Deck 18: Nonrenewable Energy
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/45
Play
Full screen (f)
Deck 18: Nonrenewable Energy
1
Which of the following is a charged particle found in the nucleus of an atom?
A)DNA
B)Proton
C)Neutron
D)Electron
A)DNA
B)Proton
C)Neutron
D)Electron
B
2
Which of the following is densest: a kilogram of feathers,a kilogram of bricks,or a kilogram of water?
A)A kilogram of water
B)A kilogram of bricks
C)A kilogram of feathers
D)All three have the same density because they all have the same mass.
A)A kilogram of water
B)A kilogram of bricks
C)A kilogram of feathers
D)All three have the same density because they all have the same mass.
B
3
Which of the following are products of photosynthesis?
A)Light and heat
B)Oxygen and sugar
C)Carbon dioxide and water
D)Water and oxygen
A)Light and heat
B)Oxygen and sugar
C)Carbon dioxide and water
D)Water and oxygen
B
4
Atoms are composed of________ ,and compounds are composed of________ .
A)cells;protons,neutrons,and electrons
B)molecules;atoms
C)protons,neutrons,and electrons;atoms
D)protons,neutrons,and electrons;molecules
A)cells;protons,neutrons,and electrons
B)molecules;atoms
C)protons,neutrons,and electrons;atoms
D)protons,neutrons,and electrons;molecules

Unlock Deck
Unlock for access to all 45 flashcards in this deck.
Unlock Deck
k this deck
5
Which of the following best represents kinetic energy?
A)A charged battery
B)The energy in the wax molecules of a candle
C)Gunpowder in a bullet
D)A hot burner on a stove
A)A charged battery
B)The energy in the wax molecules of a candle
C)Gunpowder in a bullet
D)A hot burner on a stove

Unlock Deck
Unlock for access to all 45 flashcards in this deck.
Unlock Deck
k this deck
6
When a windmill turns to generate electricity,the amount of kinetic energy input in the process___________ .
A)is unrelated to the amount of electrical energy produced
B)is less than the amount of electrical energy produced
C)is greater than the amount of electrical energy produced
D)equals the amount of electrical energy produced
A)is unrelated to the amount of electrical energy produced
B)is less than the amount of electrical energy produced
C)is greater than the amount of electrical energy produced
D)equals the amount of electrical energy produced

Unlock Deck
Unlock for access to all 45 flashcards in this deck.
Unlock Deck
k this deck
7
An___________ecologist would study the interactions between the invasive European starling and native cavity-nesting birds in the United States.
A)ecosystem
B)community
C)population
D)organismal
A)ecosystem
B)community
C)population
D)organismal

Unlock Deck
Unlock for access to all 45 flashcards in this deck.
Unlock Deck
k this deck
8
Molecules are _______.
A)chemicals that cannot be broken or separated
B)two or more atoms held together by covalent bonds
C)atoms of an element
D)basic subunits of elements
A)chemicals that cannot be broken or separated
B)two or more atoms held together by covalent bonds
C)atoms of an element
D)basic subunits of elements

Unlock Deck
Unlock for access to all 45 flashcards in this deck.
Unlock Deck
k this deck
9
_________is defined as the number of protons plus the number of neutrons in an atom.
A)Ionic number
B)Atomic number
C)Nuclear number
D)Mass number
A)Ionic number
B)Atomic number
C)Nuclear number
D)Mass number

Unlock Deck
Unlock for access to all 45 flashcards in this deck.
Unlock Deck
k this deck
10
During the process of translation,codons found in_________ are translated to build .
A)DNA;proteins
B)DNA;RNA
C)RNA;proteins
D)RNA;DNA
A)DNA;proteins
B)DNA;RNA
C)RNA;proteins
D)RNA;DNA

Unlock Deck
Unlock for access to all 45 flashcards in this deck.
Unlock Deck
k this deck
11
Acid precipitation ___________.
A)has a pH lower than pure water
B)has a low concentration of hydrogen ions
C)that measures pH = 4 is twice as acidic as precipitation that measures pH = 5
D)has a pH higher than 7
A)has a pH lower than pure water
B)has a low concentration of hydrogen ions
C)that measures pH = 4 is twice as acidic as precipitation that measures pH = 5
D)has a pH higher than 7

Unlock Deck
Unlock for access to all 45 flashcards in this deck.
Unlock Deck
k this deck
12
The pH scale is a measure of the concentration of __________in a solution.
A)sulfuric acid
B)hydrogen ions
C)water
D)total ions
A)sulfuric acid
B)hydrogen ions
C)water
D)total ions

Unlock Deck
Unlock for access to all 45 flashcards in this deck.
Unlock Deck
k this deck
13
When you burn a log in your fireplace,you are converting ________.
A)thermal energy to chemical energy
B)chemical energy to nuclear energy
C)chemical energy to thermal energy
D)thermal energy to electrical energy
A)thermal energy to chemical energy
B)chemical energy to nuclear energy
C)chemical energy to thermal energy
D)thermal energy to electrical energy

Unlock Deck
Unlock for access to all 45 flashcards in this deck.
Unlock Deck
k this deck
14
In energy conversions such as burning coal to produce heat__________,.
A)entropy decreases because heat is used to ignite the coal
B)entropy decreases because of heat released in the conversion
C)entropy is unchanged because heat is used to ignite the coal
D)entropy increases because of heat released in the conversion
A)entropy decreases because heat is used to ignite the coal
B)entropy decreases because of heat released in the conversion
C)entropy is unchanged because heat is used to ignite the coal
D)entropy increases because of heat released in the conversion

Unlock Deck
Unlock for access to all 45 flashcards in this deck.
Unlock Deck
k this deck
15
Which of the following statements defies the first law of thermodynamics?
A)When coal is burned in a power plant,some of the energy escapes as heat rather than being converted to electricity.
B)When we build a campfire,the energy stored in the molecules in the wood is released as thermal energy.
C)Autotrophs such as plants produce energy that is consumed by heterotrophs.
D)We can decrease the amount of entropy in our dorm room if we use energy to get things organized.
A)When coal is burned in a power plant,some of the energy escapes as heat rather than being converted to electricity.
B)When we build a campfire,the energy stored in the molecules in the wood is released as thermal energy.
C)Autotrophs such as plants produce energy that is consumed by heterotrophs.
D)We can decrease the amount of entropy in our dorm room if we use energy to get things organized.

Unlock Deck
Unlock for access to all 45 flashcards in this deck.
Unlock Deck
k this deck
16
All organic molecules contain what elements?
A)Potassium and sulfur
B)Hydrogen and oxygen
C)Carbon
D)Nitrogen
A)Potassium and sulfur
B)Hydrogen and oxygen
C)Carbon
D)Nitrogen

Unlock Deck
Unlock for access to all 45 flashcards in this deck.
Unlock Deck
k this deck
17
____________are atoms with the same atomic number but with different mass number.
A)Anions
B)Isotopes
C)Cations
D)Neutrons
A)Anions
B)Isotopes
C)Cations
D)Neutrons

Unlock Deck
Unlock for access to all 45 flashcards in this deck.
Unlock Deck
k this deck
18
Aerobic cellular respiration ___________.
A)releases carbon dioxide and water
B)involves a net consumption of water
C)represents a decrease in entropy
D)results in a net consumption of energy
A)releases carbon dioxide and water
B)involves a net consumption of water
C)represents a decrease in entropy
D)results in a net consumption of energy

Unlock Deck
Unlock for access to all 45 flashcards in this deck.
Unlock Deck
k this deck
19
All of the following are found in a eukaryotic cell except_____________ .
A)endoplasmic reticulum
B)Golgi apparatus
C)nucleoid region
D)mitochondria
A)endoplasmic reticulum
B)Golgi apparatus
C)nucleoid region
D)mitochondria

Unlock Deck
Unlock for access to all 45 flashcards in this deck.
Unlock Deck
k this deck
20
An atom with a charge of +1 has an atomic number of 6 and a mass number of 14.How many neutrons does it have?
A)14
B)6
C)8
D)Cannot be determined from the information given
A)14
B)6
C)8
D)Cannot be determined from the information given

Unlock Deck
Unlock for access to all 45 flashcards in this deck.
Unlock Deck
k this deck
21

-The devices shown are used to_________ .
A)convert potential energy to kinetic energy
B)convert kinetic energy to electrical energy
C)convert electrical energy to kinetic energy
D)create energy

Unlock Deck
Unlock for access to all 45 flashcards in this deck.
Unlock Deck
k this deck
22
An atom with a charge of +1 has an atomic number of 6 and a mass number of 14.How many electrons does it have?
A)8
B)5
C)6
D)7
A)8
B)5
C)6
D)7

Unlock Deck
Unlock for access to all 45 flashcards in this deck.
Unlock Deck
k this deck
23
During photosynthesis,___________.
A)sugars and carbon dioxide are produced
B)there is a net consumption of oxygen and carbon dioxide
C)there is a net consumption of water and carbon dioxide
D)carbon dioxide and oxygen are produced
A)sugars and carbon dioxide are produced
B)there is a net consumption of oxygen and carbon dioxide
C)there is a net consumption of water and carbon dioxide
D)carbon dioxide and oxygen are produced

Unlock Deck
Unlock for access to all 45 flashcards in this deck.
Unlock Deck
k this deck
24
What is the basic unit of an element?
A)Neutron
B)Isotope
C)Atom
D)Molecule
A)Neutron
B)Isotope
C)Atom
D)Molecule

Unlock Deck
Unlock for access to all 45 flashcards in this deck.
Unlock Deck
k this deck
25
An_________is the smallest unit of matter that still maintains the chemical properties of an element.
A)molecule
B)compound
C)nucleus
D)atom
A)molecule
B)compound
C)nucleus
D)atom

Unlock Deck
Unlock for access to all 45 flashcards in this deck.
Unlock Deck
k this deck
26
Which of the following reactions represents aerobic cellular respiration?
A)Water + carbon dioxide ‹ glucose + oxygen + water + energy
B)Water + carbon dioxide + energy ‹ glucose + oxygen + water
C)Glucose + oxygen ‹ water + carbon dioxide + energy
D)Nitrogen + carbon dioxide + energy ‹ methane + oxygen
A)Water + carbon dioxide ‹ glucose + oxygen + water + energy
B)Water + carbon dioxide + energy ‹ glucose + oxygen + water
C)Glucose + oxygen ‹ water + carbon dioxide + energy
D)Nitrogen + carbon dioxide + energy ‹ methane + oxygen

Unlock Deck
Unlock for access to all 45 flashcards in this deck.
Unlock Deck
k this deck
27
Which of the following best represents potential energy?
A)Cars stopped at an intersection
B)A charged battery in a flashlight
C)Sunlight shining on people on a beach
D)A breeze blowing across the top of a lake
A)Cars stopped at an intersection
B)A charged battery in a flashlight
C)Sunlight shining on people on a beach
D)A breeze blowing across the top of a lake

Unlock Deck
Unlock for access to all 45 flashcards in this deck.
Unlock Deck
k this deck
28
In the process of photosynthesis__________,.
A)kinetic energy of heat is used to combine the potential energy of oxygen and water
B)energy in light is converted to potential energy of chemical bonds
C)potential energy in light is converted to kinetic energy of chemical bonds
D)potential energy in glucose is used to generate kinetic energy in heat
A)kinetic energy of heat is used to combine the potential energy of oxygen and water
B)energy in light is converted to potential energy of chemical bonds
C)potential energy in light is converted to kinetic energy of chemical bonds
D)potential energy in glucose is used to generate kinetic energy in heat

Unlock Deck
Unlock for access to all 45 flashcards in this deck.
Unlock Deck
k this deck
29
Which of the following most accurately describes a compound?
A)A compound is a substance that is made of more than one element.
B)A compound is a combination of molecules.
C)A compound is two or more atoms held together by covalent bonds.
D)A compound is a molecule that is electrically charged.
A)A compound is a substance that is made of more than one element.
B)A compound is a combination of molecules.
C)A compound is two or more atoms held together by covalent bonds.
D)A compound is a molecule that is electrically charged.

Unlock Deck
Unlock for access to all 45 flashcards in this deck.
Unlock Deck
k this deck
30
Without regular cleanups,the tendency of a home or dorm room to become increasingly messy generally reflects________.
A)an increase in entropy
B)the loss of heat in any energy conversion
C)the conversion of kinetic energy into potential energy
D)the law of energy conservation
A)an increase in entropy
B)the loss of heat in any energy conversion
C)the conversion of kinetic energy into potential energy
D)the law of energy conservation

Unlock Deck
Unlock for access to all 45 flashcards in this deck.
Unlock Deck
k this deck
31
The first law of thermodynamics states that _____________.
A)energy can be transformed but cannot be created or destroyed
B)energy is always degraded in a chemical reaction
C)all energy always has kinetic and potential characteristics
D)entropy always decreases in normal chemical reactions
A)energy can be transformed but cannot be created or destroyed
B)energy is always degraded in a chemical reaction
C)all energy always has kinetic and potential characteristics
D)entropy always decreases in normal chemical reactions

Unlock Deck
Unlock for access to all 45 flashcards in this deck.
Unlock Deck
k this deck
32
A neutral solution has a pH of_________ .
A)14
B)5
C)1
D)7
A)14
B)5
C)1
D)7

Unlock Deck
Unlock for access to all 45 flashcards in this deck.
Unlock Deck
k this deck
33
Which of the following best represents potential energy converted to kinetic energy?
A)A windmill pumping water up into a water storage tank
B)Pulling a boat up onto a shoreline
C)Plants capturing sunlight to produce sugars using photosynthesis
D)Turning on a flashlight
A)A windmill pumping water up into a water storage tank
B)Pulling a boat up onto a shoreline
C)Plants capturing sunlight to produce sugars using photosynthesis
D)Turning on a flashlight

Unlock Deck
Unlock for access to all 45 flashcards in this deck.
Unlock Deck
k this deck
34
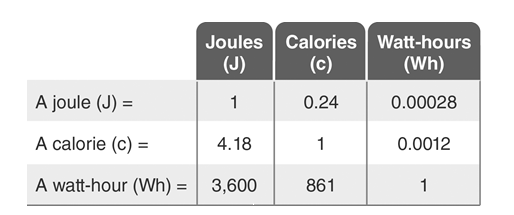
-Use the Energy Conversions table to answer the following question: An adult human has an average intake of approximately 2500 calories kilocalories)per day.Approximately how many 60-watt lightbulbs would require the same amount of energy per day as an adult human?
A)2
B)48
C)2000
D)17

Unlock Deck
Unlock for access to all 45 flashcards in this deck.
Unlock Deck
k this deck
35
Which one of the following is a molecule but not a compound?
A)CO2
B)H2O
C)O2
D)All of these would be considered both molecules and compounds.
A)CO2
B)H2O
C)O2
D)All of these would be considered both molecules and compounds.

Unlock Deck
Unlock for access to all 45 flashcards in this deck.
Unlock Deck
k this deck
36
An atom with a charge of +1 has an atomic number of 6 and a mass number of 14.How many protons does it have?
A)8
B)14
C)6
D)Cannot be determined from the information given
A)8
B)14
C)6
D)Cannot be determined from the information given

Unlock Deck
Unlock for access to all 45 flashcards in this deck.
Unlock Deck
k this deck
37
In a chemical reaction,____________.
A)atoms are rearranged
B)new atoms are formed
C)atoms are destroyed
D)new elements are formed
A)atoms are rearranged
B)new atoms are formed
C)atoms are destroyed
D)new elements are formed

Unlock Deck
Unlock for access to all 45 flashcards in this deck.
Unlock Deck
k this deck
38
Water held behind a dam is best described as a form of________ .
A)potential energy
B)kinetic energy
C)entropy
D)chemical energy
A)potential energy
B)kinetic energy
C)entropy
D)chemical energy

Unlock Deck
Unlock for access to all 45 flashcards in this deck.
Unlock Deck
k this deck
39
____________are proteins that facilitate chemical reactions such as digestion.
A)Nucleic acids
B)Enzymes
C)Anions
D)Isotopes
A)Nucleic acids
B)Enzymes
C)Anions
D)Isotopes

Unlock Deck
Unlock for access to all 45 flashcards in this deck.
Unlock Deck
k this deck
40
If the temperature of a gas increases,the ________of the gas decreases.
A)pressure
B)volume
C)number of moles
D)None of these is correct.
A)pressure
B)volume
C)number of moles
D)None of these is correct.

Unlock Deck
Unlock for access to all 45 flashcards in this deck.
Unlock Deck
k this deck
41
Hydrogen bonds involve an attraction between ____________.
A)hydrogen atoms and oxygen atoms within water molecules
B)hydrogen atoms and other hydrogen atoms
C)positive regions of one water molecule and negative regions of other water molecules
D)oxygen atoms and other oxygen atoms
A)hydrogen atoms and oxygen atoms within water molecules
B)hydrogen atoms and other hydrogen atoms
C)positive regions of one water molecule and negative regions of other water molecules
D)oxygen atoms and other oxygen atoms

Unlock Deck
Unlock for access to all 45 flashcards in this deck.
Unlock Deck
k this deck
42
Briefly explain the overall processes of photosynthesis and cellular respiration.Include a brief explanation of autotrophs and heterotrophs in your answer.

Unlock Deck
Unlock for access to all 45 flashcards in this deck.
Unlock Deck
k this deck
43
Perpetual motion machines,which by definition would not require an additional energy input,cannot exist because__________ .
A)in any energy conversion,some energy is lost to the surroundings as heat
B)energy cannot be created or destroyed
C)energy cannot be converted from one form to another
D)kinetic energy requires an input of light energy
A)in any energy conversion,some energy is lost to the surroundings as heat
B)energy cannot be created or destroyed
C)energy cannot be converted from one form to another
D)kinetic energy requires an input of light energy

Unlock Deck
Unlock for access to all 45 flashcards in this deck.
Unlock Deck
k this deck
44
Compare and discuss the first and second laws of thermodynamics.

Unlock Deck
Unlock for access to all 45 flashcards in this deck.
Unlock Deck
k this deck
45
Why is one region of a water molecule slightly negatively charged?
A)The hydrogen atoms attract protons more strongly than the oxygen atom does.
B)The hydrogen atoms attract electrons more strongly than the oxygen atom does.
C)The oxygen atom attracts electrons more strongly than the hydrogen atoms do.
D)The oxygen atom attracts protons more strongly than the hydrogen atoms do.
A)The hydrogen atoms attract protons more strongly than the oxygen atom does.
B)The hydrogen atoms attract electrons more strongly than the oxygen atom does.
C)The oxygen atom attracts electrons more strongly than the hydrogen atoms do.
D)The oxygen atom attracts protons more strongly than the hydrogen atoms do.

Unlock Deck
Unlock for access to all 45 flashcards in this deck.
Unlock Deck
k this deck


