Deck 12: Chemical Reactions
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/40
Play
Full screen (f)
Deck 12: Chemical Reactions
1
The speed of a chemical reaction is not affected by
A)the surface area of the reactants.
B)catalysts.
C)the concentration of reactants.
D)the type of bond in reaction products.
A)the surface area of the reactants.
B)catalysts.
C)the concentration of reactants.
D)the type of bond in reaction products.
the type of bond in reaction products.
2
The decomposition of an unstable compound usually
A)occurs only at low temperatures.
B)requires a catalyst to occur.
C)is endothermic.
D)is exothermic.
A)occurs only at low temperatures.
B)requires a catalyst to occur.
C)is endothermic.
D)is exothermic.
is exothermic.
3
One mole of which of the following compounds contains the largest number of fluorine atoms?
A)HF
B)SF2
C)BF3
D)XeF6
A)HF
B)SF2
C)BF3
D)XeF6
XeF6
4
If a certain reaction is endothermic, the reverse reaction
A)cannot occur.
B)may occur spontaneously.
C)cannot occur spontaneously.
D)absorbs energy.
A)cannot occur.
B)may occur spontaneously.
C)cannot occur spontaneously.
D)absorbs energy.

Unlock Deck
Unlock for access to all 40 flashcards in this deck.
Unlock Deck
k this deck
5
Approximately 4/5 of dry air consists of
A)oxygen.
B)nitrogen.
C)carbon dioxide.
D)argon.
A)oxygen.
B)nitrogen.
C)carbon dioxide.
D)argon.

Unlock Deck
Unlock for access to all 40 flashcards in this deck.
Unlock Deck
k this deck
6
The mass of 1 mole of oxygen, O2, is 32 g.The atomic mass of oxygen is
A)16 u.
B)16 g.
C)32 u.
D)32 g.
A)16 u.
B)16 g.
C)32 u.
D)32 g.

Unlock Deck
Unlock for access to all 40 flashcards in this deck.
Unlock Deck
k this deck
7
An example of slow oxidation is
A)a lighted candle.
B)the rusting of iron.
C)the lighting of a match.
D)a log burning in a fireplace.
A)a lighted candle.
B)the rusting of iron.
C)the lighting of a match.
D)a log burning in a fireplace.

Unlock Deck
Unlock for access to all 40 flashcards in this deck.
Unlock Deck
k this deck
8
The chief reason why reaction rates increase with temperature is that a rise in temperature increases the number of
A)ions.
B)polar molecules.
C)fast-moving molecules.
D)activated molecules.
A)ions.
B)polar molecules.
C)fast-moving molecules.
D)activated molecules.

Unlock Deck
Unlock for access to all 40 flashcards in this deck.
Unlock Deck
k this deck
9
According to Aristotle, every flammable material consisted of
A)earth and fire.
B)phlogiston.
C)oxygen and earth.
D)air and heat.
A)earth and fire.
B)phlogiston.
C)oxygen and earth.
D)air and heat.

Unlock Deck
Unlock for access to all 40 flashcards in this deck.
Unlock Deck
k this deck
10
An oxide decomposed by heating will give
A)oxygen and another element.
B)CO2 and water.
C)oxygen ions and metal ions.
D)a metal and water.
A)oxygen and another element.
B)CO2 and water.
C)oxygen ions and metal ions.
D)a metal and water.

Unlock Deck
Unlock for access to all 40 flashcards in this deck.
Unlock Deck
k this deck
11
Chemical reactions that give off energy are called
A)endothermic.
B)exothermic.
C)activated.
D)oxidation-reduction.
A)endothermic.
B)exothermic.
C)activated.
D)oxidation-reduction.

Unlock Deck
Unlock for access to all 40 flashcards in this deck.
Unlock Deck
k this deck
12
A mole of any compound contains Avogadro's number of
A)atoms.
B)ions.
C)molecules.
D)formula units.
A)atoms.
B)ions.
C)molecules.
D)formula units.

Unlock Deck
Unlock for access to all 40 flashcards in this deck.
Unlock Deck
k this deck
13
For a reaction that takes place near room temperature, an increase of 10 C
A)has almost no effect on the reaction speed.
B)increases the reaction speed by about 10%.
C)increases the reaction speed by about 50%.
D)approximately doubles the reaction speed.
A)has almost no effect on the reaction speed.
B)increases the reaction speed by about 10%.
C)increases the reaction speed by about 50%.
D)approximately doubles the reaction speed.

Unlock Deck
Unlock for access to all 40 flashcards in this deck.
Unlock Deck
k this deck
14
The time needed for a strong acid to neutralize a strong base in solution
A)decreases with temperature.
B)increases with temperature.
C)is virtually independent of temperature.
D)Any of these choices could be correct, depending on the particular acid and base.
A)decreases with temperature.
B)increases with temperature.
C)is virtually independent of temperature.
D)Any of these choices could be correct, depending on the particular acid and base.

Unlock Deck
Unlock for access to all 40 flashcards in this deck.
Unlock Deck
k this deck
15
When 1 g of a metal is oxidized, the resulting oxide has a mass that is
A)less than 1 g.
B)equal to 1 g.
C)more than 1 g.
D)Any of these choices could be correct, depending on the metal.
A)less than 1 g.
B)equal to 1 g.
C)more than 1 g.
D)Any of these choices could be correct, depending on the metal.

Unlock Deck
Unlock for access to all 40 flashcards in this deck.
Unlock Deck
k this deck
16
Which of the following compounds is not classed as an oxide?
A)CO2
B)SiO2
C)BaSO4
D)H2O
A)CO2
B)SiO2
C)BaSO4
D)H2O

Unlock Deck
Unlock for access to all 40 flashcards in this deck.
Unlock Deck
k this deck
17
An example of an exothermic process is the
A)dissociation of a salt in water solution.
B)neutralization of an acid by a base.
C)electrolysis of water.
D)melting of ice.
A)dissociation of a salt in water solution.
B)neutralization of an acid by a base.
C)electrolysis of water.
D)melting of ice.

Unlock Deck
Unlock for access to all 40 flashcards in this deck.
Unlock Deck
k this deck
18
The energy liberated in a chemical reaction comes from the
A)kinetic energy of atomic electrons.
B)potential energy of atomic electrons.
C)activation energy of atomic electrons.
D)equilibrium energy of atomic electrons.
A)kinetic energy of atomic electrons.
B)potential energy of atomic electrons.
C)activation energy of atomic electrons.
D)equilibrium energy of atomic electrons.

Unlock Deck
Unlock for access to all 40 flashcards in this deck.
Unlock Deck
k this deck
19
The scientist whose experiments showed that tin, upon heating, combined with a gas from the air was
A)Priestley.
B)Stahl.
C)Lavoisier.
D)Becher.
A)Priestley.
B)Stahl.
C)Lavoisier.
D)Becher.

Unlock Deck
Unlock for access to all 40 flashcards in this deck.
Unlock Deck
k this deck
20
Which of the following statements about ozone is incorrect?
A)The formula of ozone is O3.
B)Ozone molecules are more stable than those of ordinary oxygen.
C)At low altitudes ozone contributes to smog.
D)At high altitudes ozone absorbs solar ultraviolet radiation.
A)The formula of ozone is O3.
B)Ozone molecules are more stable than those of ordinary oxygen.
C)At low altitudes ozone contributes to smog.
D)At high altitudes ozone absorbs solar ultraviolet radiation.

Unlock Deck
Unlock for access to all 40 flashcards in this deck.
Unlock Deck
k this deck
21
The number of moles of atomic oxygen in 1 mole of Ca3(PO4)2 is
A)1.
B)2.
C)4.
D)8.
A)1.
B)2.
C)4.
D)8.

Unlock Deck
Unlock for access to all 40 flashcards in this deck.
Unlock Deck
k this deck
22
Oxidation-reduction reactions involve the transfer between the reacting substances of
A)electrons.
B)H+ ions.
C)O2- ions.
D)O2 molecules.
A)electrons.
B)H+ ions.
C)O2- ions.
D)O2 molecules.

Unlock Deck
Unlock for access to all 40 flashcards in this deck.
Unlock Deck
k this deck
23
The number of calcium atoms in 1 mole of calcium is
A)40.
B)1.5 * 1022.
C)3 * 1022.
D)6 * 1023.
A)40.
B)1.5 * 1022.
C)3 * 1022.
D)6 * 1023.

Unlock Deck
Unlock for access to all 40 flashcards in this deck.
Unlock Deck
k this deck
24
Most chemical reactions
A)absorb energy.
B)occur at rates that are independent of temperature.
C)are reversible.
D)produce precipitates.
A)absorb energy.
B)occur at rates that are independent of temperature.
C)are reversible.
D)produce precipitates.

Unlock Deck
Unlock for access to all 40 flashcards in this deck.
Unlock Deck
k this deck
25
The number of moles of Al needed to react with each mole of CuSO4 in the reaction 2Al + 3CuSO4 3 Cu + Al2(SO4)3 is
A)1/3.
B)1/2.
C)2/3.
D)3/2.
A)1/3.
B)1/2.
C)2/3.
D)3/2.

Unlock Deck
Unlock for access to all 40 flashcards in this deck.
Unlock Deck
k this deck
26
Ammonia gas dissolves in water and can react according to the equation NH3 + H2O  NH4+ OH-.The amount of ammonium ion in solution will be increased by which one or more of the following?
NH4+ OH-.The amount of ammonium ion in solution will be increased by which one or more of the following?
A)Increase the pressure of NH3 above the solution.
B)Pump off the gas above the solution.
C)Raise the temperature of the solution.
D)Add HCl to the solution.
E)Both A and D
 NH4+ OH-.The amount of ammonium ion in solution will be increased by which one or more of the following?
NH4+ OH-.The amount of ammonium ion in solution will be increased by which one or more of the following?A)Increase the pressure of NH3 above the solution.
B)Pump off the gas above the solution.
C)Raise the temperature of the solution.
D)Add HCl to the solution.
E)Both A and D

Unlock Deck
Unlock for access to all 40 flashcards in this deck.
Unlock Deck
k this deck
27
The number of calcium atoms in 1 g of calcium is
A)40.
B)1.5 * 1022.
C)3 * 1022.
D)6 * 1023.
A)40.
B)1.5 * 1022.
C)3 * 1022.
D)6 * 1023.

Unlock Deck
Unlock for access to all 40 flashcards in this deck.
Unlock Deck
k this deck
28
Silicon has an atomic mass of 28 u and Avogadro's number is 6 *1023 formula units/mole.The mass of 1 mole of silicon is
A)1 g.
B)28 g.
C)6 * 1023 g.
D)1.68 * 1025 g.
A)1 g.
B)28 g.
C)6 * 1023 g.
D)1.68 * 1025 g.

Unlock Deck
Unlock for access to all 40 flashcards in this deck.
Unlock Deck
k this deck
29
Chemical reactions in solution do not depend to any great extent upon
A)pressure.
B)temperature.
C)concentration.
D)catalysts.
A)pressure.
B)temperature.
C)concentration.
D)catalysts.

Unlock Deck
Unlock for access to all 40 flashcards in this deck.
Unlock Deck
k this deck
30
A chemical equilibrium occurs when the
A)mass of the products equals the mass of the reactants.
B)number of molecules of the products equals the number of molecules of the reactants.
C)concentration of the products equals the concentration of the reactants.
D)speed of the forward reaction equals the speed of the reverse reaction.
A)mass of the products equals the mass of the reactants.
B)number of molecules of the products equals the number of molecules of the reactants.
C)concentration of the products equals the concentration of the reactants.
D)speed of the forward reaction equals the speed of the reverse reaction.

Unlock Deck
Unlock for access to all 40 flashcards in this deck.
Unlock Deck
k this deck
31
The mass of Avogadro's number of silicon atoms is
A)1 g.
B)28 g.
C)6 * 1023 g.
D)1.68 * 1025 g.
A)1 g.
B)28 g.
C)6 * 1023 g.
D)1.68 * 1025 g.

Unlock Deck
Unlock for access to all 40 flashcards in this deck.
Unlock Deck
k this deck
32
An enzyme is a
A)gasoline additive that reduces pollution.
B)constituent of natural gas.
C)solvent used in electrolysis.
D)biological catalyst.
A)gasoline additive that reduces pollution.
B)constituent of natural gas.
C)solvent used in electrolysis.
D)biological catalyst.

Unlock Deck
Unlock for access to all 40 flashcards in this deck.
Unlock Deck
k this deck
33
The yield of the product C in the reversible reaction A + B 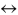 C + energy can be increased by
C + energy can be increased by
A)decreasing the temperature.
B)increasing the temperature.
C)increasing the surface area of the reactants.
D)changing the catalyst.
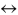 C + energy can be increased by
C + energy can be increased byA)decreasing the temperature.
B)increasing the temperature.
C)increasing the surface area of the reactants.
D)changing the catalyst.

Unlock Deck
Unlock for access to all 40 flashcards in this deck.
Unlock Deck
k this deck
34
In the electrolysis of molten NaCl, the substance liberated at the positive electrode is
A)Cl-.
B)Cl2.
C)Na+.
D)Na.
A)Cl-.
B)Cl2.
C)Na+.
D)Na.

Unlock Deck
Unlock for access to all 40 flashcards in this deck.
Unlock Deck
k this deck
35
When a gas reaction involves an increase in the number of molecules present, the yield can be increased by
A)increasing the pressure.
B)decreasing the pressure.
C)increasing the temperature.
D)decreasing the temperature.
A)increasing the pressure.
B)decreasing the pressure.
C)increasing the temperature.
D)decreasing the temperature.

Unlock Deck
Unlock for access to all 40 flashcards in this deck.
Unlock Deck
k this deck
36
Oxidation-reduction reactions are not involved in
A)electrolysis.
B)acid-base neutralization.
C)the operation of a storage battery.
D)the operation of a fuel cell.
A)electrolysis.
B)acid-base neutralization.
C)the operation of a storage battery.
D)the operation of a fuel cell.

Unlock Deck
Unlock for access to all 40 flashcards in this deck.
Unlock Deck
k this deck
37
The atomic mass of N is 14 u and that of Ca is 40 u.The proportion of N by mass in the compound Ca3N2 is
A)19 percent.
B)35 percent.
C)40 percent.
D)81 percent.
A)19 percent.
B)35 percent.
C)40 percent.
D)81 percent.

Unlock Deck
Unlock for access to all 40 flashcards in this deck.
Unlock Deck
k this deck
38
When a substance gains electrons in a chemical reaction, the substance is said to be
A)reduced.
B)oxidized.
C)dissociated.
D)activated.
A)reduced.
B)oxidized.
C)dissociated.
D)activated.

Unlock Deck
Unlock for access to all 40 flashcards in this deck.
Unlock Deck
k this deck
39
The number of moles of H2O formed when 1 mole of O2 reacts with C5H12 in the reaction C5H12 + 8O2 5CO2 + 6H2O is
A)3/4.
B)4/3.
C)6.
D)8.
A)3/4.
B)4/3.
C)6.
D)8.

Unlock Deck
Unlock for access to all 40 flashcards in this deck.
Unlock Deck
k this deck
40
The number of moles of O2 needed to react with 10 moles of C2H2 in the reaction 2C2 H2 + 5O2 4CO2 + 2 H2O is
A)5.
B)10.
C)25.
D)50.
A)5.
B)10.
C)25.
D)50.

Unlock Deck
Unlock for access to all 40 flashcards in this deck.
Unlock Deck
k this deck


