Deck 8: Periodic Relationships Among the Elements
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/144
Play
Full screen (f)
Deck 8: Periodic Relationships Among the Elements
1
Which of the following elements is found as a monatomic species in its most stable form
A) sulfur
B) oxygen
C) hydrogen
D) argon
E) phosphorus
A) sulfur
B) oxygen
C) hydrogen
D) argon
E) phosphorus
argon
2
Consider the element with the electron configuration [Kr]5s24d7. This element is
A) a representative element.
B) a transition metal.
C) a nonmetal.
D) an actinide element.
E) a noble gas.
A) a representative element.
B) a transition metal.
C) a nonmetal.
D) an actinide element.
E) a noble gas.
a transition metal.
3
As opposed to early periodic tables based on the law of octaves, modern periodic tables arrange the elements in order of increasing
A) nuclear binding energy.
B) number of neutrons.
C) atomic mass.
D) atomic number.
E) atomic size.
A) nuclear binding energy.
B) number of neutrons.
C) atomic mass.
D) atomic number.
E) atomic size.
atomic number.
4
Which of the following elements is found as a diatomic species in its most stable form
A) bromine
B) neon
C) sulfur
D) xenon
E) phosphorus
A) bromine
B) neon
C) sulfur
D) xenon
E) phosphorus

Unlock Deck
Unlock for access to all 144 flashcards in this deck.
Unlock Deck
k this deck
5
In what row and group of the periodic table would you find the element with the electron configuration [Kr]5s24d105p2
A) row 4, group 4A
B) row 4, group 5A
C) row 5, group 4A
D) row 5, group 5A
E) none of the above
A) row 4, group 4A
B) row 4, group 5A
C) row 5, group 4A
D) row 5, group 5A
E) none of the above

Unlock Deck
Unlock for access to all 144 flashcards in this deck.
Unlock Deck
k this deck
6
Which of the following is the general electron configuration for the outermost electrons of the halogens
A) ns2np6
B) ns2np5
C) ns2np6(n -1)d7
D) ns1
E) ns2np7
A) ns2np6
B) ns2np5
C) ns2np6(n -1)d7
D) ns1
E) ns2np7

Unlock Deck
Unlock for access to all 144 flashcards in this deck.
Unlock Deck
k this deck
7
The elements in Group 7A are known by what name
A) transition metals
B) halogens
C) alkali metals
D) alkaline earth metals
E) noble gases
A) transition metals
B) halogens
C) alkali metals
D) alkaline earth metals
E) noble gases

Unlock Deck
Unlock for access to all 144 flashcards in this deck.
Unlock Deck
k this deck
8
Mendeleev proposed the existence of an unknown element that he called eka-aluminum. This element is now called
A) gallium.
B) silicon.
C) magnesium.
D) boron.
E) germanium.
A) gallium.
B) silicon.
C) magnesium.
D) boron.
E) germanium.

Unlock Deck
Unlock for access to all 144 flashcards in this deck.
Unlock Deck
k this deck
9
Which of the following is the general electron configuration for the outermost electrons of elements in the alkaline earth group
A) ns1
B) ns2
C) ns2np4
D) ns2np5
E) ns2np6(n -1)d6
A) ns1
B) ns2
C) ns2np4
D) ns2np5
E) ns2np6(n -1)d6

Unlock Deck
Unlock for access to all 144 flashcards in this deck.
Unlock Deck
k this deck
10
The law of octaves states that every eighth element has similar properties when arranged in order of increasing
A) nuclear binding energy.
B) number of electrons.
C) number of neutrons.
D) atomic mass.
E) atomic number.
A) nuclear binding energy.
B) number of electrons.
C) number of neutrons.
D) atomic mass.
E) atomic number.

Unlock Deck
Unlock for access to all 144 flashcards in this deck.
Unlock Deck
k this deck
11
Which of the following is the general electron configuration for the outermost electrons of the noble gases
A) ns2np6
B) ns2np5
C) ns2np4
D) ns2np3
E) ns2
A) ns2np6
B) ns2np5
C) ns2np4
D) ns2np3
E) ns2

Unlock Deck
Unlock for access to all 144 flashcards in this deck.
Unlock Deck
k this deck
12
Which one of the following elements is a Lanthanide
A) U
B) Ce
C) Os
D) Bi
E) Cs
A) U
B) Ce
C) Os
D) Bi
E) Cs

Unlock Deck
Unlock for access to all 144 flashcards in this deck.
Unlock Deck
k this deck
13
The law of octaves was proposed by
A) G. N. Lewis.
B) John Newlands.
C) Dmitri Mendeleev.
D) J. J. Thompson.
E) Ernest Rutherford.
A) G. N. Lewis.
B) John Newlands.
C) Dmitri Mendeleev.
D) J. J. Thompson.
E) Ernest Rutherford.

Unlock Deck
Unlock for access to all 144 flashcards in this deck.
Unlock Deck
k this deck
14
The elements in Group 2A are known by what name
A) transition metals
B) halogens
C) alkali metals
D) alkaline earth metals
E) noble gases
A) transition metals
B) halogens
C) alkali metals
D) alkaline earth metals
E) noble gases

Unlock Deck
Unlock for access to all 144 flashcards in this deck.
Unlock Deck
k this deck
15
An element with the general electron configuration for its outermost electrons of ns2np1 would be in which element group
A) 2A
B) 3A
C) 4A
D) 5A
E) 8A
A) 2A
B) 3A
C) 4A
D) 5A
E) 8A

Unlock Deck
Unlock for access to all 144 flashcards in this deck.
Unlock Deck
k this deck
16
Which of the following elements is found as a monatomic species in its most stable form
A) nitrogen
B) neon
C) sulfur
D) chlorine
E) fluorine
A) nitrogen
B) neon
C) sulfur
D) chlorine
E) fluorine

Unlock Deck
Unlock for access to all 144 flashcards in this deck.
Unlock Deck
k this deck
17
The chief contribution of physicist Henry Moseley to atomic theory was
A) the discovery of the periodic law.
B) the determination of the charge of the proton.
C) the measurement of the atomic numbers of the elements.
D) the scientist who determined the electric charge of the electron.
E) the discovery of the law of octaves.
A) the discovery of the periodic law.
B) the determination of the charge of the proton.
C) the measurement of the atomic numbers of the elements.
D) the scientist who determined the electric charge of the electron.
E) the discovery of the law of octaves.

Unlock Deck
Unlock for access to all 144 flashcards in this deck.
Unlock Deck
k this deck
18
The alkali metal elements are found in _______ of the periodic table.
A) Group 1A
B) Group 2A
C) Group 3A
D) Period 7
E) Period 1
A) Group 1A
B) Group 2A
C) Group 3A
D) Period 7
E) Period 1

Unlock Deck
Unlock for access to all 144 flashcards in this deck.
Unlock Deck
k this deck
19
Which of the following is the general electron configuration for the outermost electrons of the elements in Group 5A
A) ns2np6
B) ns2np5
C) ns2np4
D) ns2np3
E) ns2np1
A) ns2np6
B) ns2np5
C) ns2np4
D) ns2np3
E) ns2np1

Unlock Deck
Unlock for access to all 144 flashcards in this deck.
Unlock Deck
k this deck
20
Which one of the following elements is a transition element
A) antimony
B) barium
C) chromium
D) potassium
E) selenium
A) antimony
B) barium
C) chromium
D) potassium
E) selenium

Unlock Deck
Unlock for access to all 144 flashcards in this deck.
Unlock Deck
k this deck
21
Which of the following make an isoelectronic pair: Cl-, O2-, F, Ca2+, Fe3+
A) Ca2+ and Fe3+
B) O2- and F
C) F and Cl-
D) Cl- and Ca2+
E) None of the above.
A) Ca2+ and Fe3+
B) O2- and F
C) F and Cl-
D) Cl- and Ca2+
E) None of the above.

Unlock Deck
Unlock for access to all 144 flashcards in this deck.
Unlock Deck
k this deck
22
Consider the element with the electron configuration [Kr]5s24d105p5. This element is
A) a representative element
B) a transition metal
C) an alkali metal
D) an actinide element
E) a noble gas
A) a representative element
B) a transition metal
C) an alkali metal
D) an actinide element
E) a noble gas

Unlock Deck
Unlock for access to all 144 flashcards in this deck.
Unlock Deck
k this deck
23
The representative elements are those with unfilled energy levels in which the "last electron" was added to
A) an s orbital.
B) an s or p orbital.
C) a d orbital.
D) a p or d orbital.
E) an f orbital.
A) an s orbital.
B) an s or p orbital.
C) a d orbital.
D) a p or d orbital.
E) an f orbital.

Unlock Deck
Unlock for access to all 144 flashcards in this deck.
Unlock Deck
k this deck
24
How many core electrons does a silicon atom have
A) 14
B) 10
C) 4
D) 8
E) 2
A) 14
B) 10
C) 4
D) 8
E) 2

Unlock Deck
Unlock for access to all 144 flashcards in this deck.
Unlock Deck
k this deck
25
Which one of the following elements forms a stable 3- anion
A) P
B) S
C) Cl
D) Al
E) Mg
A) P
B) S
C) Cl
D) Al
E) Mg

Unlock Deck
Unlock for access to all 144 flashcards in this deck.
Unlock Deck
k this deck
26
Which one of the following elements forms a stable 1- anion
A) K
B) Be
C) Al
D) O
E) I
A) K
B) Be
C) Al
D) O
E) I

Unlock Deck
Unlock for access to all 144 flashcards in this deck.
Unlock Deck
k this deck
27
How many electrons does a sulfur atom need to fill its outermost s and p subshells
A) 6
B) 8
C) 4
D) 2
E) 1
A) 6
B) 8
C) 4
D) 2
E) 1

Unlock Deck
Unlock for access to all 144 flashcards in this deck.
Unlock Deck
k this deck
28
How many electrons are in the 4p subshell of selenium
A) 0
B) 2
C) 4
D) 5
E) 6
A) 0
B) 2
C) 4
D) 5
E) 6

Unlock Deck
Unlock for access to all 144 flashcards in this deck.
Unlock Deck
k this deck
29
Which one of the following elements forms a stable 2+ cation
A) Kr
B) I
C) Se
D) Al
E) Ba
A) Kr
B) I
C) Se
D) Al
E) Ba

Unlock Deck
Unlock for access to all 144 flashcards in this deck.
Unlock Deck
k this deck
30
How many electrons does a nitrogen atom need to fill its outermost s and p subshells
A) 5
B) 3
C) 1
D) 8
E) 0
A) 5
B) 3
C) 1
D) 8
E) 0

Unlock Deck
Unlock for access to all 144 flashcards in this deck.
Unlock Deck
k this deck
31
Concerning the electron configuration of sulfur, 1s22s22p63s23p4, which of the following represents the core electrons only
A) 1s2
B) 1s22s2
C) 1s22s22p6
D) 1s22s22p63s2
E) 3s23p4
A) 1s2
B) 1s22s2
C) 1s22s22p6
D) 1s22s22p63s2
E) 3s23p4

Unlock Deck
Unlock for access to all 144 flashcards in this deck.
Unlock Deck
k this deck
32
Which two electron configurations represent elements that would have similar chemical properties
(1) 1s22s22p4 (2) 1s22s22p5 (3) [Ar]4s23d5 (4) [Ar]4s23d104p5
A) (1) and (2)
B) (1) and (3)
C) (2) and (3)
D) (2) and (4)
E) (3) and (4)
(1) 1s22s22p4 (2) 1s22s22p5 (3) [Ar]4s23d5 (4) [Ar]4s23d104p5
A) (1) and (2)
B) (1) and (3)
C) (2) and (3)
D) (2) and (4)
E) (3) and (4)

Unlock Deck
Unlock for access to all 144 flashcards in this deck.
Unlock Deck
k this deck
33
Concerning the electron configuration of fluorine, 1s22s22p5 which of the following represents the core electrons only
A) 1s2
B) 1s22s2
C) 1s22s22p5
D) 2s22p5
E) 2p5
A) 1s2
B) 1s22s2
C) 1s22s22p5
D) 2s22p5
E) 2p5

Unlock Deck
Unlock for access to all 144 flashcards in this deck.
Unlock Deck
k this deck
34
How many electrons does a bromine atom need to fill its outermost s and p subshells
A) 8
B) 7
C) 5
D) 3
E) 1
A) 8
B) 7
C) 5
D) 3
E) 1

Unlock Deck
Unlock for access to all 144 flashcards in this deck.
Unlock Deck
k this deck
35
How many core electrons does a chlorine atom have
A) 17
B) 7
C) 10
D) 18
E) 8
A) 17
B) 7
C) 10
D) 18
E) 8

Unlock Deck
Unlock for access to all 144 flashcards in this deck.
Unlock Deck
k this deck
36
How many electrons are in the 4p subshell of vanadium
A) 0
B) 2
C) 4
D) 5
E) 6
A) 0
B) 2
C) 4
D) 5
E) 6

Unlock Deck
Unlock for access to all 144 flashcards in this deck.
Unlock Deck
k this deck
37
Consider the element with the electron configuration [Xe]6s24f7. This element is
A) a representative element
B) a lanthanide element
C) a nonmetal
D) an actinide element
E) a noble gas
A) a representative element
B) a lanthanide element
C) a nonmetal
D) an actinide element
E) a noble gas

Unlock Deck
Unlock for access to all 144 flashcards in this deck.
Unlock Deck
k this deck
38
How many valence electrons does S2- have
A) 2
B) 4
C) 6
D) 16
E) 8
A) 2
B) 4
C) 6
D) 16
E) 8

Unlock Deck
Unlock for access to all 144 flashcards in this deck.
Unlock Deck
k this deck
39
How many valence electrons does P3- have
A) 3
B) 8
C) 15
D) 1
E) 18
A) 3
B) 8
C) 15
D) 1
E) 18

Unlock Deck
Unlock for access to all 144 flashcards in this deck.
Unlock Deck
k this deck
40
Concerning the electron configuration of aluminum, 1s22s22p63s23p1, which of the following represents the valence electrons only
A) 1s2
B) 1s22s2
C) 1s22s22p6
D) 1s22s22p63s2
E) 3s23p1
A) 1s2
B) 1s22s2
C) 1s22s22p6
D) 1s22s22p63s2
E) 3s23p1

Unlock Deck
Unlock for access to all 144 flashcards in this deck.
Unlock Deck
k this deck
41
The effective nuclear charge (Zeff) felt by the outermost electrons is the strongest for which of the following elements
A) B
B) C
C) N
D) O
E) F
A) B
B) C
C) N
D) O
E) F

Unlock Deck
Unlock for access to all 144 flashcards in this deck.
Unlock Deck
k this deck
42
Which one of the following does not have [Kr] as its electronic configuration
A) Se2-
B) Br-
C) Rb+
D) Y3+
E) Zn2+
A) Se2-
B) Br-
C) Rb+
D) Y3+
E) Zn2+

Unlock Deck
Unlock for access to all 144 flashcards in this deck.
Unlock Deck
k this deck
43
The effective nuclear charge (Zeff) felt by the outermost electrons is the strongest for which of the following elements
A) Al
B) Si
C) P
D) S
E) Cl
A) Al
B) Si
C) P
D) S
E) Cl

Unlock Deck
Unlock for access to all 144 flashcards in this deck.
Unlock Deck
k this deck
44
Which of the following is the electron configuration for the bromide ion
A) [Ar]
B) [Ar]4s23d104p7
C) [Kr]
D) [Kr]5s24d105p7
E) [Xe]
A) [Ar]
B) [Ar]4s23d104p7
C) [Kr]
D) [Kr]5s24d105p7
E) [Xe]

Unlock Deck
Unlock for access to all 144 flashcards in this deck.
Unlock Deck
k this deck
45
Which one of the following does not have [Xe] as its electronic configuration
A) Te2-
B) I-
C) Cs+
D) Ba2+
E) Sn4+
A) Te2-
B) I-
C) Cs+
D) Ba2+
E) Sn4+

Unlock Deck
Unlock for access to all 144 flashcards in this deck.
Unlock Deck
k this deck
46
How many 3d electrons does the manganese(II) ion, Mn2+, have
A) 3
B) 4
C) 5
D) 6
E) 7
A) 3
B) 4
C) 5
D) 6
E) 7

Unlock Deck
Unlock for access to all 144 flashcards in this deck.
Unlock Deck
k this deck
47
Which of the following is the electron configuration for the aluminum ion
A) 1s22s22p63s2
B) 1s22s22p63s23p2
C) 1s22s22p63s23p1
D) 1s22s22p6
E) 1s22s22p63s23p4
A) 1s22s22p63s2
B) 1s22s22p63s23p2
C) 1s22s22p63s23p1
D) 1s22s22p6
E) 1s22s22p63s23p4

Unlock Deck
Unlock for access to all 144 flashcards in this deck.
Unlock Deck
k this deck
48
Which of the following is the electron configuration of the iron(III) ion
A) [Ar]3d5
B) [Ar]4s13d5
C) [Ar]4s23d3
D) [Ar]3d6
E) [Ar]4s23d9
A) [Ar]3d5
B) [Ar]4s13d5
C) [Ar]4s23d3
D) [Ar]3d6
E) [Ar]4s23d9

Unlock Deck
Unlock for access to all 144 flashcards in this deck.
Unlock Deck
k this deck
49
How many 3d electrons does an Fe3+ ion have
A) 9
B) 6
C) 5
D) 4
E) 3
A) 9
B) 6
C) 5
D) 4
E) 3

Unlock Deck
Unlock for access to all 144 flashcards in this deck.
Unlock Deck
k this deck
50
The cobalt(III) ion, Co3+, has how many 3d electrons
A) 0
B) 7
C) 6
D) 5
E) 4
A) 0
B) 7
C) 6
D) 5
E) 4

Unlock Deck
Unlock for access to all 144 flashcards in this deck.
Unlock Deck
k this deck
51
Which of the atoms listed below has the smallest radius
A) Al
B) P
C) As
D) Te
E) Na
A) Al
B) P
C) As
D) Te
E) Na

Unlock Deck
Unlock for access to all 144 flashcards in this deck.
Unlock Deck
k this deck
52
Which of the following ground-state ions has unpaired electrons
A) P3-
B) V5+
C) Mg2+
D) Sc2+
E) S2+
A) P3-
B) V5+
C) Mg2+
D) Sc2+
E) S2+

Unlock Deck
Unlock for access to all 144 flashcards in this deck.
Unlock Deck
k this deck
53
Which one of the following is not isoelectronic with the others: Br-, Rb+, Se2-, Sr2+, Te2-
A) Br-
B) Rb+
C) Se2-
D) Sr2+
E) Te2-
A) Br-
B) Rb+
C) Se2-
D) Sr2+
E) Te2-

Unlock Deck
Unlock for access to all 144 flashcards in this deck.
Unlock Deck
k this deck
54
Which of the following ground-state ions has the largest number of unpaired electrons
A) Cr2+
B) Mn2+
C) Ni2+
D) Cu+
E) Co2+
A) Cr2+
B) Mn2+
C) Ni2+
D) Cu+
E) Co2+

Unlock Deck
Unlock for access to all 144 flashcards in this deck.
Unlock Deck
k this deck
55
Which one of the following pairs are isoelectronic
A) Mn2+ and Ar
B) Zn2+ and Cu2+
C) Na+ and K+
D) Cl- and S
E) K+ and Cl-
A) Mn2+ and Ar
B) Zn2+ and Cu2+
C) Na+ and K+
D) Cl- and S
E) K+ and Cl-

Unlock Deck
Unlock for access to all 144 flashcards in this deck.
Unlock Deck
k this deck
56
Which of the following is the electron configuration of a sulfide ion
A) [Ne]3s23p4
B) [Ne]
C) [Ne]3s23p1
D) [Ar]
E) [Ne]3s23p2
A) [Ne]3s23p4
B) [Ne]
C) [Ne]3s23p1
D) [Ar]
E) [Ne]3s23p2

Unlock Deck
Unlock for access to all 144 flashcards in this deck.
Unlock Deck
k this deck
57
The electron configuration of a copper(I) ion is
A) [Ar]4s23d8.
B) [Ar]4s13d9.
C) [Ar]3d10.
D) [Ar]4s23d64p2.
E) [Kr].
A) [Ar]4s23d8.
B) [Ar]4s13d9.
C) [Ar]3d10.
D) [Ar]4s23d64p2.
E) [Kr].

Unlock Deck
Unlock for access to all 144 flashcards in this deck.
Unlock Deck
k this deck
58
Which ion is isoelectronic with Ar
A) Fe2+
B) F-
C) Br-
D) Ga3+
E) Ca2+
A) Fe2+
B) F-
C) Br-
D) Ga3+
E) Ca2+

Unlock Deck
Unlock for access to all 144 flashcards in this deck.
Unlock Deck
k this deck
59
Which one of the following is not isoelectronic with Kr
A) As3+
B) Se2-
C) Rb+
D) Sr2+
E) Br-
A) As3+
B) Se2-
C) Rb+
D) Sr2+
E) Br-

Unlock Deck
Unlock for access to all 144 flashcards in this deck.
Unlock Deck
k this deck
60
The sulfide ion, S2-, is isoelectronic with which one of the following
A) O2-
B) F-
C) Na+
D) Al3+
E) K+
A) O2-
B) F-
C) Na+
D) Al3+
E) K+

Unlock Deck
Unlock for access to all 144 flashcards in this deck.
Unlock Deck
k this deck
61
Arrange the following ions in order of increasing ionic radius: K+, P3- , S2-, Cl- increasing radius
A) K+ < Cl- < S2- < P3-
B) K+ < P3- < S2- < Cl-
C) P3- < S2- < Cl- < K+
D) Cl- < S2- < P3- < K+
E) Cl- < S2- < K+ < P3-
A) K+ < Cl- < S2- < P3-
B) K+ < P3- < S2- < Cl-
C) P3- < S2- < Cl- < K+
D) Cl- < S2- < P3- < K+
E) Cl- < S2- < K+ < P3-

Unlock Deck
Unlock for access to all 144 flashcards in this deck.
Unlock Deck
k this deck
62
Which element will display an unusually large jump in ionization energy values between I3 and I4, its third and fourth ionization energies
A) Na
B) Mg
C) Al
D) Si
E) P
A) Na
B) Mg
C) Al
D) Si
E) P

Unlock Deck
Unlock for access to all 144 flashcards in this deck.
Unlock Deck
k this deck
63
Which of the elements listed below has the smallest first ionization energy
A) C
B) Ge
C) P
D) O
E) Se
A) C
B) Ge
C) P
D) O
E) Se

Unlock Deck
Unlock for access to all 144 flashcards in this deck.
Unlock Deck
k this deck
64
Which of the following elements has the greatest electron affinity (largest positive value)
A) K
B) Br
C) As
D) Ar
E) I
A) K
B) Br
C) As
D) Ar
E) I

Unlock Deck
Unlock for access to all 144 flashcards in this deck.
Unlock Deck
k this deck
65
For which of the following reactions is the enthalpy change equal to the second ionization energy of nitrogen
A) N2+(g) N3+(g) + e-
B) N2+(g) + e- N+(g)
C) N(g) N2+(g) + 2e-
D) N-(g) + e- N2-(g)
E) N+(g) N2+(g) + e-
A) N2+(g) N3+(g) + e-
B) N2+(g) + e- N+(g)
C) N(g) N2+(g) + 2e-
D) N-(g) + e- N2-(g)
E) N+(g) N2+(g) + e-

Unlock Deck
Unlock for access to all 144 flashcards in this deck.
Unlock Deck
k this deck
66
For which of the following reactions is the enthalpy change equal to the third ionization energy of vanadium
A) V2+(g) V3+(g) + e-
B) V3+(g) + e- V2+(g)
C) V(g) V3+(g) + 3e-
D) V2-(g) + e- V3-(g)
E) V3+(g) V4+(g) + e-
A) V2+(g) V3+(g) + e-
B) V3+(g) + e- V2+(g)
C) V(g) V3+(g) + 3e-
D) V2-(g) + e- V3-(g)
E) V3+(g) V4+(g) + e-

Unlock Deck
Unlock for access to all 144 flashcards in this deck.
Unlock Deck
k this deck
67
Which of the following elements has the smallest first ionization energy
A) Cl
B) Na
C) Be
D) K
E) As
A) Cl
B) Na
C) Be
D) K
E) As

Unlock Deck
Unlock for access to all 144 flashcards in this deck.
Unlock Deck
k this deck
68
Which one of the following ions has the largest radius
A) Cl-
B) K+
C) S2-
D) Na+
E) O2-
A) Cl-
B) K+
C) S2-
D) Na+
E) O2-

Unlock Deck
Unlock for access to all 144 flashcards in this deck.
Unlock Deck
k this deck
69
Which of the elements listed below has the greatest atomic radius
A) B
B) Al
C) S
D) P
E) Si
A) B
B) Al
C) S
D) P
E) Si

Unlock Deck
Unlock for access to all 144 flashcards in this deck.
Unlock Deck
k this deck
70
The successive ionization energies of a certain element are I1= 589.5 kJ/mol, I2 =1145 kJ/mol, I3= 4900 kJ/mol, I4 = 6500 kJ/mol, and I5 = 8100 kJ/mol. This pattern of ionization energies suggests that the unknown element is
A) K
B) Si
C) As
D) Ca
E) S
A) K
B) Si
C) As
D) Ca
E) S

Unlock Deck
Unlock for access to all 144 flashcards in this deck.
Unlock Deck
k this deck
71
Which of the following elements has the greatest electron affinity (largest positive value)
A) Mg
B) Al
C) Si
D) P
E) S
A) Mg
B) Al
C) Si
D) P
E) S

Unlock Deck
Unlock for access to all 144 flashcards in this deck.
Unlock Deck
k this deck
72
Arrange the following ions in order of decreasing ionic radius: Al3+, Mg2+, Na+, O2-.decreasing radius
A) Al3+ > Mg2+ > O2- > Na+
B) Al3+ > Mg2+ > Na+ > O2-
C) Na+ > Mg2+ > Al3+ > O2-
D) O2- > Al3+ > Mg2+ > Na+
E) O2- > Na+ > Mg2+ > Al3+
A) Al3+ > Mg2+ > O2- > Na+
B) Al3+ > Mg2+ > Na+ > O2-
C) Na+ > Mg2+ > Al3+ > O2-
D) O2- > Al3+ > Mg2+ > Na+
E) O2- > Na+ > Mg2+ > Al3+

Unlock Deck
Unlock for access to all 144 flashcards in this deck.
Unlock Deck
k this deck
73
Which of the following elements has the smallest ionization energy
A) Li
B) Na
C) Be
D) K
E) Rb
A) Li
B) Na
C) Be
D) K
E) Rb

Unlock Deck
Unlock for access to all 144 flashcards in this deck.
Unlock Deck
k this deck
74
Which of the elements listed below has the highest first ionization energy
A) He
B) Ne
C) Ar
D) Kr
E) Xe
A) He
B) Ne
C) Ar
D) Kr
E) Xe

Unlock Deck
Unlock for access to all 144 flashcards in this deck.
Unlock Deck
k this deck
75
Which of the elements listed below has the following pattern for its first six ionization energies (I1 = first ionization energy, I2 = second ionization energy, etc.) 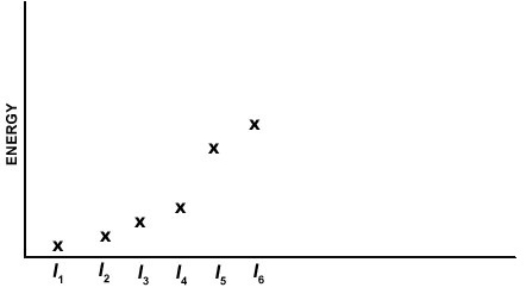
A) Ca
B) Si
C) Al
D) Se
E) P

A) Ca
B) Si
C) Al
D) Se
E) P

Unlock Deck
Unlock for access to all 144 flashcards in this deck.
Unlock Deck
k this deck
76
For phosphorus atoms, which ionization energy will show an exceptionally large increase over the previous ionization energy
A) 2nd
B) 3rd
C) 4th
D) 5th
E) 6th
A) 2nd
B) 3rd
C) 4th
D) 5th
E) 6th

Unlock Deck
Unlock for access to all 144 flashcards in this deck.
Unlock Deck
k this deck
77
The successive ionization energies of a certain element are I1= 577.9 kJ/mol, I2 = 1820 kJ/mol, I3= 2750 kJ/mol, I4 = 11,600 kJ/mol, and I5 = 14,800 kJ/mol. This pattern of ionization energies suggests that the unknown element is
A) K
B) Al
C) Cl
D) Se
E) Kr
A) K
B) Al
C) Cl
D) Se
E) Kr

Unlock Deck
Unlock for access to all 144 flashcards in this deck.
Unlock Deck
k this deck
78
Which of the atoms listed below has the largest radius
A) Cl
B) I
C) P
D) Sb
E) Se
A) Cl
B) I
C) P
D) Sb
E) Se

Unlock Deck
Unlock for access to all 144 flashcards in this deck.
Unlock Deck
k this deck
79
For silicon atoms, which ionization energy will show an exceptionally large increase over the preceding ionization energy
A) 2nd
B) 3rd
C) 4th
D) 5th
E) 6th
A) 2nd
B) 3rd
C) 4th
D) 5th
E) 6th

Unlock Deck
Unlock for access to all 144 flashcards in this deck.
Unlock Deck
k this deck
80
Which of the elements listed below has the highest first ionization energy
A) C
B) Ge
C) P
D) O
E) Se
A) C
B) Ge
C) P
D) O
E) Se

Unlock Deck
Unlock for access to all 144 flashcards in this deck.
Unlock Deck
k this deck


