing:
A) silicon atoms.
B) cations.
C) electrons.
D) oxygen atoms.
A) silicon atoms.
B) cations.
C) electrons.
D) oxygen atoms.

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
49
Draw and label the atoms in a silicon-oxygen tetrahedron.

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
50
The diamonds we typically see today in engagement rings do not display their natural crystal faces but rather ________,which are made by grinding the gem on a spinning lap.
A) striations
B) facets
C) cleavage planes
D) facades
A) striations
B) facets
C) cleavage planes
D) facades

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
51
What type of silicate is the mineral mica?
A) chain silicate
B) framework silicate
C) sheet silicate
D) ring silicate
A) chain silicate
B) framework silicate
C) sheet silicate
D) ring silicate

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
52
What are cleavage and fracture? How are these physical properties similar? How are they different?

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
53
Why are minerals often referred to as "the building blocks of Earth?"

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
54
Some physical properties are more useful for certain minerals than others.Explain how color alone may fail to identify some minerals.

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
55
Pyrite (FeS2)is in which mineral class?
A) silicates
B) carbonates
C) oxides
D) sulfides
A) silicates
B) carbonates
C) oxides
D) sulfides

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
56
Which classes do the common minerals quartz and calcite belong to? What are some of the properties that would distinguish quartz from calcite?

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
57
Diamonds are usually found in a rock called
A) graphite.
B) kimberlite.
C) gabbro.
D) diorite.
A) graphite.
B) kimberlite.
C) gabbro.
D) diorite.

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
58
How are minerals classified? List the major classes of minerals and their pertinent anions.

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
59
Based on the definition of a mineral,explain why a diamond created in a laboratory is not considered a mineral.

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck
60
Calcite (CaCO3)is in which mineral class?
A) silicates
B) carbonates
C) oxides
D) sulfides
A) silicates
B) carbonates
C) oxides
D) sulfides

Unlock Deck
Unlock for access to all 60 flashcards in this deck.
Unlock Deck
k this deck


