Deck 6: Metabolism: Energy and Enzymes
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/55
Play
Full screen (f)
Deck 6: Metabolism: Energy and Enzymes
1
A living organism represents stored energy in the form of chemical compounds.When an organism dies,what happens to this stored energy?
A)All chemicals immediately lose their high-energy bonds.
B)All molecules immediately degrade into basic elements.
C)All energy immediately leaves,and that is one manifestation that the organism is dead.
D)The chemical compounds in cells lose their organization over time because there is no longer an input of energy to maintain the organized state.
E)The chemical compounds remain exactly intact and ready to start up again unless digested by a consumer or decay organism.
A)All chemicals immediately lose their high-energy bonds.
B)All molecules immediately degrade into basic elements.
C)All energy immediately leaves,and that is one manifestation that the organism is dead.
D)The chemical compounds in cells lose their organization over time because there is no longer an input of energy to maintain the organized state.
E)The chemical compounds remain exactly intact and ready to start up again unless digested by a consumer or decay organism.
D
2
Endergonic reactions:
A)release energy.
B)have a negative G and occur spontaneously.
C)can only occur if there is an input of energy.
D)have products with less free energy than the reactants.
E)All of the choices are correct.
A)release energy.
B)have a negative G and occur spontaneously.
C)can only occur if there is an input of energy.
D)have products with less free energy than the reactants.
E)All of the choices are correct.
can only occur if there is an input of energy.
3
Which form of energy is NOT correctly associated with the related example?
A)kinetic energy: fat molecules
B)kinetic energy: movement of muscles
C)chemical energy: glucose
D)potential energy: water held behind a dam
E)potential energy: ATP
A)kinetic energy: fat molecules
B)kinetic energy: movement of muscles
C)chemical energy: glucose
D)potential energy: water held behind a dam
E)potential energy: ATP
A
4
ATP is considered a high-energy compound because under cellular conditions,7.3 kcal per mole of energy is released when a bond is broken between:
A)the base adenine and the sugar ribose.
B)the adenosine and the phosphate groups.
C)the base adenine and the phosphate groups.
D)the adenosine diphosphate and the third phosphate.
E)All of the bonds release energy as ATP is completely broken down.
A)the base adenine and the sugar ribose.
B)the adenosine and the phosphate groups.
C)the base adenine and the phosphate groups.
D)the adenosine diphosphate and the third phosphate.
E)All of the bonds release energy as ATP is completely broken down.

Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
5
Which of these statements is NOT a consequence of the second law of thermodynamics?
A)While the total amount of energy is unchanged,the energy lost as heat is no longer useful to the cell in doing work.
B)Reactions that occur spontaneously are those that increase the amount of useful energy in a system.
C)The amount of disorder in the universe is always increasing.
D)To maintain organization of a cell,a continual input of energy is required.
A)While the total amount of energy is unchanged,the energy lost as heat is no longer useful to the cell in doing work.
B)Reactions that occur spontaneously are those that increase the amount of useful energy in a system.
C)The amount of disorder in the universe is always increasing.
D)To maintain organization of a cell,a continual input of energy is required.

Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
6
Which statement is NOT true about how various conditions will effect the activity of an enzyme?
A)Higher temperatures generally increase the activity of an enzyme up to a point.
B)Above a certain range of temperatures,the protein of an enzyme is denatured.
C)A change in pH can cause an enzyme to be inactivated.
D)An enzyme's activity is generally reduced by an increase in substrate concentration.
E)When sufficient substrate is available,the active site will nearly always be occupied.
A)Higher temperatures generally increase the activity of an enzyme up to a point.
B)Above a certain range of temperatures,the protein of an enzyme is denatured.
C)A change in pH can cause an enzyme to be inactivated.
D)An enzyme's activity is generally reduced by an increase in substrate concentration.
E)When sufficient substrate is available,the active site will nearly always be occupied.

Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
7
Which best describes the first law of thermodynamics?
A)Energy is changed from one form to another with a loss of usable energy.
B)Energy is not created nor destroyed,but it can change from one energy form to another.
C)Energy can be created from matter or used to produce matter.
D)Some useful energy is lost as heat whenever an energy transfer occurs.
E)Energy transfers are always 100% efficient in changing energy from one useful form to another.
A)Energy is changed from one form to another with a loss of usable energy.
B)Energy is not created nor destroyed,but it can change from one energy form to another.
C)Energy can be created from matter or used to produce matter.
D)Some useful energy is lost as heat whenever an energy transfer occurs.
E)Energy transfers are always 100% efficient in changing energy from one useful form to another.

Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
8
Which statement is NOT true about enzyme inhibition?
A)In competitive inhibition,the inhibitor binds to the active site of the enzyme.
B)In noncompetitive inhibition,the inhibitor binds to the allosteric site of the substrate.
C)In irreversible inhibition,a poison binds to the enzyme so that it can never work again.
D)Most inhibitors act in a reversible fashion.
E)All of the statements are true.
A)In competitive inhibition,the inhibitor binds to the active site of the enzyme.
B)In noncompetitive inhibition,the inhibitor binds to the allosteric site of the substrate.
C)In irreversible inhibition,a poison binds to the enzyme so that it can never work again.
D)Most inhibitors act in a reversible fashion.
E)All of the statements are true.

Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
9
All of the biochemical pathways in a cell constitute
A)coupling reactions.
B)free energy.
C)endergonic reactions only.
D)exergonic reactions only.
E)metabolism.
A)coupling reactions.
B)free energy.
C)endergonic reactions only.
D)exergonic reactions only.
E)metabolism.

Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
10
A coenzyme is
A)an ionic cofactor that interacts with an enzyme to allow it to work.
B)a protein cofactor that interacts with an enzyme to allow it to work.
C)a nonprotein organic cofactor that interacts with an enzyme to allow it to work.
D)an ionic cofactor that interacts with an enzyme to inhibit it.
E)a protein cofactor that interacts with an enzyme to inhibit it.
A)an ionic cofactor that interacts with an enzyme to allow it to work.
B)a protein cofactor that interacts with an enzyme to allow it to work.
C)a nonprotein organic cofactor that interacts with an enzyme to allow it to work.
D)an ionic cofactor that interacts with an enzyme to inhibit it.
E)a protein cofactor that interacts with an enzyme to inhibit it.

Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
11
Which of the following is NOT a form of potential energy?
A)food
B)water in a dam
C)a muscle contracting
D)All of the choices are not potential energy.
A)food
B)water in a dam
C)a muscle contracting
D)All of the choices are not potential energy.

Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
12
The activity of an enzyme might be increased by all of the following except ________.
A)increase in substrate concentration
B)a vitamin
C)a 2-4 degree increase in temperature
D)the presence of lead
A)increase in substrate concentration
B)a vitamin
C)a 2-4 degree increase in temperature
D)the presence of lead

Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
13
ATP is considered to be
A)an enzyme used widely in all kinds of cells.
B)a coenzyme used to inhibit or activate different enzymes.
C)a molecule that carries a great deal of chemical energy in a chemical bond.
D)the precursor of a high-energy membrane-bounded protein.
A)an enzyme used widely in all kinds of cells.
B)a coenzyme used to inhibit or activate different enzymes.
C)a molecule that carries a great deal of chemical energy in a chemical bond.
D)the precursor of a high-energy membrane-bounded protein.

Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
14
Which statement describes the currently accepted theory of how an enzyme and its substrate fit together?
A)As the product is released,the enzyme breaks down.
B)The enzyme is like a key that fits into the substrate,which is like a lock.
C)The active site is permanently changed by its interaction with the substrate.
D)As the substrate binds to the enzyme,the shape of the active site changes to accommodate the reaction.
A)As the product is released,the enzyme breaks down.
B)The enzyme is like a key that fits into the substrate,which is like a lock.
C)The active site is permanently changed by its interaction with the substrate.
D)As the substrate binds to the enzyme,the shape of the active site changes to accommodate the reaction.

Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
15
Energy coupling of endergonic and exergonic reactions within cells
A)permits biological reactions to proceed at temperatures consistent with life.
B)uses heat released by one reaction to fuel the other reaction.
C)utilizes ATP to carry energy between the exergonic and endergonic reactions.
D)All of the choices are correct.
A)permits biological reactions to proceed at temperatures consistent with life.
B)uses heat released by one reaction to fuel the other reaction.
C)utilizes ATP to carry energy between the exergonic and endergonic reactions.
D)All of the choices are correct.

Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
16
The subunits from which ATP is made are:
A)ADP and phosphate.
B)FAD and NAD+.
C)FAD and NADPH.
D)ADP and FAD.
E)ADP and NAD+.
A)ADP and phosphate.
B)FAD and NAD+.
C)FAD and NADPH.
D)ADP and FAD.
E)ADP and NAD+.

Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
17
Coupling occurs when the energy released by an exergonic reaction is:
A)used to drive another exergonic reaction.
B)used to drive an endergonic reaction.
C)lost as nonusable heat to the environment.
D)used to decrease the entropy of the universe.
E)All of the choices are correct.
A)used to drive another exergonic reaction.
B)used to drive an endergonic reaction.
C)lost as nonusable heat to the environment.
D)used to decrease the entropy of the universe.
E)All of the choices are correct.

Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
18
Which best describes the second law of thermodynamics?
A)Energy is not created nor destroyed,but it can change into matter.
B)Energy is not created nor destroyed,but it can change from one energy form to another.
C)Energy can be created from matter or used to produce matter.
D)Some useful energy is lost as heat whenever an energy transfer occurs.
E)Energy transfers are always 100% efficient in changing energy from one useful form to another.
A)Energy is not created nor destroyed,but it can change into matter.
B)Energy is not created nor destroyed,but it can change from one energy form to another.
C)Energy can be created from matter or used to produce matter.
D)Some useful energy is lost as heat whenever an energy transfer occurs.
E)Energy transfers are always 100% efficient in changing energy from one useful form to another.

Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
19
Which organelles contain functioning ATP synthetase complexes in their membranes?
A)Golgi complexes and lysosomes.
B)Mitochondria and chloroplasts.
C)Endoplasmic reticulum and vesicles.
D)Vacuoles and vesicles.
E)Mitochondria and endoplasmic reticulum.
A)Golgi complexes and lysosomes.
B)Mitochondria and chloroplasts.
C)Endoplasmic reticulum and vesicles.
D)Vacuoles and vesicles.
E)Mitochondria and endoplasmic reticulum.

Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
20
What establishes the electrochemical gradient across a membrane to provide energy for ATP production?
A)The chloroplast's electron transport system provides the ions.
B)Hydrogen ions naturally collect on the outside of the organelle membrane.
C)Hydrogen ions are pumped across the membrane by carrier proteins of the electron transport chain.
D)All of the choices establish the electrochemical gradient.
A)The chloroplast's electron transport system provides the ions.
B)Hydrogen ions naturally collect on the outside of the organelle membrane.
C)Hydrogen ions are pumped across the membrane by carrier proteins of the electron transport chain.
D)All of the choices establish the electrochemical gradient.

Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
21
In this reaction,the reactant (s)are _____________ and the coenzyme NAD is ____________. 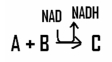
A)reduced; oxidized
B)oxidized; oxidized
C)reduced; reduced
D)oxidized; reduced

A)reduced; oxidized
B)oxidized; oxidized
C)reduced; reduced
D)oxidized; reduced

Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
22
Study the graph at right.The letter 'B ' depicts the _____,while 'C' depicts ________. 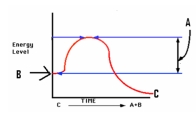
A)Energy of Activation; Energy of products
B)Energy of products; Energy of Activation
C)Energy of reactants; Energy of products
D)Reactant concentration; Activation Energy

A)Energy of Activation; Energy of products
B)Energy of products; Energy of Activation
C)Energy of reactants; Energy of products
D)Reactant concentration; Activation Energy

Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
23
Of the following,which process will not denature a protein?
A)heating to temperatures above 100 C
B)addition of strong acids or strong bases
C)phosphorylation
D)addition of distilled water
A)heating to temperatures above 100 C
B)addition of strong acids or strong bases
C)phosphorylation
D)addition of distilled water

Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
24
In this pathway,B C is coupled with ADP ATP.Categorize the reactions as endergonic or exergonic. 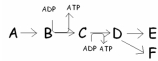
A)B C is endergonic and ADP
ATP is exergonic.
B)ADP ATP is endergonic and B
C is exergonic.
C)Both B C and ADP
ATP are endergonic.
D)Both B C and ADP
ATP are exergonic.

A)B C is endergonic and ADP
ATP is exergonic.
B)ADP ATP is endergonic and B
C is exergonic.
C)Both B C and ADP
ATP are endergonic.
D)Both B C and ADP
ATP are exergonic.

Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
25
Astrophysicists explain that eventually the sun will swell to become a red giant,engulf the earth and "burn out" with all forms of energy dispersing in a final "heat death." Compared with conditions today,the entropy of the universe then will
A)have increased greatly.
B)have decreased greatly.
C)remain the same because energy cannot be created or destroyed.
A)have increased greatly.
B)have decreased greatly.
C)remain the same because energy cannot be created or destroyed.

Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
26
__________ represents the first reactant of this metabolic pathway and ________ represents the endproduct (s)of the pathway? 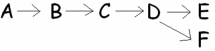
A)A; E
B)B; E and F
C)A; E and F
D)A and B; D and E
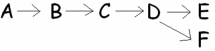
A)A; E
B)B; E and F
C)A; E and F
D)A and B; D and E

Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
27
If a change in pH alters an allosteric site where an inhibitor binds,but doesn't change the active site for the intended substrate,it would be possible for an enzymatically controlled reaction to occur as normal.

Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
28
If there are twelve different intermediate products produced in the stages of a metabolic pathway within a cell,we can expect that there
A)is one enzyme that carries this process through to the end product.
B)is one enzyme for degradation and another enzyme for synthesis.
C)may not be any enzymes involved if this is a natural cell product.
D)must be twelve different raw materials combined in the cell by one enzyme.
E)are about twelve enzymes,at least one responsible for each step in the metabolic pathway.
A)is one enzyme that carries this process through to the end product.
B)is one enzyme for degradation and another enzyme for synthesis.
C)may not be any enzymes involved if this is a natural cell product.
D)must be twelve different raw materials combined in the cell by one enzyme.
E)are about twelve enzymes,at least one responsible for each step in the metabolic pathway.

Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
29
While eating a container of yogurt,you have to leave,so you store the yogurt in the refrigerator.A day later you return and find the surface of the yogurt is no longer smooth but has broken into several liquified products.You correctly guess that enzymes from your saliva,via the spoon,have continued digesting the yogurt in your absence.What will happen over time?
A)The reaction will soon stop because the amount of saliva is small,and you would have to add more saliva to continue the degradation.
B)The reaction will continue,since the enzyme is not denatured by the reaction.
C)The reaction will continue until half is digested and then stop because the reaction between substrate and product will be balanced.
D)Absolutely no degradation of the yogurt will occur naturally unless in the presence of this enzyme. In
A)The reaction will soon stop because the amount of saliva is small,and you would have to add more saliva to continue the degradation.
B)The reaction will continue,since the enzyme is not denatured by the reaction.
C)The reaction will continue until half is digested and then stop because the reaction between substrate and product will be balanced.
D)Absolutely no degradation of the yogurt will occur naturally unless in the presence of this enzyme. In

Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
30
An automobile engine is about 20 - 30% efficient in converting chemical energy to mechanical energy.Cells are about 39% efficient in the transformation of glucose to ATP.The rest of the energy is lost as heat.This is illustrative of the:
A)First Law of Thermodynamics.
B)Second Law of Thermodynamics.
C)Third Law of Thermodynamics.
D)The Cell Theory.
A)First Law of Thermodynamics.
B)Second Law of Thermodynamics.
C)Third Law of Thermodynamics.
D)The Cell Theory.

Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
31
In order to roll a rock down a hillside,you must first push it up out of the hole in which it rests.Pushing the rock is analogous to the energy of activation of a chemical reaction.

Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
32
If the reaction graphed at right is coupled with D E,then D E is: 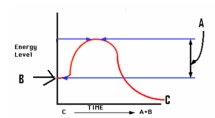
A)exergonic
B)endergonic
C)spontaneous
D)none of the above

A)exergonic
B)endergonic
C)spontaneous
D)none of the above

Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
33
In the electron transport systems of chloroplasts and mitochondria,
A)the system consists of a series of membrane bound carriers that transfer electrons from one carrier to another.
B)high energy electrons enter the system and low energy electrons exit the system.
C)energy release occurs when the electron transfers from one carrier to another.
D)All of the choices are correct.
A)the system consists of a series of membrane bound carriers that transfer electrons from one carrier to another.
B)high energy electrons enter the system and low energy electrons exit the system.
C)energy release occurs when the electron transfers from one carrier to another.
D)All of the choices are correct.

Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
34
Which of the following is consistent with the laws of physics governing energy?
A)When a liter of gasoline is burned in a car engine,100% of its energy goes into moving the car along the road.
B)You eat a "quarter-pounder" hamburger and assemble exactly a quarter-pound of additional body weight on your body.
C)Eventually sunlight that is absorbed on the earth returns to space as dispersed heat.
D)A calorie of sunlight becomes a calorie of plant tissue that,eaten by you,becomes a calorie of heat lost in muscle "power."
E)Chemical bonds are a case of converting energy to matter; breaking the bonds converts matter to energy.
A)When a liter of gasoline is burned in a car engine,100% of its energy goes into moving the car along the road.
B)You eat a "quarter-pounder" hamburger and assemble exactly a quarter-pound of additional body weight on your body.
C)Eventually sunlight that is absorbed on the earth returns to space as dispersed heat.
D)A calorie of sunlight becomes a calorie of plant tissue that,eaten by you,becomes a calorie of heat lost in muscle "power."
E)Chemical bonds are a case of converting energy to matter; breaking the bonds converts matter to energy.

Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
35
While science is not yet able to describe the phenomenon of "thinking" in physical terms,we can be certain that it is a process involving the metabolism of brain cells.With positron emission tomography (PET scan)it is possible to inject short-lived isotopes and image the regions of the brain that have the most active metabolism during various mental activities.For different mental functions,different regions and amounts of nerve cells become active.However,
A)the cellular energy expended in "thinking" must be less than the chemical bond energy supplied in food to these brain cells.
B)"thought" cannot be linked to cell processes because energy is not related to matter.
C)since thoughts can occur over and over,the requirement for a continual input of energy to prevent entropy does not apply to this cell activity.
D)"thinking" is beyond the scope of science to study.
A)the cellular energy expended in "thinking" must be less than the chemical bond energy supplied in food to these brain cells.
B)"thought" cannot be linked to cell processes because energy is not related to matter.
C)since thoughts can occur over and over,the requirement for a continual input of energy to prevent entropy does not apply to this cell activity.
D)"thinking" is beyond the scope of science to study.

Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
36
Which of the following labels is incorrectly identified. 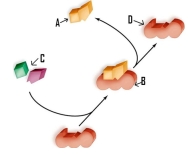
A)A refers to the products.
B)B refers to the substrate-enzyme complex.
C)C refers to the reactants.
D)D refers to the product.

A)A refers to the products.
B)B refers to the substrate-enzyme complex.
C)C refers to the reactants.
D)D refers to the product.

Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
37
The metabolic pathway at right involves how many possible chemical reactions? 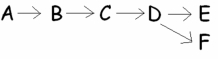
A)five
B)six
C)one
D)three

A)five
B)six
C)one
D)three

Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
38
Lactose is milk sugar,and humans produce substantial lactase enzyme to digest it when we are infants.However,we soon lose some or even all of our lactase after childhood.In such cases,undigested lactose passes to the lower intestine where bacteria break it down into lactic acid and CO2,causing painful gas and bloating.This problem could be avoided by
A)avoiding all dairy products containing lactose.
B)taking lactase enzyme tablets when consuming lactose products.
C)taking any enzyme tablets when consuming dairy products.
D)consuming lactose in tablet form.
E)Both taking lactase enzyme and avoiding all dairy products would be correct.
A)avoiding all dairy products containing lactose.
B)taking lactase enzyme tablets when consuming lactose products.
C)taking any enzyme tablets when consuming dairy products.
D)consuming lactose in tablet form.
E)Both taking lactase enzyme and avoiding all dairy products would be correct.

Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
39
Identify the graph that depicts the reaction C
A)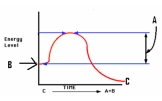
B)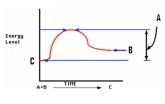
C)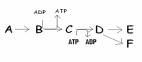
A)

B)

C)


Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
40
Study the figure at right.What does the letter A depict? 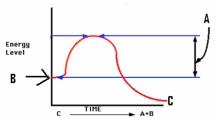
A)Energy of the reactant
B)Energy of the products
C)Energy of activation
D)Substrate concentration

A)Energy of the reactant
B)Energy of the products
C)Energy of activation
D)Substrate concentration

Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
41
Feedback inhibition is the process that turns off an enzyme in a metabolic pathway as the result of inhibitory actions of a product of the pathway.

Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
42
The energy for ATP synthesis in chemiosmotic phosphorylation comes from the movement of hydrogen ions across a membrane down a concentration gradient.

Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
43
If an organism runs photosynthesis for its main form of energy,the removal of what substrate would limit it's growth?
A)6H2O
B)C6H12O6
C)6O2
D)6CO-
A)6H2O
B)C6H12O6
C)6O2
D)6CO-

Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
44
While we are sitting down to lunch we are consuming _______ energy which will then be converted into ______ energy as we work until dinner time.
A)potential,kinetic
B)kinetic,potential
C)kinetic,free
D)potential,stored
A)potential,kinetic
B)kinetic,potential
C)kinetic,free
D)potential,stored

Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
45
Which of the following substrates are required to run cellular respiration?
A)glucose & oxygen
B)glucose & carbon dioxide
C)sunlight & oxygen
D)energy & glucose
A)glucose & oxygen
B)glucose & carbon dioxide
C)sunlight & oxygen
D)energy & glucose

Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
46
An enzyme is a globular protein that inhibits the formation of chemical bonds within the enzyme's substrate(s)causing the rate of the reaction to slow down.

Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
47
During the conversion of glucose into a free form of energy only a small percentage is converted into useable ATP.What is the rest of the energy converted into?
A)heat
B)CO2
C)H2O
D)CO-
A)heat
B)CO2
C)H2O
D)CO-

Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
48
During oxidation NAD+ will accept ______ but only _______.
A)2 electrons,1 hydrogen
B)1 electron,2 hydrogens
C)2 electrons,2 hydrogens
D)1 electron,1 hydrogen
A)2 electrons,1 hydrogen
B)1 electron,2 hydrogens
C)2 electrons,2 hydrogens
D)1 electron,1 hydrogen

Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
49
What is the coenzyme used during the cellular respiration pathway to produce oxidation?
A)NAD+
B)NAD-
C)NADP+
D)CO_
A)NAD+
B)NAD-
C)NADP+
D)CO_

Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
50
What are the end products of photosynthesis?
A)glucose & oxygen
B)glucose & ATP
C)carbon dioxide & energy
D)carbon dioxide & water
A)glucose & oxygen
B)glucose & ATP
C)carbon dioxide & energy
D)carbon dioxide & water

Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
51
Which of the following statements about high energy electrons and NADH is correct?
A)NAD+ will capture two electrons and one H+ in order to make NADH.
B)NAD+ will capture one electron and two H+ in order to make NADH.
C)NAD+ will capture 2 electrons and one H+ in order to make NADPH.
D)NAD+ does not require electrons or H+ in order to make NADH.
A)NAD+ will capture two electrons and one H+ in order to make NADH.
B)NAD+ will capture one electron and two H+ in order to make NADH.
C)NAD+ will capture 2 electrons and one H+ in order to make NADPH.
D)NAD+ does not require electrons or H+ in order to make NADH.

Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
52
Which of the following is an example of potential energy?
A)a snickers bar
B)an apple growing on a tree
C)a glass of milk
D)all are examples of potential energy
A)a snickers bar
B)an apple growing on a tree
C)a glass of milk
D)all are examples of potential energy

Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
53
Identify the missing substrate in the reaction that will oxidize NAD+.NAD+ + 2e + _____ = NADH
A)H+
B)3H+
C)OH_
D)2H+
A)H+
B)3H+
C)OH_
D)2H+

Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
54
Which substrate is missing: 6CO2 + ______ + energy = C6H12O6 + 6O2
A)6H2O
B)4H2O
C)4O2
D)glucose
A)6H2O
B)4H2O
C)4O2
D)glucose

Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck
55
Which of the following conditions is in defiance of the Second Law of Thermodynamics?
A)As our body converts glucose into ATP some of the energy is given off in the form of heat.
B)The temperature of your car's engine begins to increase as you drive to work.
C)When we are cold our body shivers as a response to the decrease in body temperature.
D)None of these are in defiance of the Second Law of Thermodynamics.
A)As our body converts glucose into ATP some of the energy is given off in the form of heat.
B)The temperature of your car's engine begins to increase as you drive to work.
C)When we are cold our body shivers as a response to the decrease in body temperature.
D)None of these are in defiance of the Second Law of Thermodynamics.

Unlock Deck
Unlock for access to all 55 flashcards in this deck.
Unlock Deck
k this deck


