Deck 13: Chemical Kinetics
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/132
Play
Full screen (f)
Deck 13: Chemical Kinetics
1
For the following reaction, P(C6H14)/ t was found to be -6.2 * 10-3 atm/s.C6H14(g) C6H6(g) + 4H2(g) Determine P(H2)/ t for this reaction at the same time.
A) 6.2 * 10-3 atm/s
B) 1.6 * 10-3 atm/s
C) 2.5 * 10-2 atm/s
D) -1.6 * 10-3 atm/s
E) -2.5 * 10-2 atm/s
A) 6.2 * 10-3 atm/s
B) 1.6 * 10-3 atm/s
C) 2.5 * 10-2 atm/s
D) -1.6 * 10-3 atm/s
E) -2.5 * 10-2 atm/s
2.5 * 10-2 atm/s
2
Use the following data to determine the rate law for the reaction shown below.2NO + H2 N2O + H2O
A) rate = k[NO]
B) rate = k[NO]2
C) rate = k[NO][H2]
D) rate = k[NO]2[H2]
E) rate = k[NO]2[H2]2
A) rate = k[NO]
B) rate = k[NO]2
C) rate = k[NO][H2]
D) rate = k[NO]2[H2]
E) rate = k[NO]2[H2]2
B
3
For the reaction A + 3B 2C, the rate of disappearance of B given by ( [B]/ t) may also be expressed as
A)( [B]/ t) = - (1/3) [A]/ t.
B)( [B]/ t) = - 3 [A]/ t.
C)( [B]/ t) = 3 [A]/ t.
D)( [B]/ t) = (1/3) [A]/ t.
E)( [B]/ t) = - [A]/ t.
A)( [B]/ t) = - (1/3) [A]/ t.
B)( [B]/ t) = - 3 [A]/ t.
C)( [B]/ t) = 3 [A]/ t.
D)( [B]/ t) = (1/3) [A]/ t.
E)( [B]/ t) = - [A]/ t.
( [B]/ t) = 3 [A]/ t.
4
Chlorine dioxide reacts in basic water to form chlorite and chlorate according to the following chemical equation: 2ClO2(aq) + 2OH-(aq) ClO2-(aq) + ClO3-(aq) + H2O(l)
Under a certain set of conditions, the initial rate of disappearance of chlorine dioxide was determined to be 2.30 x 10-1 M/s. What is the initial rate of appearance of chlorite ion under those same conditions
A) 5.75 x 10-2 M/s
B) 1.15 x 10-1 M/s
C) 2.30 x 10-1 M/s
D) 4.60 x 10-1 M/s
E) 9.20 x 10-1 M/s
Under a certain set of conditions, the initial rate of disappearance of chlorine dioxide was determined to be 2.30 x 10-1 M/s. What is the initial rate of appearance of chlorite ion under those same conditions
A) 5.75 x 10-2 M/s
B) 1.15 x 10-1 M/s
C) 2.30 x 10-1 M/s
D) 4.60 x 10-1 M/s
E) 9.20 x 10-1 M/s

Unlock Deck
Unlock for access to all 132 flashcards in this deck.
Unlock Deck
k this deck
5
Concerning the rate law, Rate = k[A]2[B], what are appropriate units for the rate constant k
A) s-1
B) M-1s-1
C) M-2s-1
D) M/s
E) M2/s
A) s-1
B) M-1s-1
C) M-2s-1
D) M/s
E) M2/s

Unlock Deck
Unlock for access to all 132 flashcards in this deck.
Unlock Deck
k this deck
6
The reaction A + 2B products has the rate law, rate = k[A][B]3. If the concentration of B is doubled while that of A is unchanged, by what factor will the rate of reaction increase
A) 2
B) 4
C) 6
D) 8
E) 9
A) 2
B) 4
C) 6
D) 8
E) 9

Unlock Deck
Unlock for access to all 132 flashcards in this deck.
Unlock Deck
k this deck
7
For the overall chemical reaction shown below, which one of the following statements can be rightly assumed
2H2S(g) + O2(g) 2S(s) + 2H2O(l)
A) The reaction is third-order overall.
B) The reaction is second-order overall.
C) The rate law is, rate = k[H2S]2 [O2].
D) The rate law is, rate = k[H2S] [O2].
E) The rate law cannot be determined from the information given.
2H2S(g) + O2(g) 2S(s) + 2H2O(l)
A) The reaction is third-order overall.
B) The reaction is second-order overall.
C) The rate law is, rate = k[H2S]2 [O2].
D) The rate law is, rate = k[H2S] [O2].
E) The rate law cannot be determined from the information given.

Unlock Deck
Unlock for access to all 132 flashcards in this deck.
Unlock Deck
k this deck
8
The reaction A + 2B products was found to have the rate law, rate = k[A] [B]2. Predict by what factor the rate of reaction will increase when the concentration of A is doubled and the concentration of B is also doubled.
A) 2
B) 4
C) 6
D) 8
E) 9
A) 2
B) 4
C) 6
D) 8
E) 9

Unlock Deck
Unlock for access to all 132 flashcards in this deck.
Unlock Deck
k this deck
9
Concerning the rate law, Rate = k[A]0, what are appropriate units for the rate constant k
A) s-1
B) M-1s-1
C) M-2s-1
D) M/s
E) M2/s
A) s-1
B) M-1s-1
C) M-2s-1
D) M/s
E) M2/s

Unlock Deck
Unlock for access to all 132 flashcards in this deck.
Unlock Deck
k this deck
10
For the reaction BrO3- + 5Br-+ 6H+ 3Br2 + 3H2O at a particular time, - [BrO3-]/ t = 1.5 * 10-2 M/s. What is - [Br-]/ t at the same instant
A) 13 M/s
B) 7.5 * 10-2 M/s
C) 1.5 * 10-2 M/s
D) 3.0 * 10-3 M/s
E) 330 M/s
A) 13 M/s
B) 7.5 * 10-2 M/s
C) 1.5 * 10-2 M/s
D) 3.0 * 10-3 M/s
E) 330 M/s

Unlock Deck
Unlock for access to all 132 flashcards in this deck.
Unlock Deck
k this deck
11
Chlorine dioxide reacts in basic water to form chlorite and chlorate according to the following chemical equation: 2ClO2(aq) + 2OH-(aq) ClO2-(aq) + ClO3-(aq) + H2O(l)
A kinetic study of this reaction under a certain set of conditions yielded the data below. Which one of the following is the rate law for this reaction
A) rate = k[ClO2][OH-]
B) rate = k[ClO2]2[OH-]
C) rate = k[ClO2][OH-]2
D) rate = k[ClO2]2[OH-]2
E) rate = k[ClO2]4[OH-]
A kinetic study of this reaction under a certain set of conditions yielded the data below. Which one of the following is the rate law for this reaction
A) rate = k[ClO2][OH-]
B) rate = k[ClO2]2[OH-]
C) rate = k[ClO2][OH-]2
D) rate = k[ClO2]2[OH-]2
E) rate = k[ClO2]4[OH-]

Unlock Deck
Unlock for access to all 132 flashcards in this deck.
Unlock Deck
k this deck
12
Appropriate units for a first-order rate constant are
A) M/s.
B) 1/M·s.
C) 1/s.
D) 1/M2·s.
A) M/s.
B) 1/M·s.
C) 1/s.
D) 1/M2·s.

Unlock Deck
Unlock for access to all 132 flashcards in this deck.
Unlock Deck
k this deck
13
It takes 42.0 min for the concentration of a reactant in a first-order reaction to drop from 0.45 M to 0.32 M at 25 C. How long will it take for the reaction to be 90% complete
A) 13.0 min
B) 86.0 min
C) 137 min
D) 222 min
E) 284 min
A) 13.0 min
B) 86.0 min
C) 137 min
D) 222 min
E) 284 min

Unlock Deck
Unlock for access to all 132 flashcards in this deck.
Unlock Deck
k this deck
14
For the reaction C6H14(g) C6H6(g) + 4H2(g), P(H2)/ t was found to be 2.5 * 10-2 atm/s, where P(H2) is the change in pressure of hydrogen. Determine P(C6H14)/ t for this reaction at the same time.
A) 2.5 * 10-2 atm/s
B) -6.2 * 10-3 atm/s
C) -2.5 * 10-2 atm/s
D) 0.10 atm/s
E) 6.2 * 10-3 atm/s
A) 2.5 * 10-2 atm/s
B) -6.2 * 10-3 atm/s
C) -2.5 * 10-2 atm/s
D) 0.10 atm/s
E) 6.2 * 10-3 atm/s

Unlock Deck
Unlock for access to all 132 flashcards in this deck.
Unlock Deck
k this deck
15
For the hypothetical reaction A + 3B 2C, the rate of appearance of C given by ( [C]/ t) may also be expressed as
A)( [C]/ t) = [A]/ t
B)( [C]/ t) = -(3/2) [B]/ t
C)( [C]/ t) = -(2/3) [B]/ t
D)( [C]/ t) = -(1/2) [A]/ t
A)( [C]/ t) = [A]/ t
B)( [C]/ t) = -(3/2) [B]/ t
C)( [C]/ t) = -(2/3) [B]/ t
D)( [C]/ t) = -(1/2) [A]/ t

Unlock Deck
Unlock for access to all 132 flashcards in this deck.
Unlock Deck
k this deck
16
Concerning the rate law, Rate = k[A][B], what are appropriate units for the rate constant k
A) s-1
B) M-1s-1
C) M-2s-1
D) M/s
E) M2/s
A) s-1
B) M-1s-1
C) M-2s-1
D) M/s
E) M2/s

Unlock Deck
Unlock for access to all 132 flashcards in this deck.
Unlock Deck
k this deck
17
The reaction A + 2B products has been found to have the rate law, rate = k[A] [B]2. While holding the concentration of A constant, the concentration of B is increased from x to 3x. Predict by what factor the rate of reaction increases.
A) 3
B) 6
C) 9
D) 27
E) 30
A) 3
B) 6
C) 9
D) 27
E) 30

Unlock Deck
Unlock for access to all 132 flashcards in this deck.
Unlock Deck
k this deck
18
Concerning the rate law, Rate = k[A][B][C], what are appropriate units for the rate constant k
A) s-1
B) M-1s-1
C) M-2s-1
D) M/s
E) M2/s
A) s-1
B) M-1s-1
C) M-2s-1
D) M/s
E) M2/s

Unlock Deck
Unlock for access to all 132 flashcards in this deck.
Unlock Deck
k this deck
19
Nitric oxide gas (NO) reacts with chlorine gas according to the chemical equation given below.NO + 1/2Cl2 NOCl
The following initial rates of reaction have been measured for the given reagent concentrations. Which of the following is the rate law (rate equation) for this reaction
A) rate = k[NO]
B) rate = k[NO][Cl2]1/2
C) rate = k[NO][Cl2]
D) rate = k[NO]2[Cl2]
E) rate = k[NO]2[Cl2]2
The following initial rates of reaction have been measured for the given reagent concentrations. Which of the following is the rate law (rate equation) for this reaction
A) rate = k[NO]
B) rate = k[NO][Cl2]1/2
C) rate = k[NO][Cl2]
D) rate = k[NO]2[Cl2]
E) rate = k[NO]2[Cl2]2

Unlock Deck
Unlock for access to all 132 flashcards in this deck.
Unlock Deck
k this deck
20
The reaction A + 2B products was found to follow the rate law: rate = k[A]2[B]. Predict by what factor the rate of reaction will increase when the concentration of A is doubled, the concentration of B is tripled, and the temperature remains constant.
A) 5
B) 6
C) 12
D) 18
E) None of these.
A) 5
B) 6
C) 12
D) 18
E) None of these.

Unlock Deck
Unlock for access to all 132 flashcards in this deck.
Unlock Deck
k this deck
21
The half life for a first order reaction is 45 min. What is the rate constant in units of s-1
A) 0.015 s-1
B) 65 s-1
C) 2.6 x 10-4 s-1
D) 3.9 x 103 s-1
E) 1.9 x 103 s-1
A) 0.015 s-1
B) 65 s-1
C) 2.6 x 10-4 s-1
D) 3.9 x 103 s-1
E) 1.9 x 103 s-1

Unlock Deck
Unlock for access to all 132 flashcards in this deck.
Unlock Deck
k this deck
22
A reaction was experimentally determined to follow the rate law, Rate = k[A]2 where k = 0.456 s-1M-1. Starting with [A]o = 0.500 M, how many seconds will it take for [A]t = 0.250 M
A) 2.85 x 10-2 s
B) 1.14 x 10-1 s
C) 1.52 s
D) 4.39 s
E) 5.48x10-1 s
A) 2.85 x 10-2 s
B) 1.14 x 10-1 s
C) 1.52 s
D) 4.39 s
E) 5.48x10-1 s

Unlock Deck
Unlock for access to all 132 flashcards in this deck.
Unlock Deck
k this deck
23
The following initial rate data apply to the reaction below.F2(g) + 2Cl2O(g) 2FClO2(g) + Cl2(g) Which of the following is the rate law (rate equation) for this reaction
A) rate = k[F2]2[Cl2O]4
B) rate = k[F2]2[Cl2O]
C) rate = k[F2][Cl2O]
D) rate = k[F2][Cl2O]2
E) rate = k[F2]2[Cl2O]2
A) rate = k[F2]2[Cl2O]4
B) rate = k[F2]2[Cl2O]
C) rate = k[F2][Cl2O]
D) rate = k[F2][Cl2O]2
E) rate = k[F2]2[Cl2O]2

Unlock Deck
Unlock for access to all 132 flashcards in this deck.
Unlock Deck
k this deck
24
A reaction was experimentally determined to follow the rate law, Rate = k[A] where k = 0.15 s-1. Starting with [A]o = 0.225M, how many seconds will it take for [A]t = 0.0350M
A) 3.4 x 10-2 s
B) 5.3 x 10-3 s
C) 12 s
D) 160 s
E) 1.3 s
A) 3.4 x 10-2 s
B) 5.3 x 10-3 s
C) 12 s
D) 160 s
E) 1.3 s

Unlock Deck
Unlock for access to all 132 flashcards in this deck.
Unlock Deck
k this deck
25
The isomerization of cyclopropane to form propene is a first-order reaction. 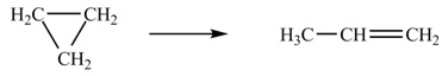 At 760 K, 15% of a sample of cyclopropane changes to propene in 6.8 min. What is the half-life of cyclopropane at 760 K
At 760 K, 15% of a sample of cyclopropane changes to propene in 6.8 min. What is the half-life of cyclopropane at 760 K
A) 3.4 * 10-2 min
B) 2.5 min
C) 23 min
D) 29 min
E) 230 min
 At 760 K, 15% of a sample of cyclopropane changes to propene in 6.8 min. What is the half-life of cyclopropane at 760 K
At 760 K, 15% of a sample of cyclopropane changes to propene in 6.8 min. What is the half-life of cyclopropane at 760 KA) 3.4 * 10-2 min
B) 2.5 min
C) 23 min
D) 29 min
E) 230 min

Unlock Deck
Unlock for access to all 132 flashcards in this deck.
Unlock Deck
k this deck
26
At 700 K, the rate constant for the following reaction is 6.2 * 10-4 min-1. 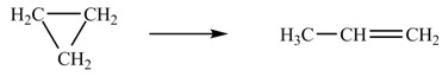 How many minutes are required for 20% of a sample of cyclopropane to isomerize to propene
How many minutes are required for 20% of a sample of cyclopropane to isomerize to propene
A) 1,120 min
B) 360 min
C) 3710 min
D) 1.4 * 10-4 min
E) 280 min
 How many minutes are required for 20% of a sample of cyclopropane to isomerize to propene
How many minutes are required for 20% of a sample of cyclopropane to isomerize to propeneA) 1,120 min
B) 360 min
C) 3710 min
D) 1.4 * 10-4 min
E) 280 min

Unlock Deck
Unlock for access to all 132 flashcards in this deck.
Unlock Deck
k this deck
27
A certain reaction, reaction A products, is first order with respect toA. Starting with [A] = 0.250M, it takes 45 min to reduce the concentration of A to 0.110M. What is its rate constant for this reaction
A) 7.9 x 10-3 min-1
B) 1.1 x 10-1 min-1
C) 3.0 x 10-4 min-1
D) 1.8 x 10-2 min-1
A) 7.9 x 10-3 min-1
B) 1.1 x 10-1 min-1
C) 3.0 x 10-4 min-1
D) 1.8 x 10-2 min-1

Unlock Deck
Unlock for access to all 132 flashcards in this deck.
Unlock Deck
k this deck
28
A city's water supply is contaminated with a toxin at a concentration of 0.63 mg/L. For the water to be safe for drinking, the concentration of this toxin must be below 1.5 x 10-3 mg/L. Fortunately, this toxin decomposes to a safe mixture of products by first-order kinetics with a rate constant of 0.27 day-1. How long will it take for the water to be safe to drink
A) 2.2 days
B) 2.6 days
C) 20 days
D) 22 days
E) 27 days
A) 2.2 days
B) 2.6 days
C) 20 days
D) 22 days
E) 27 days

Unlock Deck
Unlock for access to all 132 flashcards in this deck.
Unlock Deck
k this deck
29
The half life for a first order reaction is 27 min. How long will it take for 4 half lives to occur
A) 53,000 min
B) 108 min
C) 81 min
D) 260 min
E) Not enough information given
A) 53,000 min
B) 108 min
C) 81 min
D) 260 min
E) Not enough information given

Unlock Deck
Unlock for access to all 132 flashcards in this deck.
Unlock Deck
k this deck
30
Ammonium ion (NH4+) reacts with nitrite ion (NO2-) to yield nitrogen gas and liquid water. The following initial rates of reaction have been measured for the given reactant concentrations. Which of the following is the rate law (rate equation) for this reaction
A) rate = k [NH4+] [NO2-]4
B) rate = k [NH4+] [NO2-]
C) rate = k [NH4+] [NO2-]2
D) rate = k [NH4+]2 [NO2-]
E) rate = k [NH4+]1/2 [NO2-]1/4
A) rate = k [NH4+] [NO2-]4
B) rate = k [NH4+] [NO2-]
C) rate = k [NH4+] [NO2-]2
D) rate = k [NH4+]2 [NO2-]
E) rate = k [NH4+]1/2 [NO2-]1/4

Unlock Deck
Unlock for access to all 132 flashcards in this deck.
Unlock Deck
k this deck
31
The isomerization of cyclopropane to propene follows first-order kinetics. 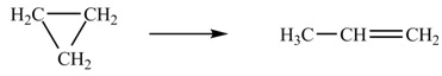 At 700 K, the rate constant for this reaction is 6.2 * 10-4 min-1. How many minutes are required for 10.0% of a sample of cyclopropane to isomerize to propene
At 700 K, the rate constant for this reaction is 6.2 * 10-4 min-1. How many minutes are required for 10.0% of a sample of cyclopropane to isomerize to propene
A) 16,100 min
B) 170 min
C) 3,710 min
D) 1.43 * 10-3 min
E) 1,120 min
 At 700 K, the rate constant for this reaction is 6.2 * 10-4 min-1. How many minutes are required for 10.0% of a sample of cyclopropane to isomerize to propene
At 700 K, the rate constant for this reaction is 6.2 * 10-4 min-1. How many minutes are required for 10.0% of a sample of cyclopropane to isomerize to propeneA) 16,100 min
B) 170 min
C) 3,710 min
D) 1.43 * 10-3 min
E) 1,120 min

Unlock Deck
Unlock for access to all 132 flashcards in this deck.
Unlock Deck
k this deck
32
Appropriate units for a second-order rate constant are
A) M/s
B) 1/M·s
C) 1/s
D) 1/M2·s
A) M/s
B) 1/M·s
C) 1/s
D) 1/M2·s

Unlock Deck
Unlock for access to all 132 flashcards in this deck.
Unlock Deck
k this deck
33
The data below were determined for the reaction shown below.S2O82- + 3I - (aq) 2SO42- + I3-
The rate law for this reaction must be:
A) rate = k[S2O82- ][I -]3
B) rate = k[S2O82-]
C) rate = k[S2O82-]2[I -]2
D) rate = k[I -]
E) rate = k[S2O82-][I -]
The rate law for this reaction must be:
A) rate = k[S2O82- ][I -]3
B) rate = k[S2O82-]
C) rate = k[S2O82-]2[I -]2
D) rate = k[I -]
E) rate = k[S2O82-][I -]

Unlock Deck
Unlock for access to all 132 flashcards in this deck.
Unlock Deck
k this deck
34
Benzoyl chloride, C6H5COCl, reacts with water to form benzoic acid, C6H5COOH, and hydrochloric acid. This first-order reaction is 25% complete after 26 s. How much longer would one have to wait in order to obtain 99% conversion of benzoyl chloride to benzoic acid
A) 393 s
B) 419 s
C) 183 s
D) 293 s
E) 209 s
A) 393 s
B) 419 s
C) 183 s
D) 293 s
E) 209 s

Unlock Deck
Unlock for access to all 132 flashcards in this deck.
Unlock Deck
k this deck
35
Nitric oxide reacts with chlorine to form nitrosyl chloride, NOCl. Use the following data to determine the rate equation for the reaction.NO + 1/2Cl2 NOCl
A) rate = k[NO]
B) rate = k[NO][Cl2]1/2
C) rate = k[NO][Cl2]
D) rate = k[NO]2[Cl2]
E) rate = k[NO]2[Cl2]2
A) rate = k[NO]
B) rate = k[NO][Cl2]1/2
C) rate = k[NO][Cl2]
D) rate = k[NO]2[Cl2]
E) rate = k[NO]2[Cl2]2

Unlock Deck
Unlock for access to all 132 flashcards in this deck.
Unlock Deck
k this deck
36
A city's water supply is contaminated with a toxin at a concentration of 0.63 mg/L. Fortunately, this toxin decomposes to a safe mixture of products by first-order kinetics with a rate constant of 0.27 day-1. How long will it take for half of the toxin to decompose
A) 0.17 days
B) 0.27 days
C) 0.38 days
D) 2.3 days
E) 2.6 days
A) 0.17 days
B) 0.27 days
C) 0.38 days
D) 2.3 days
E) 2.6 days

Unlock Deck
Unlock for access to all 132 flashcards in this deck.
Unlock Deck
k this deck
37
The isomerization of cyclopropane to form propene is a first-order reaction. 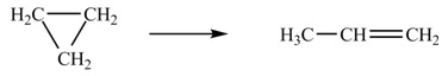 At 760 K, 85% of a sample of cyclopropane changes to propene in 79.0 min. Determine the rate constant for this reaction at 760 K.
At 760 K, 85% of a sample of cyclopropane changes to propene in 79.0 min. Determine the rate constant for this reaction at 760 K.
A) 3.66 * 10-2 min-1
B) 1.04 * 10-2 min-1
C) 2.42 min-1
D) 2.06 * 10-3 min-1
E) 2.40 * 10-2 min-1
 At 760 K, 85% of a sample of cyclopropane changes to propene in 79.0 min. Determine the rate constant for this reaction at 760 K.
At 760 K, 85% of a sample of cyclopropane changes to propene in 79.0 min. Determine the rate constant for this reaction at 760 K.A) 3.66 * 10-2 min-1
B) 1.04 * 10-2 min-1
C) 2.42 min-1
D) 2.06 * 10-3 min-1
E) 2.40 * 10-2 min-1

Unlock Deck
Unlock for access to all 132 flashcards in this deck.
Unlock Deck
k this deck
38
aa

Unlock Deck
Unlock for access to all 132 flashcards in this deck.
Unlock Deck
k this deck
38
The first-order decomposition, A products, has a rate constant of 0.150 s-1. Starting with [A]o = 0.350 M, how much time is required for [A]t = 0.125 M
A) 6.86 s
B) 2.98 s
C) 34 s
D) 1.50 s
E) 4.62 s
A) 6.86 s
B) 2.98 s
C) 34 s
D) 1.50 s
E) 4.62 s

Unlock Deck
Unlock for access to all 132 flashcards in this deck.
Unlock Deck
k this deck
39
At 25 C the rate constant for the first-order decomposition of a pesticide solution is 6.40 *10-3 min-1. If the starting concentration of pesticide is 0.0314 M, what concentration will remain after 62.0 min at 25 C
A) 1.14 * 10-1 M
B) 47.4 M
C) -8.72.0 M
D) 2.11 * 10-2 M
E) 2.68* 10-2 M
A) 1.14 * 10-1 M
B) 47.4 M
C) -8.72.0 M
D) 2.11 * 10-2 M
E) 2.68* 10-2 M

Unlock Deck
Unlock for access to all 132 flashcards in this deck.
Unlock Deck
k this deck
40
A certain first-order reaction A B is 25% complete in 42 min at 25 C. What is the half-life of the reaction
A) 21 min
B) 42 min
C) 84 min
D) 20 min
E) 101 min
A) 21 min
B) 42 min
C) 84 min
D) 20 min
E) 101 min

Unlock Deck
Unlock for access to all 132 flashcards in this deck.
Unlock Deck
k this deck
41
For what order reaction does the half-life get longer as the initial concentration increases
A) zero order
B) first order
C) second order
D) none of them because half-life is always independent of the initial concentration
A) zero order
B) first order
C) second order
D) none of them because half-life is always independent of the initial concentration

Unlock Deck
Unlock for access to all 132 flashcards in this deck.
Unlock Deck
k this deck
42
A reaction is experimentally found to follow the rate law, Rate = k[A]2 where k = 0.130 M-1min-1. Starting with [A]o = 2.50 M, how many seconds will it take for [A]t = 1.25 M
A) 3.08 s
B) 185 s
C) 5.33 s
A) 3.08 s
B) 185 s
C) 5.33 s

Unlock Deck
Unlock for access to all 132 flashcards in this deck.
Unlock Deck
k this deck
43
The reaction 2NO2(g) 2NO(g) + O2(g) is suspected to be second order in NO2. Which of the following kinetic plots would be the most useful to confirm whether or not the reaction is second order
A) A plot of [NO2]-1 vs. t
B) A plot of ln [NO2]-1 vs. t
C) A plot of ln [NO2] vs. t
D) A plot of [NO2]2 vs. t
E) A plot of [NO2] vs. t
A) A plot of [NO2]-1 vs. t
B) A plot of ln [NO2]-1 vs. t
C) A plot of ln [NO2] vs. t
D) A plot of [NO2]2 vs. t
E) A plot of [NO2] vs. t

Unlock Deck
Unlock for access to all 132 flashcards in this deck.
Unlock Deck
k this deck
44
In general, to calculate the rate constant for a second order reaction with respect to A, given an initial and final concentration as well as the time required for this change, which equation should be used
A) ln([A]t/[A]o) = - kt
B) t1/2 = ln2/k
C) [A]t = -kt + [A]o
D) 1/[A]t = kt + 1/[A]o
E) Rate = k[A]
A) ln([A]t/[A]o) = - kt
B) t1/2 = ln2/k
C) [A]t = -kt + [A]o
D) 1/[A]t = kt + 1/[A]o
E) Rate = k[A]

Unlock Deck
Unlock for access to all 132 flashcards in this deck.
Unlock Deck
k this deck
45
The thermal decomposition of acetaldehyde, CH3CHO CH4 + CO, is a second-order reaction. The following data were obtained at 518 C. Based on the data given, what is the half-life for the disappearance of acetaldehyde
A) 1.5 * 105 s
B) 410 s
C) 5.4 * 107 s
D) 520 s
E) 305 s
A) 1.5 * 105 s
B) 410 s
C) 5.4 * 107 s
D) 520 s
E) 305 s

Unlock Deck
Unlock for access to all 132 flashcards in this deck.
Unlock Deck
k this deck
46
Concerning the decomposition of A, A products, which of the following methods could be used to determine the order of the reaction with respect to A
I. Plot [A] vs time, ln[A] vs time, and 1/[A] vs time and identify which plot yields a straight line.
II. Vary the concentration of A and note by what factor the rate changes.
III. Identify if successive half lives of A double, halve, or stay constant.
A) I only
B) II only
C) III only
D) I and III
E) I, II, and III
I. Plot [A] vs time, ln[A] vs time, and 1/[A] vs time and identify which plot yields a straight line.
II. Vary the concentration of A and note by what factor the rate changes.
III. Identify if successive half lives of A double, halve, or stay constant.
A) I only
B) II only
C) III only
D) I and III
E) I, II, and III

Unlock Deck
Unlock for access to all 132 flashcards in this deck.
Unlock Deck
k this deck
47
For the chemical reaction A B + C, a plot of [A]t versus time is found to give a straight line with a negative slope. What is the order of reaction with respect to A
A) zero
B) first
C) second
D) third
E) Such a plot cannot reveal the order of the reaction.
A) zero
B) first
C) second
D) third
E) Such a plot cannot reveal the order of the reaction.

Unlock Deck
Unlock for access to all 132 flashcards in this deck.
Unlock Deck
k this deck
48
For a second order reaction, the half-life is equal to
A) t1/2 = 0.693/k.
B) t1/2 = k/0.693.
C) t1/2 = 1/k[A]o.
D) t1/2 = k.
E) t1/2 = [A]o/2k.
A) t1/2 = 0.693/k.
B) t1/2 = k/0.693.
C) t1/2 = 1/k[A]o.
D) t1/2 = k.
E) t1/2 = [A]o/2k.

Unlock Deck
Unlock for access to all 132 flashcards in this deck.
Unlock Deck
k this deck
49
Which one of the following changes would alter the rate constant (k) for the reaction 2A + B products
A) increasing the concentration of A
B) increasing the concentration of B
C) increasing the temperature
D) measuring k again after the reaction has run for a while
A) increasing the concentration of A
B) increasing the concentration of B
C) increasing the temperature
D) measuring k again after the reaction has run for a while

Unlock Deck
Unlock for access to all 132 flashcards in this deck.
Unlock Deck
k this deck
50
A certain reaction A products is second order with respect toA. If it takes 45 min to reduce the concentration of A from 0.350 M to 0.125 M, what is the rate constant for this reaction
A) 3.8 x 10-4 M-1min-1
B) 1.9 x 10-3 M-1min-1
C) 5.0 x 10-3 M-1min-1
D) 2.3 x 10-2 M-1min-1
E) 1.1 x 10-1 M-1min-1
A) 3.8 x 10-4 M-1min-1
B) 1.9 x 10-3 M-1min-1
C) 5.0 x 10-3 M-1min-1
D) 2.3 x 10-2 M-1min-1
E) 1.1 x 10-1 M-1min-1

Unlock Deck
Unlock for access to all 132 flashcards in this deck.
Unlock Deck
k this deck
51
The graphs below all refer to the same reaction. What is the order of this reaction 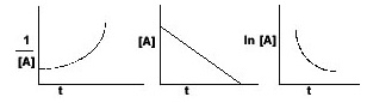
A) zero order
B) first order
C) second order
D) unable to predict

A) zero order
B) first order
C) second order
D) unable to predict

Unlock Deck
Unlock for access to all 132 flashcards in this deck.
Unlock Deck
k this deck
52
For the reaction X + Y Z, the reaction rate is found to depend only upon the concentration of X. A plot of 1/X verses time gives a straight line. ![<strong>For the reaction X + Y \rarr Z, the reaction rate is found to depend only upon the concentration of X. A plot of 1/X verses time gives a straight line. What is the rate law for this reaction</strong> A) rate = k [X] B) rate = k [X]<sup>2</sup> C) rate = k [X][Y] D) rate = k [X]<sup>2</sup>[Y]](https://storage.examlex.com/TB3247/11ea7498_3a28_459f_a57a_95cf16b600f4_TB3247_00.jpg) What is the rate law for this reaction
What is the rate law for this reaction
A) rate = k [X]
B) rate = k [X]2
C) rate = k [X][Y]
D) rate = k [X]2[Y]
![<strong>For the reaction X + Y \rarr Z, the reaction rate is found to depend only upon the concentration of X. A plot of 1/X verses time gives a straight line. What is the rate law for this reaction</strong> A) rate = k [X] B) rate = k [X]<sup>2</sup> C) rate = k [X][Y] D) rate = k [X]<sup>2</sup>[Y]](https://storage.examlex.com/TB3247/11ea7498_3a28_459f_a57a_95cf16b600f4_TB3247_00.jpg) What is the rate law for this reaction
What is the rate law for this reactionA) rate = k [X]
B) rate = k [X]2
C) rate = k [X][Y]
D) rate = k [X]2[Y]

Unlock Deck
Unlock for access to all 132 flashcards in this deck.
Unlock Deck
k this deck
53
The thermal decomposition of acetaldehyde, CH3CHO CH4 + CO, is a second-order reaction. The following data were obtained at 518 C. Calculate the rate constant for the decomposition of acetaldehyde from the above data.
A) 2.2 * 10-3/s
B) 0.70 mmHg/s
C) 2.2 * 10-3/mmHg·s
D) 6.7 *10-6/mmHg·s
E) 5.2 * 10-5/mmHg·s
A) 2.2 * 10-3/s
B) 0.70 mmHg/s
C) 2.2 * 10-3/mmHg·s
D) 6.7 *10-6/mmHg·s
E) 5.2 * 10-5/mmHg·s

Unlock Deck
Unlock for access to all 132 flashcards in this deck.
Unlock Deck
k this deck
54
At 25 C, the second-order reaction NOCl(g) NO(g) + 1/2Cl2(g)is 50% complete after 5.82 hours when the initial concentration of NOCl is 4.46 mol/L. How long will it take for the reaction to be 75% complete
A) 8.22 hr
B) 11.6 hr
C) 15.5 hr
D) 17.5 hr
E) 23.0 hr
A) 8.22 hr
B) 11.6 hr
C) 15.5 hr
D) 17.5 hr
E) 23.0 hr

Unlock Deck
Unlock for access to all 132 flashcards in this deck.
Unlock Deck
k this deck
55
A reaction is experimentally found to follow the rate law, Rate = k[A]2 where k = 0.355 M-1min-1. Starting with [A]o = 1.55 M, how many seconds will it take for [A]t = 0.150M
A) 6.58 s
B) 395 s
C) 6.02 s
D) 17.0 s
E) 1.02 x 103 s
A) 6.58 s
B) 395 s
C) 6.02 s
D) 17.0 s
E) 1.02 x 103 s

Unlock Deck
Unlock for access to all 132 flashcards in this deck.
Unlock Deck
k this deck
56
For the chemical reaction A C, a plot of 1/[A]t versus time was found to give a straight line with a positive slope. What is the order of reaction
A) zeroth
B) first
C) second
D) Such a plot cannot reveal the order of the reaction.
A) zeroth
B) first
C) second
D) Such a plot cannot reveal the order of the reaction.

Unlock Deck
Unlock for access to all 132 flashcards in this deck.
Unlock Deck
k this deck
57
In general, to calculate the rate constant for a first order decomposition reaction, A products, given the time needed to decrease the initial concentration by one half, which equation should be used
A) t1/2 = 1/(k[A]o)
B) t1/2 = ln2/k
C) [A]t = -kt + [A]o
D) 1/[A]t = kt + 1/[A]o
E) Rate = k[A]
A) t1/2 = 1/(k[A]o)
B) t1/2 = ln2/k
C) [A]t = -kt + [A]o
D) 1/[A]t = kt + 1/[A]o
E) Rate = k[A]

Unlock Deck
Unlock for access to all 132 flashcards in this deck.
Unlock Deck
k this deck
58
In general, to calculate the time required for a given initial concentration to decrease by 35% given the rate constant with units of 1/M-1s-1, which equation should be used
A) ln([A]t/[A]o) = - kt
B) t1/2 = ln2/k
C) [A]t = -kt + [A]o
D) 1/[A]t = kt + 1/[A]o
E) Rate = k[A]
A) ln([A]t/[A]o) = - kt
B) t1/2 = ln2/k
C) [A]t = -kt + [A]o
D) 1/[A]t = kt + 1/[A]o
E) Rate = k[A]

Unlock Deck
Unlock for access to all 132 flashcards in this deck.
Unlock Deck
k this deck
59
A certain reaction A products is second order with respect to A with a rate constant, k, 0.122 M-1min-1. Starting with [A]o = 1.01M, how many seconds will it take for A to reach a concentration of 0.750M
A) 2.81 s
B) 14.9 s
C) 893 s
D) 169 s
E) 128 s
A) 2.81 s
B) 14.9 s
C) 893 s
D) 169 s
E) 128 s

Unlock Deck
Unlock for access to all 132 flashcards in this deck.
Unlock Deck
k this deck
60
In general, to calculate the activation energy for an elementary step given the rate constants at two different temperatures, which equation should be used
A) ln([A]t/[A]o) = - kt
B) t1/2 = ln2/k
C) ln(k1/k2) = Ea/R((T1 - T2)/T1T2)
D) 1/[A]t = kt + 1/[A]o
E) Rate = k[A]
A) ln([A]t/[A]o) = - kt
B) t1/2 = ln2/k
C) ln(k1/k2) = Ea/R((T1 - T2)/T1T2)
D) 1/[A]t = kt + 1/[A]o
E) Rate = k[A]

Unlock Deck
Unlock for access to all 132 flashcards in this deck.
Unlock Deck
k this deck
61
Calculate the activation energy, in kJ/mol, for the redox reaction Sn2+ + 2Co3+ Sn4+ + 2Co2+.
A) 59.2
B) 0.477
C) 5.37
D) 163 kJ
E) 48.1 kJ
A) 59.2
B) 0.477
C) 5.37
D) 163 kJ
E) 48.1 kJ

Unlock Deck
Unlock for access to all 132 flashcards in this deck.
Unlock Deck
k this deck
62
According to the collision theory, all collisions do not lead to reaction. Which choice gives both reasons why not all collisions between reactant molecules lead to reaction
1. The total energy of two colliding molecules is less than some minimum amount of energy.
2. Molecules cannot react with each other unless a catalyst is present.
3. Molecules that are improperly oriented during collision will not react.
4. Solids cannot react with gases.
A) 1 and 2
B) 1 and 3
C) 1 and 4
D) 2 and 3
E) 3 and 4
1. The total energy of two colliding molecules is less than some minimum amount of energy.
2. Molecules cannot react with each other unless a catalyst is present.
3. Molecules that are improperly oriented during collision will not react.
4. Solids cannot react with gases.
A) 1 and 2
B) 1 and 3
C) 1 and 4
D) 2 and 3
E) 3 and 4

Unlock Deck
Unlock for access to all 132 flashcards in this deck.
Unlock Deck
k this deck
63
The activation energy for the following first-order reaction is 102 kJ/mol.N2O5(g) 2NO2(g) + 1/2O2(g) The value of the rate constant (k) is 1.35 * 10-4 s-1 at 35 C. What is the value of k at 0 C
A) 8.2 * 10-7 s-1
B) 1.9 * 10-5 s-1
C) 4.2 * 10-5 s-1
D) 2.2 * 10-2 s-1
E) none of these
A) 8.2 * 10-7 s-1
B) 1.9 * 10-5 s-1
C) 4.2 * 10-5 s-1
D) 2.2 * 10-2 s-1
E) none of these

Unlock Deck
Unlock for access to all 132 flashcards in this deck.
Unlock Deck
k this deck
64
The reaction C4H10 C2H6 + C2H4 has an activation energy (Ea) of 350 kJ/mol, and the Ea of the reverse reaction is 260 kJ/mol. Estimate H, in kJ/mol, for the reaction as written above.
A) -90 kJ/mol
B) +90 kJ/mol
C) 350 kJ/mol
D) -610 kJ/mol
E) +610 kJ/mol
A) -90 kJ/mol
B) +90 kJ/mol
C) 350 kJ/mol
D) -610 kJ/mol
E) +610 kJ/mol

Unlock Deck
Unlock for access to all 132 flashcards in this deck.
Unlock Deck
k this deck
65
An increase in the temperature of the reactants causes an increase in the rate of reaction. The best explanation for this behavior is that as the temperature increases,
A) the concentration of reactants increases.
B) the activation energy decreases.
C) the collision frequency increases.
D) the fraction of collisions with total kinetic energy greater than Ea increases.
E) the activation energy increases.
A) the concentration of reactants increases.
B) the activation energy decreases.
C) the collision frequency increases.
D) the fraction of collisions with total kinetic energy greater than Ea increases.
E) the activation energy increases.

Unlock Deck
Unlock for access to all 132 flashcards in this deck.
Unlock Deck
k this deck
66
For the chemical reaction system described by the diagram below, which statement is true 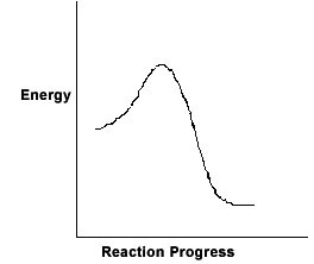 If the Ea for the forward reaction is 25 kJ/mol and the enthalpy of reaction is -95 kJ/mol, what is Ea for the reverse reaction
If the Ea for the forward reaction is 25 kJ/mol and the enthalpy of reaction is -95 kJ/mol, what is Ea for the reverse reaction
A) 120 kJ/mol
B) 70 kJ/mol
C) 95 kJ/mol
D) 25 kJ/mol
E) -70 kJ/mol
 If the Ea for the forward reaction is 25 kJ/mol and the enthalpy of reaction is -95 kJ/mol, what is Ea for the reverse reaction
If the Ea for the forward reaction is 25 kJ/mol and the enthalpy of reaction is -95 kJ/mol, what is Ea for the reverse reactionA) 120 kJ/mol
B) 70 kJ/mol
C) 95 kJ/mol
D) 25 kJ/mol
E) -70 kJ/mol

Unlock Deck
Unlock for access to all 132 flashcards in this deck.
Unlock Deck
k this deck
67
For the chemical reaction system described by the diagram below, which statement is true 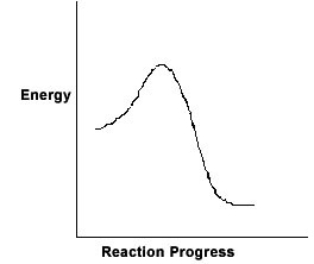
A) The forward reaction is endothermic.
B) The activation energy for the forward reaction is greater than the activation energy for the reverse reaction.
C) At equilibrium, the activation energy for the forward reaction is equal to the activation energy for the reverse reaction.
D) The activation energy for the reverse reaction is greater than the activation energy for the forward reaction.
E) The reverse reaction is exothermic.

A) The forward reaction is endothermic.
B) The activation energy for the forward reaction is greater than the activation energy for the reverse reaction.
C) At equilibrium, the activation energy for the forward reaction is equal to the activation energy for the reverse reaction.
D) The activation energy for the reverse reaction is greater than the activation energy for the forward reaction.
E) The reverse reaction is exothermic.

Unlock Deck
Unlock for access to all 132 flashcards in this deck.
Unlock Deck
k this deck
68
Given that Ea for a certain biological reaction is 48 kJ/mol and that the rate constant is 2.5 *10-2 s-1 at 15 C, what is the rate constant at 37 C
A) 2.7 * 10-2 s-1
B) 2.5 * 10-1 s-1
C) 1.0 * 10-1 s-1
D) 6.0 * 10-3 s-1
E) 1.1 s-1
A) 2.7 * 10-2 s-1
B) 2.5 * 10-1 s-1
C) 1.0 * 10-1 s-1
D) 6.0 * 10-3 s-1
E) 1.1 s-1

Unlock Deck
Unlock for access to all 132 flashcards in this deck.
Unlock Deck
k this deck
69
When the concentrations of reactant molecules are increased, the rate of reaction increases. The best explanation for this phenomenon is that as the reactant concentration increases,
A) the average kinetic energy of molecules increases.
B) the frequency of molecular collisions increases.
C) the rate constant increases.
D) the activation energy increases.
E) the order of reaction increases.
A) the average kinetic energy of molecules increases.
B) the frequency of molecular collisions increases.
C) the rate constant increases.
D) the activation energy increases.
E) the order of reaction increases.

Unlock Deck
Unlock for access to all 132 flashcards in this deck.
Unlock Deck
k this deck
70
The Arrhenius equation is k = Ae-Ea/RT. The slope of a plot of ln k vs. 1/T is equal to
A) -k
B) k
C) Ea
D) -Ea /R
E) A
A) -k
B) k
C) Ea
D) -Ea /R
E) A

Unlock Deck
Unlock for access to all 132 flashcards in this deck.
Unlock Deck
k this deck
71
The isomerization of methyl isocyanide, CH3NC CH3CN, follows first-order kinetics. The half-lives were found to be 161 min at 199 C and 12.5 min at 230 C. Calculate the activation energy for this reaction.
A) 6.17 * 10-3 kJ/mol
B) 31.4 kJ/mol
C) 78.2 kJ/mol
D) 124 kJ/mol
E) 163 kJ/mol
A) 6.17 * 10-3 kJ/mol
B) 31.4 kJ/mol
C) 78.2 kJ/mol
D) 124 kJ/mol
E) 163 kJ/mol

Unlock Deck
Unlock for access to all 132 flashcards in this deck.
Unlock Deck
k this deck
72
The following mechanism has been suggested for the reaction: Identify all intermediates included in this mechanism.
A) H+ and I -
B) H+ and HOI
C) HOI and OH-
D) H+ only
E) H2O and OH-
A) H+ and I -
B) H+ and HOI
C) HOI and OH-
D) H+ only
E) H2O and OH-

Unlock Deck
Unlock for access to all 132 flashcards in this deck.
Unlock Deck
k this deck
73
The activation energy for the reaction O + O3 2O2 is 25 kJ/mol, and the enthalpy change is H = -388 kJ/mol. What is the activation energy for the decomposition of O2 by the reverse reaction
A) 413 kJ
B) 388 kJ
C) 363 kJ
D) 50 kJ
E) 25 kJ
A) 413 kJ
B) 388 kJ
C) 363 kJ
D) 50 kJ
E) 25 kJ

Unlock Deck
Unlock for access to all 132 flashcards in this deck.
Unlock Deck
k this deck
74
The activation energy for the following reaction is 60. kJ/mol.Sn2+ + 2Co3+ Sn4+ + 2Co2+ By what factor (how many times) will the rate constant increase when the temperature is raised from 10 C to 28 C
A) 1.002
B) 4.6
C) 5.6
D) 2.8
E) 696
A) 1.002
B) 4.6
C) 5.6
D) 2.8
E) 696

Unlock Deck
Unlock for access to all 132 flashcards in this deck.
Unlock Deck
k this deck
75
What is the slope of an Arrhenius plot for the following reaction
2NOCl 2NO + Cl2
A) 8.18 * 10-2 K
B) 5.06 * 10-2 K
C) -1.22 * 104 K
D) -1.96 * 104 K
E) not enough information to calculate the slope
2NOCl 2NO + Cl2
A) 8.18 * 10-2 K
B) 5.06 * 10-2 K
C) -1.22 * 104 K
D) -1.96 * 104 K
E) not enough information to calculate the slope

Unlock Deck
Unlock for access to all 132 flashcards in this deck.
Unlock Deck
k this deck
76
If Ea for a certain biological reaction is 50. kJ/mol, by what factor (how many times) will the rate of this reaction increase when body temperature increases from 37 C (normal) to 40 C (fever)
A) 1.15
B) 1.20
C) 2.0 * 105
D) 1.0002
E) 2.0
A) 1.15
B) 1.20
C) 2.0 * 105
D) 1.0002
E) 2.0

Unlock Deck
Unlock for access to all 132 flashcards in this deck.
Unlock Deck
k this deck
77
At 25 C, by what factor is the reaction rate increased by a catalyst that reduces the activation energy of the reaction by 1.00 kJ/mol
A) 1.63
B) 123
C) 1.04
D) 1.50
E) 2.53
A) 1.63
B) 123
C) 1.04
D) 1.50
E) 2.53

Unlock Deck
Unlock for access to all 132 flashcards in this deck.
Unlock Deck
k this deck
78
The activation energy for the reaction CH3CO CH3 + CO is 71 kJ/mol. How many times greater is the rate constant for this reaction at 170 C than at 150 C
A) 0.40
B) 1.1
C) 2.5
D) 4.0
E) 5.0
A) 0.40
B) 1.1
C) 2.5
D) 4.0
E) 5.0

Unlock Deck
Unlock for access to all 132 flashcards in this deck.
Unlock Deck
k this deck
79
At 30 C, by how much is a reaction's activation energy decreased by the addition of a catalyst if the catalyst triples the reaction rate
A) 2.77 kJ/mol
B) 274 J/mol
C) 2.70 J/mol
D) 119 J/mol
E) 1.20 kJ/mol
A) 2.77 kJ/mol
B) 274 J/mol
C) 2.70 J/mol
D) 119 J/mol
E) 1.20 kJ/mol

Unlock Deck
Unlock for access to all 132 flashcards in this deck.
Unlock Deck
k this deck


