Deck 14: The Chemistry of Solutes and Solutions
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/57
Play
Full screen (f)
Deck 14: The Chemistry of Solutes and Solutions
1
Several processes contribute to the dissolving of a solid salt such as NaCl(s) in water. A large exothermic contribution is supplied by the
A) entropy.
B) heat of mixing.
C) hydration energy.
D) lattice energy.
E) solubilization.
A) entropy.
B) heat of mixing.
C) hydration energy.
D) lattice energy.
E) solubilization.
hydration energy.
2
A solution with solute concentration less than the solubility is said to be ____; ____ solute will dissolve in such as solution.
A) undersaturated; more
B) undersaturated; no more
C) saturated; more
D) saturated; no more
E) supersaturated; more
A) undersaturated; more
B) undersaturated; no more
C) saturated; more
D) saturated; no more
E) supersaturated; more
undersaturated; more
3
The ____ of a substance is defined as the maximum amount that dissolves in a given amount of solvent at a specified temperature.
A) concentration
B) solubility
C) density
D) molarity
E) lattice energy
A) concentration
B) solubility
C) density
D) molarity
E) lattice energy
solubility
4
Starting with a saturated solution of sodium chloride in water at room temperature, adding solid sodium chloride will
A) increase the concentration of the solution
B) decrease the concentration of the solution
C) increase the anion concentration and decrease the cation concentration of the solution
D) decrease the anion concentration and increase the cation concentration of the solution
E) have no effect on the concentration of the solution
A) increase the concentration of the solution
B) decrease the concentration of the solution
C) increase the anion concentration and decrease the cation concentration of the solution
D) decrease the anion concentration and increase the cation concentration of the solution
E) have no effect on the concentration of the solution

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
5
Two liquids which mix together in all proportions are said to be ____; they mix because ____.
A) miscible; their intermolecular interactions are dissimilar
B) miscible; their intermolecular interactions are similar
C) miscible; their densities are dissimilar
D) immiscible; their intermolecular interactions are similar
E) immiscible; their intermolecular interactions are dissimilar
A) miscible; their intermolecular interactions are dissimilar
B) miscible; their intermolecular interactions are similar
C) miscible; their densities are dissimilar
D) immiscible; their intermolecular interactions are similar
E) immiscible; their intermolecular interactions are dissimilar

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
6
A solution with solute concentration equal to the solubility is said to be ____; ____ solute will dissolve in such a solution.
A) undersaturated; more
B) undersaturated; no more
C) saturated; more
D) saturated; no more
E) supersaturated; more
A) undersaturated; more
B) undersaturated; no more
C) saturated; more
D) saturated; no more
E) supersaturated; more

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
7
Which of the following represents a solution?
A) anesthesia gas
B) carbonated water
C) stainless steel
D) ocean water
E) all of these
A) anesthesia gas
B) carbonated water
C) stainless steel
D) ocean water
E) all of these

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
8
Salts containing only doubly or triply charged ions are often insoluble in water. This could be because
A) the ions are highly charged, and a large number of water molecules is organized around them, so DS is negative.
B) the ions are highly charged, hence the lattice energy is very large, and DH therefore tends to be negative.
C) the ions are very large, hence the lattice energy is very large, and DH is positive.
D) both a and b.
E) both a and c.
A) the ions are highly charged, and a large number of water molecules is organized around them, so DS is negative.
B) the ions are highly charged, hence the lattice energy is very large, and DH therefore tends to be negative.
C) the ions are very large, hence the lattice energy is very large, and DH is positive.
D) both a and b.
E) both a and c.

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
9
Ethyl alcohol and water dissolve in each other in all proportions. Therefore,
A) ethyl alcohol is miscible with water
B) ethyl alcohol is immiscible with water
C) water is miscible with ethyl alcohol
D) water is immiscible with ethyl alcohol
E) both a and c are correct
A) ethyl alcohol is miscible with water
B) ethyl alcohol is immiscible with water
C) water is miscible with ethyl alcohol
D) water is immiscible with ethyl alcohol
E) both a and c are correct

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
10
The overall enthalpy change during the process of forming a solution involves three terms: separating solvent molecules (DH solvent), separating solute particles (DH solute), and forming new solute-solvent associations (DH solution). The overall process of forming a solution is endothermic if
A) |DH solute + DH solvent| > |DH solution|.
B) |DH solute + DH solvent| = |DH solution|.
C) |DH solute + DH solvent| < |DH solution|.
D) DH solute + DH solvent = 0.
E) DH solution = 0.
A) |DH solute + DH solvent| > |DH solution|.
B) |DH solute + DH solvent| = |DH solution|.
C) |DH solute + DH solvent| < |DH solution|.
D) DH solute + DH solvent = 0.
E) DH solution = 0.

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
11
Solutions form when efficient intermolecular forces can form between solvent molecules and solute molecules or ions. What kind of intermolecular forces could be responsible?
A) dipole-dipole
B) ion-dipole
C) London
D) hydrogen bonds
E) any of a, b, c, or d
A) dipole-dipole
B) ion-dipole
C) London
D) hydrogen bonds
E) any of a, b, c, or d

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
12
When a solution is supersaturated the concentration of the solute is ____ the solubility, and ____ solute will dissolve.
A) less than; more
B) equal to; no more
C) greater than; more
D) less than; no more
E) greater than; no more
A) less than; more
B) equal to; no more
C) greater than; more
D) less than; no more
E) greater than; no more

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
13
If two liquids are miscible, they ____ because ____.
A) mix together; their intermolecular interactions are similar
B) do not mix together; their intermolecular interactions are similar
C) do not mix together; their intermolecular interactions are dissimilar
D) mix together; their intermolecular interactions are dissimilar
E) do not mix together; their densities are dissimilar
A) mix together; their intermolecular interactions are similar
B) do not mix together; their intermolecular interactions are similar
C) do not mix together; their intermolecular interactions are dissimilar
D) mix together; their intermolecular interactions are dissimilar
E) do not mix together; their densities are dissimilar

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
14
The process of dissolving is favored if the ____ interactions are weaker than the ____ interactions.
A) solute-solvent; solute-solute and solvent-solvent
B) solvent-solvent; solute-solute and solute-solvent
C) solute-solute and solvent-solvent; solute-solvent
D) solute-solvent and solvent-solvent; solute-solute
E) solute-solute; solute-solvent and solvent-solvent
A) solute-solvent; solute-solute and solvent-solvent
B) solvent-solvent; solute-solute and solute-solvent
C) solute-solute and solvent-solvent; solute-solvent
D) solute-solvent and solvent-solvent; solute-solute
E) solute-solute; solute-solvent and solvent-solvent

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
15
If a liquid is miscible with water, some part of the molecule is ____ and therefore ____.
A) nonpolar; hydrophobic
B) nonpolar; hydrophilic
C) bipolar; insoluble
D) polar; hydrophilic
E) polar; hydrophobic
A) nonpolar; hydrophobic
B) nonpolar; hydrophilic
C) bipolar; insoluble
D) polar; hydrophilic
E) polar; hydrophobic

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
16
The process of forming a solution occurs in three parts: separating solvent molecules, separating solute particles, and forming new solute-solvent associations. These three processes are, respectively,
A) endothermic, endothermic, and endothermic.
B) endothermic, endothermic, and exothermic.
C) endothermic, exothermic, and endothermic.
D) exothermic, exothermic, and endothermic.
E) exothermic, exothermic, and exothermic.
A) endothermic, endothermic, and endothermic.
B) endothermic, endothermic, and exothermic.
C) endothermic, exothermic, and endothermic.
D) exothermic, exothermic, and endothermic.
E) exothermic, exothermic, and exothermic.

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
17
Which of the following represents a type of solution?
A) gas in gas
B) gas in liquid
C) solid in solid
D) solid in liquid
E) all of these
A) gas in gas
B) gas in liquid
C) solid in solid
D) solid in liquid
E) all of these

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
18
Which of the following is correct?
A) substances with similar noncovalent forces are likely to be soluble in each other
B) solutes do not readily dissolve in solvents whose noncovalent forces are quite different from their own
C) weaker solute-solvent attractions favor solubility
D) stronger solute-solute and stronger solvent-solvent attractions reduce solubility
E) a, b and d are correct
A) substances with similar noncovalent forces are likely to be soluble in each other
B) solutes do not readily dissolve in solvents whose noncovalent forces are quite different from their own
C) weaker solute-solvent attractions favor solubility
D) stronger solute-solute and stronger solvent-solvent attractions reduce solubility
E) a, b and d are correct

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
19
Thermal pollution affects fish and other marine wildlife by
A) increasing the growth of marine plants.
B) increasing the uptake of toxic metals.
C) raising the body temperature of fish to a dangerous level.
D) decreasing the solubility of oxygen in water.
E) hindering the growth of marine plants.
A) increasing the growth of marine plants.
B) increasing the uptake of toxic metals.
C) raising the body temperature of fish to a dangerous level.
D) decreasing the solubility of oxygen in water.
E) hindering the growth of marine plants.

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
20
Sand is insoluble in both polar and nonpolar solvents because
A) the interactions between the particles of sand are much stronger than the interactions between nonpolar molecules.
B) the interactions between the particles of sand are much weaker than the interactions between polar molecules.
C) the interactions between the particles of sand are much weaker than the interactions between nonpolar molecules.
D) the interactions between the particles of sand are much stronger than the interactions between polar molecules.
E) its atoms are held together by a three-dimensional network of strong covalent bonds.
A) the interactions between the particles of sand are much stronger than the interactions between nonpolar molecules.
B) the interactions between the particles of sand are much weaker than the interactions between polar molecules.
C) the interactions between the particles of sand are much weaker than the interactions between nonpolar molecules.
D) the interactions between the particles of sand are much stronger than the interactions between polar molecules.
E) its atoms are held together by a three-dimensional network of strong covalent bonds.

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
21
Molarity (M) is defined as moles of solute per ____ of ____.
A) mole; solution
B) kg; solvent
C) kg; solution
D) L; solvent
E) L; solution
A) mole; solution
B) kg; solvent
C) kg; solution
D) L; solvent
E) L; solution

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
22
Calculate the concentration in ppm of Cd in a 2.00 mL sample which has been shown to contain 16.4 mg Cd. Assume the sample has a density of 1.00 g/mL.
A) 8.20 × 10-3
B) 8.20
C) 32.8
D) 8.20 × 103
E) 3.28 × 104
A) 8.20 × 10-3
B) 8.20
C) 32.8
D) 8.20 × 103
E) 3.28 × 104

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
23
Which units for concentration are not defined in terms of the mass or volume of any substance?
A) molarity
B) mole fraction
C) molality
D) parts per million
E) weight %
A) molarity
B) mole fraction
C) molality
D) parts per million
E) weight %

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
24
At a particular temperature, as the pressure increases the solubility of a gas in water will
A) always decrease
B) always increase
C) remain the same
D) be inversely proportional to the pressure
E) be directly proportional to the temperature
A) always decrease
B) always increase
C) remain the same
D) be inversely proportional to the pressure
E) be directly proportional to the temperature

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
25
Molality (m) is defined as moles of solute per ____ of ____.
A) mole; solution
B) kg; solvent
C) kg; solution
D) L; solvent
E) L; solution
A) mole; solution
B) kg; solvent
C) kg; solution
D) L; solvent
E) L; solution

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
26
For inorganic compounds, solubility in water is
A) always increased by an increase in temperature
B) always decreased by an increase in temperature
C) usually decreased by an increase in temperature
D) usually increased by an increase in temperature
E) not affected by a change in temperature
A) always increased by an increase in temperature
B) always decreased by an increase in temperature
C) usually decreased by an increase in temperature
D) usually increased by an increase in temperature
E) not affected by a change in temperature

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
27
Which of the following refers to a specific value of concentration at a given temperature?
A) saturated
B) unsaturated
C) supersaturated
D) concentrated
E) immiscible
A) saturated
B) unsaturated
C) supersaturated
D) concentrated
E) immiscible

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
28
Assuming that its density is 1.000 g/mL, what is the mass % concentration of a 0.1432 M AgNO3 solution?
A) 2.864 %
B) 2.433 %
C) 2.204 %
D) 1.974 %
E) 1.432 %
A) 2.864 %
B) 2.433 %
C) 2.204 %
D) 1.974 %
E) 1.432 %

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
29
The concentration unit one part per billion (one ppb) is equivalent to one ____ of solute per ____ of solution.
A) g
B) mm
C) mg
D) kg
A) g
B) mm
C) mg
D) kg

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
30
Increasing the temperature of water containing a dissolved gas will almost always
A) decrease the solubility of the gas
B) increase the solubility of the gas
C) have no effect on the solubility of the gas
D) decrease the solubility of the gas only if the gas is one that naturally occurs in the atmosphere
E) increase the solubility of the gas only if the gas is one that naturally occurs in the atmosphere
A) decrease the solubility of the gas
B) increase the solubility of the gas
C) have no effect on the solubility of the gas
D) decrease the solubility of the gas only if the gas is one that naturally occurs in the atmosphere
E) increase the solubility of the gas only if the gas is one that naturally occurs in the atmosphere

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
31
Determine the chloride ion concentration in a solution containing 50.0 g MgCl2 dissolved in 1500 mL of solution.
A) 0.350 M
B) 0.700 M
C) 1.40 M
D) 3.17 M
E) 6.34 M
A) 0.350 M
B) 0.700 M
C) 1.40 M
D) 3.17 M
E) 6.34 M

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
32
Isotonic saline solution has a mass % NaCl concentration of 0.900 %. Assuming its density is 1.00 g/mL, what is its molarity?
A) 9.00 × 10-3 M
B) 0.0900 M
C) 0.154 M
D) 0.900 M
E) 1.80 M
A) 9.00 × 10-3 M
B) 0.0900 M
C) 0.154 M
D) 0.900 M
E) 1.80 M

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
33
Which unit of concentration is dependent on temperature?
A) mole%
B) ppm
C) molality
D) molarity
E) mass%
A) mole%
B) ppm
C) molality
D) molarity
E) mass%

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
34
If 750. mL of a certain solution contains 50.0 g Na2SO4, the sodium ion concentration, [Na+], is
A) 0.264 M
B) 0.315 M
C) 0.469 M
D) 0.560 M
E) 0.939 M
A) 0.264 M
B) 0.315 M
C) 0.469 M
D) 0.560 M
E) 0.939 M

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
35
The pressure inside a soda bottle is two atmospheres (1520 mm Hg). If exactly half this pressure is generated by CO2, what is the solubility of carbon dioxide in a 2 L bottle of root beer at 40 C? The Henry's Law constant for CO2 is 4.45 × 10-5 M/mm Hg.
A) 2.93 × 10-8 M
B) 9.90 × 10-5 M
C) 1.78 × 10-3 M
D) 0.0338 M
E) 0.0676 M
A) 2.93 × 10-8 M
B) 9.90 × 10-5 M
C) 1.78 × 10-3 M
D) 0.0338 M
E) 0.0676 M

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
36
When a soda bottle is opened, there is rapid bubbling of gas; the solubility of a gas in a liquid decreases if the pressure of the gas is decreased. This is because
A) the pressure decrease is always accompanied by a temperature decrease.
B) a increase in temperature is caused by the pressure decrease.
C) the probability of collisions between the gas and the liquid surface is decreased.
D) the energy released from the bubbling process cools the gas.
E) the volume of the system must decrease if the pressure decreases.
A) the pressure decrease is always accompanied by a temperature decrease.
B) a increase in temperature is caused by the pressure decrease.
C) the probability of collisions between the gas and the liquid surface is decreased.
D) the energy released from the bubbling process cools the gas.
E) the volume of the system must decrease if the pressure decreases.

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
37
To convert from ____ to ____, one must multiply by ____.
A) parts per million; parts per billion; 10-3
B) parts per thousand; parts per billion; 103
C) parts per million; weight percent; 10-4
D) parts per billion; parts per trillion; 106
E) parts per thousand; weight percent; 10
A) parts per million; parts per billion; 10-3
B) parts per thousand; parts per billion; 103
C) parts per million; weight percent; 10-4
D) parts per billion; parts per trillion; 106
E) parts per thousand; weight percent; 10

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
38
The solubility of a gas in a liquid is ____ the pressure of the gas; this is ____.
A) proportional to; Henry's Law
B) inversely proportional to; Henry's Law
C) proportional to; Raoult's Law
D) inversely proportional to; Raoult's Law
E) proportional to the square of; Raoult's Law
A) proportional to; Henry's Law
B) inversely proportional to; Henry's Law
C) proportional to; Raoult's Law
D) inversely proportional to; Raoult's Law
E) proportional to the square of; Raoult's Law

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
39
A concentrated antifreeze solution contains 580. g ethylene glycol mixed with 540. g of water. The weight percent of water in this solution is
A) 27.0 %
B) 48.2 %
C) 51.8 %
D) 93.1 %
E) 107 %
A) 27.0 %
B) 48.2 %
C) 51.8 %
D) 93.1 %
E) 107 %

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
40
If 50. mL of solution containing 2.0 × 103 ppm sodium ion is diluted to 4.0 L, what is the final concentration of sodium ion?
A) 2.5 ppm
B) 2.5 × 104 ppb
C) 2.5%
D) 2.5 mg/L
E) all of these are correct
A) 2.5 ppm
B) 2.5 × 104 ppb
C) 2.5%
D) 2.5 mg/L
E) all of these are correct

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
41
In order to calculate the molality of a solution from its molarity, what other information would be needed?
A) molar mass of solute and molar mass of solvent
B) molar mass of solute and density of solvent
C) molar mass of solute and density of solution
D) molar mass of solvent and density of solution
E) molar mass of solvent and density of solute
A) molar mass of solute and molar mass of solvent
B) molar mass of solute and density of solvent
C) molar mass of solute and density of solution
D) molar mass of solvent and density of solution
E) molar mass of solvent and density of solute

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
42
The osmotic pressure of a solution of Mg(NO3)2 at a given temperature is ____ that of a solution of NaNO3 of identical concentration at the same temperature.
A) half
B) two-thirds
C) the same as
D) one and a half times
E) twice
A) half
B) two-thirds
C) the same as
D) one and a half times
E) twice

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
43
Explain what is meant by
a. osmosis.
b. osmotic pressure.
c. reverse osmosis.
a. osmosis.
b. osmotic pressure.
c. reverse osmosis.

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
44
Osmotic pressure is the
A) pressure that must be applied to a solution in order to prevent osmosis from the pure solvent.
B) pressure that must be applied to a solution in order to cause osmosis to occur from the pure solvent.
C) correction factor applied when a sample of gas is collected by water displacement.
D) increase in vapor pressure when a solution is compared to a pure solvent.
E) decrease in vapor pressure when a solution is compared to a pure solvent.
A) pressure that must be applied to a solution in order to prevent osmosis from the pure solvent.
B) pressure that must be applied to a solution in order to cause osmosis to occur from the pure solvent.
C) correction factor applied when a sample of gas is collected by water displacement.
D) increase in vapor pressure when a solution is compared to a pure solvent.
E) decrease in vapor pressure when a solution is compared to a pure solvent.

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
45
A coolant consisting of a 50/50 mixture by volume of ethylene glycol and water utilizes the
A) colligative properties of solutions
B) freezing point elevation constant of water
C) boiling point elevation constant of water
D) boiling point depression constant of water
E) a and c
A) colligative properties of solutions
B) freezing point elevation constant of water
C) boiling point elevation constant of water
D) boiling point depression constant of water
E) a and c

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
46
Given that the value of kf for water is 1.86 C/m, what is the freezing point of an aqueous solution of NaCl prepared by mixing 427.0 g solute with 1.000 kg of water?
A) -4.36 C
B) -7.94 C
C) -13.6 C
D) -15.7 C
E) -18.3 C
A) -4.36 C
B) -7.94 C
C) -13.6 C
D) -15.7 C
E) -18.3 C

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
47
Drinkable water can be produced from seawater by the process of ____, using a membrane which is permeable to ____ but not to ____.
A) osmosis; water; ions
B) reverse osmosis; water; ions
C) reverse osmosis; sodium ions; chloride ions
D) osmosis; ions; water
E) reverse osmosis; ions; water
A) osmosis; water; ions
B) reverse osmosis; water; ions
C) reverse osmosis; sodium ions; chloride ions
D) osmosis; ions; water
E) reverse osmosis; ions; water

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
48
The freezing points of the following aqueous solutions, from highest to lowest, are: 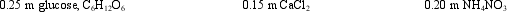
A) C6H12O6 > NH4NO3 > CaCl2
B) C6H12O6 > CaCl2 > NH4NO3
C) CaCl2 > C6H12O6 > NH4NO3
D) CaCl2 > NH4NO3 > C6H12O6
E) NH4NO3 > C6H12O6 > CaCl2
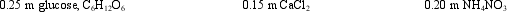
A) C6H12O6 > NH4NO3 > CaCl2
B) C6H12O6 > CaCl2 > NH4NO3
C) CaCl2 > C6H12O6 > NH4NO3
D) CaCl2 > NH4NO3 > C6H12O6
E) NH4NO3 > C6H12O6 > CaCl2

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
49
The driving force for osmosis is
A) temperature.
B) pressure.
C) identity of the solute.
D) enthalpy.
E) entropy.
A) temperature.
B) pressure.
C) identity of the solute.
D) enthalpy.
E) entropy.

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
50
In living systems, a solution with a higher solute concentration than the surrounding cells is said to be ____. This causes water to flow ____ the cells by osmosis.
A) hypertonic; into
B) hypertonic; out of
C) catatonic; into
D) hypotonic; into
E) hypotonic; out of
A) hypertonic; into
B) hypertonic; out of
C) catatonic; into
D) hypotonic; into
E) hypotonic; out of

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
51
What is the vapor pressure of a 45.0 % solution of glucose, C6H12O6, at 90.0 C, given that the vapor pressure of pure water at that temperature is 526 mm Hg?
A) 486 mm Hg
B) 289 mmHg
C) 237 mm Hg
D) 78.2 mm Hg
E) 39.8 mm Hg
A) 486 mm Hg
B) 289 mmHg
C) 237 mm Hg
D) 78.2 mm Hg
E) 39.8 mm Hg

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
52
A 0.1 M solution of Na2SO4 contains
A) 9.6 × 103 mg/L SO42-
B) 4.6 × 106 ppb Na+
C) 14.2. g/L sodium sulfate
D) 0.3 mol/L ions
E) all of these are correct
A) 9.6 × 103 mg/L SO42-
B) 4.6 × 106 ppb Na+
C) 14.2. g/L sodium sulfate
D) 0.3 mol/L ions
E) all of these are correct

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
53
Consider these three primary alcohols:
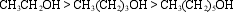 The three alcohols have similar molecular structures. However, their solubilities in water decrease dramatically, in the order shown. Explain this.
The three alcohols have similar molecular structures. However, their solubilities in water decrease dramatically, in the order shown. Explain this.
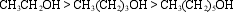 The three alcohols have similar molecular structures. However, their solubilities in water decrease dramatically, in the order shown. Explain this.
The three alcohols have similar molecular structures. However, their solubilities in water decrease dramatically, in the order shown. Explain this.
Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
54
The solubility in water of magnesium fluoride, MgF2, is 7.3 mg/100 mL. Is a solution containing 33 mg MgF2 in 475 mL water unsaturated, saturated, or supersaturated? Show a calculation to support your answer.

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
55
Calculate the boiling point of a mixture where 95.0 g of formic acid, H2CO2, is dissolved in 250 g of acetic acid. Acetic acid has a Kb of 2.93 C/m and boils at 118 C. Assume that formic acid does not ionize when dissolved in acetic acid, and that formic acid is non-volatile.
A) 118 C
B) 142 C
C) 136 C
D) 115 C
E) 126 C
A) 118 C
B) 142 C
C) 136 C
D) 115 C
E) 126 C

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
56
An aqueous solution of phosphoric acid, H3PO4, contains 285 g H3PO4 in 400 mL solution, and has a density of 1.35 g/mL. Calculate
a. the weight %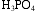 in this solution.
in this solution.
b. The concentration in mol/L of this solution.
a. the weight %
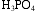 in this solution.
in this solution.b. The concentration in mol/L of this solution.

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck
57
An aqueous solution at 27 C contains 3.60 g of a protein in a 200. mL sample. The osmotic pressure is 0.0203 atm. What is the molar mass of the protein?
A) 21.8 g/mol
B) 78.6 g/mol
C) 8.70 × 102 g/mol
D) 2.03 × 103 g/mol
E) 2.18 × 104 g/mol
A) 21.8 g/mol
B) 78.6 g/mol
C) 8.70 × 102 g/mol
D) 2.03 × 103 g/mol
E) 2.18 × 104 g/mol

Unlock Deck
Unlock for access to all 57 flashcards in this deck.
Unlock Deck
k this deck


