Deck 4: Reactions in Aqueous Solution
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/156
Play
Full screen (f)
Deck 4: Reactions in Aqueous Solution
1
Based on the solubility rules, which one of the following compounds should be insoluble in water?
A)NaCl
B)MgBr2
C)FeCl2
D)AgBr
E)ZnCl2
A)NaCl
B)MgBr2
C)FeCl2
D)AgBr
E)ZnCl2
AgBr
2
Which of the following compounds is a strong electrolyte?
A)H2O
B)CH3OH
C)CH3CH2OH
D)HF
E)NaF
A)H2O
B)CH3OH
C)CH3CH2OH
D)HF
E)NaF
NaF
3
Based on the solubility rules, which one of the following should be soluble in water?
A)Hg2Cl2
B)Na2S
C)Ag2CO3
D)Ag2S
E)BaSO4
A)Hg2Cl2
B)Na2S
C)Ag2CO3
D)Ag2S
E)BaSO4
Na2S
4
Based on the solubility rules, which one of the following should be soluble in water?
A)AgBr
B)AgCl
C)Ag2CO3
D)AgNO3
E)Ag2S
A)AgBr
B)AgCl
C)Ag2CO3
D)AgNO3
E)Ag2S

Unlock Deck
Unlock for access to all 156 flashcards in this deck.
Unlock Deck
k this deck
5
Based on the solubility rules, which of the following will occur when solutions of ZnSO4(aq)and MgCl2(aq)are mixed?
A)ZnCl2 will precipitate; Mg2+ and SO42- will be spectator ions.
B)ZnSO4 will precipitate; Mg2+ and Cl- will be spectator ions.
C)MgSO4 will precipitate; Zn2+ and Cl- will be spectator ions.
D)MgCl2 will precipitate; Zn2+ and SO42- will be spectator ions.
E)No precipitate will form.
A)ZnCl2 will precipitate; Mg2+ and SO42- will be spectator ions.
B)ZnSO4 will precipitate; Mg2+ and Cl- will be spectator ions.
C)MgSO4 will precipitate; Zn2+ and Cl- will be spectator ions.
D)MgCl2 will precipitate; Zn2+ and SO42- will be spectator ions.
E)No precipitate will form.

Unlock Deck
Unlock for access to all 156 flashcards in this deck.
Unlock Deck
k this deck
6
Which of the following compounds is a weak electrolyte?
A)HNO3
B)NaNO3
C)HNO2
D)NaNO2
E)NaOH
A)HNO3
B)NaNO3
C)HNO2
D)NaNO2
E)NaOH

Unlock Deck
Unlock for access to all 156 flashcards in this deck.
Unlock Deck
k this deck
7
Which of the following compounds is a nonelectrolyte?
A)NaF
B)HNO3
C)CH3COOH (acetic acid)
D)NaOH
E)C6H12O6 (glucose)
A)NaF
B)HNO3
C)CH3COOH (acetic acid)
D)NaOH
E)C6H12O6 (glucose)

Unlock Deck
Unlock for access to all 156 flashcards in this deck.
Unlock Deck
k this deck
8
The distinguishing characteristic of all electrolyte solutions is that they
A)contain molecules.
B)conduct electricity.
C)react with other solutions.
D)always contain acids.
E)conduct heat.
A)contain molecules.
B)conduct electricity.
C)react with other solutions.
D)always contain acids.
E)conduct heat.

Unlock Deck
Unlock for access to all 156 flashcards in this deck.
Unlock Deck
k this deck
9
Identify the major ionic species present in an aqueous solution of Na2CO3.
A)Na2+, CO32-
B)Na2+, C2 -, O3
C)Na+, C4+, O32-
D)Na+, C+, O2-
E)Na+, CO32-
A)Na2+, CO32-
B)Na2+, C2 -, O3
C)Na+, C4+, O32-
D)Na+, C+, O2-
E)Na+, CO32-

Unlock Deck
Unlock for access to all 156 flashcards in this deck.
Unlock Deck
k this deck
10
Based on the solubility rules, which one of the following should be soluble in water?
A)CaSO4
B)BaSO4
C)PbSO4
D)K2SO4
E)AgCl
A)CaSO4
B)BaSO4
C)PbSO4
D)K2SO4
E)AgCl

Unlock Deck
Unlock for access to all 156 flashcards in this deck.
Unlock Deck
k this deck
11
Based on the solubility rules, which one of the following compounds should be insoluble in water?
A)CaCO3
B)(NH4)2CO3
C)Na2CO3
D)K2CO3
E)KNO3
A)CaCO3
B)(NH4)2CO3
C)Na2CO3
D)K2CO3
E)KNO3

Unlock Deck
Unlock for access to all 156 flashcards in this deck.
Unlock Deck
k this deck
12
Based on the solubility rules, which one of the following compounds should be insoluble in water?
A)Na2SO4
B)BaSO4
C)CuSO4
D)MgSO4
E)Rb2SO4
A)Na2SO4
B)BaSO4
C)CuSO4
D)MgSO4
E)Rb2SO4

Unlock Deck
Unlock for access to all 156 flashcards in this deck.
Unlock Deck
k this deck
13
Identify the major ionic species present in an aqueous solution of K2SO4.
A)K2+, S6+, O48-
B)K2+, S6+, 4O2-
C)2K+, S6+, O48-
D)2K+, S6+, 4O2-
E)2K+, SO42-
A)K2+, S6+, O48-
B)K2+, S6+, 4O2-
C)2K+, S6+, O48-
D)2K+, S6+, 4O2-
E)2K+, SO42-

Unlock Deck
Unlock for access to all 156 flashcards in this deck.
Unlock Deck
k this deck
14
Based on the solubility rules, which one of the following should be soluble in water?
A)(NH4)3PO4
B)Ca3(PO4)2
C)AlPO4
D)Ag3PO4
E)Mg3(PO4)2
A)(NH4)3PO4
B)Ca3(PO4)2
C)AlPO4
D)Ag3PO4
E)Mg3(PO4)2

Unlock Deck
Unlock for access to all 156 flashcards in this deck.
Unlock Deck
k this deck
15
Based on the solubility rules, which of the following will occur if solutions of CuSO4(aq)and BaCl2(aq)are mixed?
A)CuCl2 will precipitate; Ba2+ and SO42 - are spectator ions.
B)CuSO4 will precipitate; Ba2+ and Cl - are spectator ions.
C)BaSO4 will precipitate; Cu2+ and Cl- are spectator ions.
D)BaCl2 will precipitate; Cu2+ and SO42 - are spectator ions.
E)No precipitate will form.
A)CuCl2 will precipitate; Ba2+ and SO42 - are spectator ions.
B)CuSO4 will precipitate; Ba2+ and Cl - are spectator ions.
C)BaSO4 will precipitate; Cu2+ and Cl- are spectator ions.
D)BaCl2 will precipitate; Cu2+ and SO42 - are spectator ions.
E)No precipitate will form.

Unlock Deck
Unlock for access to all 156 flashcards in this deck.
Unlock Deck
k this deck
16
Which of the following compounds is a nonelectrolyte?
A)NaOH
B)HNO3
C)C2H6O (ethanol)
D)KF
E)CH3COOH (acetic acid)
A)NaOH
B)HNO3
C)C2H6O (ethanol)
D)KF
E)CH3COOH (acetic acid)

Unlock Deck
Unlock for access to all 156 flashcards in this deck.
Unlock Deck
k this deck
17
Which of the following compounds is a weak electrolyte?
A)HCl
B)CH3COOH (acetic acid)
C)C6H12O6 (glucose)
D)O2
E)NaCl
A)HCl
B)CH3COOH (acetic acid)
C)C6H12O6 (glucose)
D)O2
E)NaCl

Unlock Deck
Unlock for access to all 156 flashcards in this deck.
Unlock Deck
k this deck
18
Based on the solubility rules, which of the following will occur when a solution containing about 0.1 g of Pb(NO3)2(aq)is mixed with a solution containing 0.1 g of KI(aq)/100 mL?
A)KNO3 will precipitate; Pb2+ and I- are spectator ions.
B)No precipitate will form.
C)Pb(NO3)2 will precipitate; K+ and I- are spectator ions.
D)PbI2 will precipitate; K+ and NO3- are spectator ions.
E)Pb2+ and I- are spectator ions, and PbI2 will precipitate.
A)KNO3 will precipitate; Pb2+ and I- are spectator ions.
B)No precipitate will form.
C)Pb(NO3)2 will precipitate; K+ and I- are spectator ions.
D)PbI2 will precipitate; K+ and NO3- are spectator ions.
E)Pb2+ and I- are spectator ions, and PbI2 will precipitate.

Unlock Deck
Unlock for access to all 156 flashcards in this deck.
Unlock Deck
k this deck
19
Which of the following compounds is a weak electrolyte?
A)HCl
B)NH3
C)C6H12O6 (glucose)
D)N2
E)KCl
A)HCl
B)NH3
C)C6H12O6 (glucose)
D)N2
E)KCl

Unlock Deck
Unlock for access to all 156 flashcards in this deck.
Unlock Deck
k this deck
20
Which of the following compounds is a strong electrolyte?
A)H2O
B)N2
C)CH3COOH (acetic acid)
D)CH3CH2OH (ethanol)
E)KOH
A)H2O
B)N2
C)CH3COOH (acetic acid)
D)CH3CH2OH (ethanol)
E)KOH

Unlock Deck
Unlock for access to all 156 flashcards in this deck.
Unlock Deck
k this deck
21
The oxidation number of Mn in KMnO4 is
A)+8.
B)+7.
C)+5.
D)-7.
E)-8.
A)+8.
B)+7.
C)+5.
D)-7.
E)-8.

Unlock Deck
Unlock for access to all 156 flashcards in this deck.
Unlock Deck
k this deck
22
The oxidation number of Cr in Cr2O72- is
A)-12.
B)-7.
C)-2.
D)+6.
E)+7.
A)-12.
B)-7.
C)-2.
D)+6.
E)+7.

Unlock Deck
Unlock for access to all 156 flashcards in this deck.
Unlock Deck
k this deck
23
For which one of the following acids is chlorine in the +5 oxidation state?
A)HCl
B)HClO
C)HClO2
D)HClO3
E)HClO4
A)HCl
B)HClO
C)HClO2
D)HClO3
E)HClO4

Unlock Deck
Unlock for access to all 156 flashcards in this deck.
Unlock Deck
k this deck
24
The oxidation number of Fe in K3Fe(CN)6 is
A)+3.
B)+2.
C)+1.
D)-3.
E)-4.
A)+3.
B)+2.
C)+1.
D)-3.
E)-4.

Unlock Deck
Unlock for access to all 156 flashcards in this deck.
Unlock Deck
k this deck
25
What is the chemical formula of the salt produced by the neutralization of hydrobromic acid with magnesium hydroxide?
A)MgBr
B)Mg2Br3
C)Mg3Br2
D)Mg2Br
E)MgBr2
A)MgBr
B)Mg2Br3
C)Mg3Br2
D)Mg2Br
E)MgBr2

Unlock Deck
Unlock for access to all 156 flashcards in this deck.
Unlock Deck
k this deck
26
What is the chemical formula of the salt produced by the complete neutralization of sodium hydroxide with sulfuric acid?
A)Na2SO4
B)Na2(SO4)3
C)Na(SO4)2
D)NaSO3
E)Na3SO4
A)Na2SO4
B)Na2(SO4)3
C)Na(SO4)2
D)NaSO3
E)Na3SO4

Unlock Deck
Unlock for access to all 156 flashcards in this deck.
Unlock Deck
k this deck
27
What is the correct formula of the salt formed in the neutralization reaction of hydrochloric acid with calcium hydroxide?
A)CaO
B)CaCl2
C)CaH2
D)CaCl
E)CaClH
A)CaO
B)CaCl2
C)CaH2
D)CaCl
E)CaClH

Unlock Deck
Unlock for access to all 156 flashcards in this deck.
Unlock Deck
k this deck
28
Which of the following compounds is a weak acid?
A)HF
B)HCl
C)HBr
D)HI
E)HClO4
A)HF
B)HCl
C)HBr
D)HI
E)HClO4

Unlock Deck
Unlock for access to all 156 flashcards in this deck.
Unlock Deck
k this deck
29
The oxidation number of N in NaNO3 is
A)+6.
B)+5.
C)+3.
D)-3.
E)None of the above.
A)+6.
B)+5.
C)+3.
D)-3.
E)None of the above.

Unlock Deck
Unlock for access to all 156 flashcards in this deck.
Unlock Deck
k this deck
30
What is the chemical formula of the salt produced by the neutralization of potassium hydroxide with sulfuric acid?
A)KSO3
B)K2(SO4)3
C)K2SO4
D)K(SO4)2
E)KSO4
A)KSO3
B)K2(SO4)3
C)K2SO4
D)K(SO4)2
E)KSO4

Unlock Deck
Unlock for access to all 156 flashcards in this deck.
Unlock Deck
k this deck
31
The highest possible oxidation number of nitrogen is
A)+8.
B)+5.
C)+3.
D)+1.
E)-3.
A)+8.
B)+5.
C)+3.
D)+1.
E)-3.

Unlock Deck
Unlock for access to all 156 flashcards in this deck.
Unlock Deck
k this deck
32
Identify the major ionic species present in an aqueous solution of H2SO4.
A)S6+, O36- (plus H2O as a neutral species)
B)H+, OH-, S6+, 3O2-
C)2H+, S6+, 4O2-
D)H+, HSO4-
E)2H+, SO42-
A)S6+, O36- (plus H2O as a neutral species)
B)H+, OH-, S6+, 3O2-
C)2H+, S6+, 4O2-
D)H+, HSO4-
E)2H+, SO42-

Unlock Deck
Unlock for access to all 156 flashcards in this deck.
Unlock Deck
k this deck
33
Which of the following is the correct net ionic equation for the reaction that occurs when solutions of Pb(NO3)2 and NH4Cl are mixed?
A)Pb(NO3)2(aq)+ 2NH4Cl(aq) NH4NO3(aq)+ PbCl2(s)
B)Pb2+(aq)+ 2Cl-(aq) PbCl2(s)
C)Pb2+(aq)+ 2NO3- (aq)+ 2NH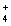 (aq)+ 2Cl-(aq) 2NH
(aq)+ 2Cl-(aq) 2NH 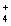 (aq)+ 2NO3- (aq)+ PbCl2(s)
(aq)+ 2NO3- (aq)+ PbCl2(s)
D)NH4+(aq)+ NO3- (aq) 2NH4NO3(s)
E)No reaction occurs when the solutions are mixed.
A)Pb(NO3)2(aq)+ 2NH4Cl(aq) NH4NO3(aq)+ PbCl2(s)
B)Pb2+(aq)+ 2Cl-(aq) PbCl2(s)
C)Pb2+(aq)+ 2NO3- (aq)+ 2NH
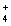 (aq)+ 2Cl-(aq) 2NH
(aq)+ 2Cl-(aq) 2NH 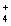 (aq)+ 2NO3- (aq)+ PbCl2(s)
(aq)+ 2NO3- (aq)+ PbCl2(s)D)NH4+(aq)+ NO3- (aq) 2NH4NO3(s)
E)No reaction occurs when the solutions are mixed.

Unlock Deck
Unlock for access to all 156 flashcards in this deck.
Unlock Deck
k this deck
34
Identify the major ions present in an aqueous LiOH solution.
A)Li2+, O- , H-
B)Li+, OH-
C)LiO-, H+
D)Li+, O2- , H+
E)Li- , OH+
A)Li2+, O- , H-
B)Li+, OH-
C)LiO-, H+
D)Li+, O2- , H+
E)Li- , OH+

Unlock Deck
Unlock for access to all 156 flashcards in this deck.
Unlock Deck
k this deck
35
The oxidation number of S in K2SO4 is
A)+6.
B)+4.
C)+2.
D)-1.
E)None of the above.
A)+6.
B)+4.
C)+2.
D)-1.
E)None of the above.

Unlock Deck
Unlock for access to all 156 flashcards in this deck.
Unlock Deck
k this deck
36
The oxidation number of Cl in ClO3- is
A)-1.
B)+7.
C)+5.
D)+3.
E)None of the above.
A)-1.
B)+7.
C)+5.
D)+3.
E)None of the above.

Unlock Deck
Unlock for access to all 156 flashcards in this deck.
Unlock Deck
k this deck
37
What is the chemical formula of the salt produced by the neutralization of nitric acid with calcium hydroxide?
A)CaNO3
B)Ca2(NO3)3
C)Ca3(NO3)2
D)Ca2NO3
E)Ca(NO3)2
A)CaNO3
B)Ca2(NO3)3
C)Ca3(NO3)2
D)Ca2NO3
E)Ca(NO3)2

Unlock Deck
Unlock for access to all 156 flashcards in this deck.
Unlock Deck
k this deck
38
The oxidation number of Cl in ClO4- is
A)-1.
B)+1.
C)+3.
D)+5.
E)None of the above.
A)-1.
B)+1.
C)+3.
D)+5.
E)None of the above.

Unlock Deck
Unlock for access to all 156 flashcards in this deck.
Unlock Deck
k this deck
39
Identify the major ions present in an aqueous HNO3 solution.
A)HN+, O2-
B)OH- , NO3-
C)OH- , NO
D)H+, N3-, O2-
E)H+, NO3-
A)HN+, O2-
B)OH- , NO3-
C)OH- , NO
D)H+, N3-, O2-
E)H+, NO3-

Unlock Deck
Unlock for access to all 156 flashcards in this deck.
Unlock Deck
k this deck
40
The common constituent in all acid solutions is
A)H2.
B)H+.
C)OH-.
D)H2SO4.
E)Cl-.
A)H2.
B)H+.
C)OH-.
D)H2SO4.
E)Cl-.

Unlock Deck
Unlock for access to all 156 flashcards in this deck.
Unlock Deck
k this deck
41
Select the compound in which sulfur has its highest possible oxidation number.
A)H2S
B)SO2
C)SCl2
D)H2SO3
E)Na2SO4
A)H2S
B)SO2
C)SCl2
D)H2SO3
E)Na2SO4

Unlock Deck
Unlock for access to all 156 flashcards in this deck.
Unlock Deck
k this deck
42
What element is reduced in the following chemical reaction?
Cu + 2H2SO4 CuSO4 + SO2 + 2H2O
A)Cu
B)H
C)S
D)O
E)H2O
Cu + 2H2SO4 CuSO4 + SO2 + 2H2O
A)Cu
B)H
C)S
D)O
E)H2O

Unlock Deck
Unlock for access to all 156 flashcards in this deck.
Unlock Deck
k this deck
43
The highest possible oxidation number of carbon is
A)+8.
B)+6.
C)+4.
D)+2.
E)-4.
A)+8.
B)+6.
C)+4.
D)+2.
E)-4.

Unlock Deck
Unlock for access to all 156 flashcards in this deck.
Unlock Deck
k this deck
44
In the following chemical reaction the oxidizing agent is:
5S + 6KNO3 + 2CaCO3 3K2SO4 + 2CaSO4 + CO2 + 3N2
A)S
B)N2
C)KNO3
D)CaSO4
E)CaCO3
5S + 6KNO3 + 2CaCO3 3K2SO4 + 2CaSO4 + CO2 + 3N2
A)S
B)N2
C)KNO3
D)CaSO4
E)CaCO3

Unlock Deck
Unlock for access to all 156 flashcards in this deck.
Unlock Deck
k this deck
45
The oxidation number of N in N2H4 is
A)+4.
B)-4.
C)+2.
D)-2.
E)0.
A)+4.
B)-4.
C)+2.
D)-2.
E)0.

Unlock Deck
Unlock for access to all 156 flashcards in this deck.
Unlock Deck
k this deck
46
Which of the following is an example of a disproportionation reaction?
A)2C2H6(g)+ 7O2(g) 4CO2(g)+ 6H2O(l)
B)2KBr(aq)+ Cl2(g) 2KCl(aq)+ Br2(l)
C)2H2O2(aq) 2H2O(l)+ O2(g)
D)CaBr2(aq)+ H2SO4(aq) CaSO4(s)+ 2HBr(g)
E)2Al(s)+ 3H2SO4(aq) Al2(SO4)3(aq)+ 3H2(g)
A)2C2H6(g)+ 7O2(g) 4CO2(g)+ 6H2O(l)
B)2KBr(aq)+ Cl2(g) 2KCl(aq)+ Br2(l)
C)2H2O2(aq) 2H2O(l)+ O2(g)
D)CaBr2(aq)+ H2SO4(aq) CaSO4(s)+ 2HBr(g)
E)2Al(s)+ 3H2SO4(aq) Al2(SO4)3(aq)+ 3H2(g)

Unlock Deck
Unlock for access to all 156 flashcards in this deck.
Unlock Deck
k this deck
47
What element is oxidized in the following chemical reaction?
H2SO4 + Cd(OH)2 2H2O + CdSO4
A)H
B)S
C)O
D)Cd
E)this is not a redox reaction
H2SO4 + Cd(OH)2 2H2O + CdSO4
A)H
B)S
C)O
D)Cd
E)this is not a redox reaction

Unlock Deck
Unlock for access to all 156 flashcards in this deck.
Unlock Deck
k this deck
48
Which of the following equations does not represent an oxidation-reduction reaction?
A)3Al + 6HCl 3H2 + AlCl3
B)2H2O 2H2 + O2
C)2NaCl + Pb(NO3)2 PbCl2 + 3NaNO3
D)2NaI + Br2 2NaBr + I2
E)Cu(NO3)2 + Zn Zn(NO3)2 + Cu
A)3Al + 6HCl 3H2 + AlCl3
B)2H2O 2H2 + O2
C)2NaCl + Pb(NO3)2 PbCl2 + 3NaNO3
D)2NaI + Br2 2NaBr + I2
E)Cu(NO3)2 + Zn Zn(NO3)2 + Cu

Unlock Deck
Unlock for access to all 156 flashcards in this deck.
Unlock Deck
k this deck
49
Identify the oxidizing agent in the following chemical reaction.
2MnO4- + 5H2SO3 2Mn2+ + 5SO42- + 4H+ + 3H2O
A)MnO4-
B)H2SO3
C)Mn2+
D)SO42-
E)H+
2MnO4- + 5H2SO3 2Mn2+ + 5SO42- + 4H+ + 3H2O
A)MnO4-
B)H2SO3
C)Mn2+
D)SO42-
E)H+

Unlock Deck
Unlock for access to all 156 flashcards in this deck.
Unlock Deck
k this deck
50
In the following chemical reaction the oxidizing agent is:
5H2O2 + 2MnO4- + 6H+ 2Mn2+ + 8H2O + 5O2
A)H2O2
B)MnO4-
C)H+
D)Mn2+
E)O2
5H2O2 + 2MnO4- + 6H+ 2Mn2+ + 8H2O + 5O2
A)H2O2
B)MnO4-
C)H+
D)Mn2+
E)O2

Unlock Deck
Unlock for access to all 156 flashcards in this deck.
Unlock Deck
k this deck
51
What element is oxidized in the following chemical reaction?
NiO2 + Cd + 2H2O Ni(OH)2 + Cd(OH)2
A)Ni
B)Cd
C)O
D)H
E)This is not a redox reaction.
NiO2 + Cd + 2H2O Ni(OH)2 + Cd(OH)2
A)Ni
B)Cd
C)O
D)H
E)This is not a redox reaction.

Unlock Deck
Unlock for access to all 156 flashcards in this deck.
Unlock Deck
k this deck
52
In the following redox reaction 4NH3 + 3Ca(ClO)2 2N2 + 6H2O + 3CaCl2
Which element is oxidized and which is reduced?
A)H is oxidized and N is reduced
B)N is oxidized and Cl is reduced
C)N is oxidized and O is reduced
D)Cl is oxidized and O is reduced
E)Cl is oxidized and N is reduced
Which element is oxidized and which is reduced?
A)H is oxidized and N is reduced
B)N is oxidized and Cl is reduced
C)N is oxidized and O is reduced
D)Cl is oxidized and O is reduced
E)Cl is oxidized and N is reduced

Unlock Deck
Unlock for access to all 156 flashcards in this deck.
Unlock Deck
k this deck
53
Which choice gives the correct oxidation numbers for all three elements in Ca(ClO)2 in the order that the elements are shown in the formula?
A)+2, +1, -2
B)+2, -2, +1
C)+2, -3, +2
D)-2, +2, -1
E)-2, +3, -2
A)+2, +1, -2
B)+2, -2, +1
C)+2, -3, +2
D)-2, +2, -1
E)-2, +3, -2

Unlock Deck
Unlock for access to all 156 flashcards in this deck.
Unlock Deck
k this deck
54
Identify the elements that are oxidized and reduced in the following reaction. KClO3(aq)+ 6HBr(aq) KCl(aq)+ 3Br2(l)+ 3H2O(l)
A)Br is oxidized and Cl is reduced
B)Cl is oxidized and H is reduced
C)H is oxidized and O is reduced
D)O is oxidized and Cl is reduced
E)Cl is oxidized and Br is reduced
A)Br is oxidized and Cl is reduced
B)Cl is oxidized and H is reduced
C)H is oxidized and O is reduced
D)O is oxidized and Cl is reduced
E)Cl is oxidized and Br is reduced

Unlock Deck
Unlock for access to all 156 flashcards in this deck.
Unlock Deck
k this deck
55
Predict the products of the following single replacement reaction.
Fe(s)+ CuSO4(aq)
A)Cu(s)+ FeSO4(aq)
B)Fe(s)+ Cu(s)+ SO4(aq)
C)CuS(s)+ Fe2SO4(aq)
D)FeCuSO4(aq)
E)FeO(s)+ CuSO3(aq)
Fe(s)+ CuSO4(aq)
A)Cu(s)+ FeSO4(aq)
B)Fe(s)+ Cu(s)+ SO4(aq)
C)CuS(s)+ Fe2SO4(aq)
D)FeCuSO4(aq)
E)FeO(s)+ CuSO3(aq)

Unlock Deck
Unlock for access to all 156 flashcards in this deck.
Unlock Deck
k this deck
56
Which one of the following is a redox reaction?
A)2Al(s)+ 3H2SO4(aq) Al2(SO4)3(aq)+ 3H2(g)
B)2KBr(aq)+ Pb(NO3)2(aq) 2KNO3(aq)+ PbBr2(s)
C)CaBr2(aq)+ H2SO4(aq) CaSO4(s)+ 2HBr(g)
D)H+(aq)+ OH- (aq) H2O(l)
E)CO32- (aq)+ HSO4-(aq) HCO3- (aq)+ SO42- (aq)
A)2Al(s)+ 3H2SO4(aq) Al2(SO4)3(aq)+ 3H2(g)
B)2KBr(aq)+ Pb(NO3)2(aq) 2KNO3(aq)+ PbBr2(s)
C)CaBr2(aq)+ H2SO4(aq) CaSO4(s)+ 2HBr(g)
D)H+(aq)+ OH- (aq) H2O(l)
E)CO32- (aq)+ HSO4-(aq) HCO3- (aq)+ SO42- (aq)

Unlock Deck
Unlock for access to all 156 flashcards in this deck.
Unlock Deck
k this deck
57
What element is oxidized in the following chemical reaction?
3Cu + 8HNO3 3Cu(NO3)2 + 2NO + 4H2O
A)Cu
B)H
C)N
D)O
E)H2O
3Cu + 8HNO3 3Cu(NO3)2 + 2NO + 4H2O
A)Cu
B)H
C)N
D)O
E)H2O

Unlock Deck
Unlock for access to all 156 flashcards in this deck.
Unlock Deck
k this deck
58
Identify the reducing agent in the following chemical reaction.
Cd + NiO2 + 2H2O Cd(OH)2 + Ni(OH)2
A)Cd
B)NiO2
C)H2O
D)Cd(OH)2
E)Ni(OH)2
Cd + NiO2 + 2H2O Cd(OH)2 + Ni(OH)2
A)Cd
B)NiO2
C)H2O
D)Cd(OH)2
E)Ni(OH)2

Unlock Deck
Unlock for access to all 156 flashcards in this deck.
Unlock Deck
k this deck
59
Which choice gives the correct oxidation numbers for all three elements in Rb2SO3 in the order that the elements are shown in the formula?
A)-2, +6, -2
B)-1, +4, -3
C)+2, +4, -2
D)+1, +4, -2
E)+1, +6, -6
A)-2, +6, -2
B)-1, +4, -3
C)+2, +4, -2
D)+1, +4, -2
E)+1, +6, -6

Unlock Deck
Unlock for access to all 156 flashcards in this deck.
Unlock Deck
k this deck
60
Identify the reducing agent in the following chemical reaction.
5Fe2+(aq)+ MnO4-(aq) + 8H+(aq) 5Fe3+(aq)+ Mn2+(aq)+ 4H2O(l)
A)Fe2+
B)MnO4-
C)H+
D)Mn2+
E)Fe3+
5Fe2+(aq)+ MnO4-(aq) + 8H+(aq) 5Fe3+(aq)+ Mn2+(aq)+ 4H2O(l)
A)Fe2+
B)MnO4-
C)H+
D)Mn2+
E)Fe3+

Unlock Deck
Unlock for access to all 156 flashcards in this deck.
Unlock Deck
k this deck
61
When 20.0 mL of a 0.250 M (NH4)2S solution is added to 150.0 mL of a solution of Cu(NO3)2, a CuS precipitate forms. The precipitate is then filtered from the solution, dried, and weighed. If the recovered CuS is found to have a mass of 0.3491 g, what was the concentration of copper ions in the original Cu(NO3)2 solution?
A)3.65 * 10-3 M
B)1.22 * 10-2 M
C)3.33 * 10-2 M
D)4.87 * 10-2 M
E)2.43 * 10-2 M
A)3.65 * 10-3 M
B)1.22 * 10-2 M
C)3.33 * 10-2 M
D)4.87 * 10-2 M
E)2.43 * 10-2 M

Unlock Deck
Unlock for access to all 156 flashcards in this deck.
Unlock Deck
k this deck
62
A 350. mL sample of 0.276M HNO3 is partially neutralized by 125 mL of 0.0120M Ca(OH)2. Find the concentration of nitric acid in the resulting solution.
A)0.210 M
B)0.00632 M
C)0.203 M
D)0.0240 M
E)0.197 M
A)0.210 M
B)0.00632 M
C)0.203 M
D)0.0240 M
E)0.197 M

Unlock Deck
Unlock for access to all 156 flashcards in this deck.
Unlock Deck
k this deck
63
What mass of Na2SO4 is needed to prepare 350. mL of a solution having a sodium ion concentration of 0.125 M?
A)3.11 g
B)24.9 g
C)12.4 g
D)6.21 g
E)8.88 g
A)3.11 g
B)24.9 g
C)12.4 g
D)6.21 g
E)8.88 g

Unlock Deck
Unlock for access to all 156 flashcards in this deck.
Unlock Deck
k this deck
64
A 3.682 g sample of KClO3 is dissolved in enough water to give 375. mL of solution. What is the chlorate ion concentration in this solution?
A)3.00 * 10-2 M
B)4.41 * 10-2 M
C)0.118 M
D)1.65 * 10-2 M
E)8.01 * 10-2 M
A)3.00 * 10-2 M
B)4.41 * 10-2 M
C)0.118 M
D)1.65 * 10-2 M
E)8.01 * 10-2 M

Unlock Deck
Unlock for access to all 156 flashcards in this deck.
Unlock Deck
k this deck
65
Which of the following represents an acid-base neutralization reaction?
A)2Al(s)+ 3H2SO4(aq) Al2(SO4)3(aq)+ 3H2(g)
B)SO2(g)+ H2O(l) H2SO3(g)
C)LiOH(aq)+ HNO3(aq) LiNO3(aq)+ H2O(l)
D)2KBr(aq)+ Cl2(g) 2KCl(aq)+ Br2(l)
E)CaBr2(aq)+ H2SO4(aq) CaSO4(s)+ 2HBr(g)
A)2Al(s)+ 3H2SO4(aq) Al2(SO4)3(aq)+ 3H2(g)
B)SO2(g)+ H2O(l) H2SO3(g)
C)LiOH(aq)+ HNO3(aq) LiNO3(aq)+ H2O(l)
D)2KBr(aq)+ Cl2(g) 2KCl(aq)+ Br2(l)
E)CaBr2(aq)+ H2SO4(aq) CaSO4(s)+ 2HBr(g)

Unlock Deck
Unlock for access to all 156 flashcards in this deck.
Unlock Deck
k this deck
66
Which of the following represents a precipitation reaction?
A)2H2(g)+ O2(g) 2H2O(l)
B)CaBr2(aq)+ H2SO4(aq) CaSO4(s)+ 2HBr(g)
C)2KNO3(s) 2KNO2(s)+ O2(g)
D)2KBr(aq)+ Cl2(g) 2KCl(aq)+ Br2(l)
E)2Al(s)+ 3H2SO4(aq) Al2(SO4)3(aq)+ 3H2(g)
A)2H2(g)+ O2(g) 2H2O(l)
B)CaBr2(aq)+ H2SO4(aq) CaSO4(s)+ 2HBr(g)
C)2KNO3(s) 2KNO2(s)+ O2(g)
D)2KBr(aq)+ Cl2(g) 2KCl(aq)+ Br2(l)
E)2Al(s)+ 3H2SO4(aq) Al2(SO4)3(aq)+ 3H2(g)

Unlock Deck
Unlock for access to all 156 flashcards in this deck.
Unlock Deck
k this deck
67
Which of the following represents a hydrogen displacement reaction?
A)2C2H6(g)+ 7O2(g) 4CO2(g)+ 6H2O(l)
B)2KBr(aq)+ Cl2(g) 2KCl(aq)+ Br2(l)
C)N2(g)+ 3H2(g) 2NH3(g)
D)CaBr2(aq)+ H2SO4(aq) CaSO4(s)+ 2HBr(g)
E)2Al(s)+ 3H2SO4(aq) Al2(SO4)3(aq)+ 3H2(g)
A)2C2H6(g)+ 7O2(g) 4CO2(g)+ 6H2O(l)
B)2KBr(aq)+ Cl2(g) 2KCl(aq)+ Br2(l)
C)N2(g)+ 3H2(g) 2NH3(g)
D)CaBr2(aq)+ H2SO4(aq) CaSO4(s)+ 2HBr(g)
E)2Al(s)+ 3H2SO4(aq) Al2(SO4)3(aq)+ 3H2(g)

Unlock Deck
Unlock for access to all 156 flashcards in this deck.
Unlock Deck
k this deck
68
When 50.0 mL of a 0.3000 M AgNO3 solution is added to 50.0 mL of a solution of MgCl2, an AgCl precipitate forms immediately. The precipitate is then filtered from the solution, dried, and weighed. If the recovered AgCl is found to have a mass of 0.1183 g, what is the concentration of magnesium ions in the original MgCl2 solution?
A)0.300 M
B)8.25 * 10-3 M
C)1.65 * 10-2 M
D)2.06 * 10-5 M
E)4.13 * 10-3 M
A)0.300 M
B)8.25 * 10-3 M
C)1.65 * 10-2 M
D)2.06 * 10-5 M
E)4.13 * 10-3 M

Unlock Deck
Unlock for access to all 156 flashcards in this deck.
Unlock Deck
k this deck
69
Which of the following represents a combustion reaction?
A)2C2H6(g)+ 7O2(g) 4CO2(g)+ 6H2O(l)
B)LiOH(aq)+ HNO3(aq) LiNO3(aq)+ H2O(l)
C)N2(g)+ 3H2(g) 2NH3(g)
D)2Na(s)+ 2H2O(l) 2NaOH(aq)+ H2(g)
E)2Al(s)+ 3H2SO4(aq) Al2(SO4)3(aq)+ 3H2(g)
A)2C2H6(g)+ 7O2(g) 4CO2(g)+ 6H2O(l)
B)LiOH(aq)+ HNO3(aq) LiNO3(aq)+ H2O(l)
C)N2(g)+ 3H2(g) 2NH3(g)
D)2Na(s)+ 2H2O(l) 2NaOH(aq)+ H2(g)
E)2Al(s)+ 3H2SO4(aq) Al2(SO4)3(aq)+ 3H2(g)

Unlock Deck
Unlock for access to all 156 flashcards in this deck.
Unlock Deck
k this deck
70
What mass of K2CO3 is needed to prepare 200. mL of a solution having a potassium ion concentration of 0.150 M?
A)4.15 g
B)10.4 g
C)13.8 g
D)2.07 g
E)1.49 g
A)4.15 g
B)10.4 g
C)13.8 g
D)2.07 g
E)1.49 g

Unlock Deck
Unlock for access to all 156 flashcards in this deck.
Unlock Deck
k this deck
71
A 250. mL sample of 0.0328M HCl is partially neutralized by the addition of 100. mL of 0.0245M NaOH. Find the concentration of hydrochloric acid in the resulting solution.
A)0.00700 M
B)0.0164 M
C)0.0383 M
D)0.0230 M
E)0.0575 M
A)0.00700 M
B)0.0164 M
C)0.0383 M
D)0.0230 M
E)0.0575 M

Unlock Deck
Unlock for access to all 156 flashcards in this deck.
Unlock Deck
k this deck
72
A 20.00 mL sample of 0.1015 M nitric acid is introduced into a flask, and water is added until the volume of the solution reaches 250. mL. What is the concentration of nitric acid in the final solution?
A)1.27 M
B)8.12 * 10-3 M
C)0.406 M
D)3.25 * 10-2 M
E)5.08 * 10-4 M
A)1.27 M
B)8.12 * 10-3 M
C)0.406 M
D)3.25 * 10-2 M
E)5.08 * 10-4 M

Unlock Deck
Unlock for access to all 156 flashcards in this deck.
Unlock Deck
k this deck
73
When solid iron(II)hydroxide is added to water, the resulting solution contains 1.4 * 10-3g of dissolved iron(II)hydroxide per liter of solution. What is the hydroxide ion concentration in this solution?
A)7.8 * 10-6 M
B)1.6 * 10-5 M
C)2.5 * 10-10 M
D)3.1 *10-5 M
E)4.0 * 10-3 M
A)7.8 * 10-6 M
B)1.6 * 10-5 M
C)2.5 * 10-10 M
D)3.1 *10-5 M
E)4.0 * 10-3 M

Unlock Deck
Unlock for access to all 156 flashcards in this deck.
Unlock Deck
k this deck
74
A 4.691 g sample of MgCl2 is dissolved in enough water to give 750. mL of solution. What is the magnesium ion concentration in this solution?
A)3.70 * 10-2 M
B)1.05 * 10-2 M
C)6.57 * 10-2 M
D)4.93 * 10-2 M
E)0.131 M
A)3.70 * 10-2 M
B)1.05 * 10-2 M
C)6.57 * 10-2 M
D)4.93 * 10-2 M
E)0.131 M

Unlock Deck
Unlock for access to all 156 flashcards in this deck.
Unlock Deck
k this deck
75
A 50.0 mL sample of 0.436 M NH4NO3 is diluted with water to a total volume of 250.0 mL. What is the ammonium nitrate concentration in the resulting solution?
A)21.8 M
B)0.459 M
C)2.18 * 10-2 M
D)8.72 * 10-2 M
E)0.109 M
A)21.8 M
B)0.459 M
C)2.18 * 10-2 M
D)8.72 * 10-2 M
E)0.109 M

Unlock Deck
Unlock for access to all 156 flashcards in this deck.
Unlock Deck
k this deck
76
Which of the following represents a halogen displacement reaction?
A)2KBr(aq)+ Cl2(g) 2KCl(aq)+ Br2(l)
B)2Na(s)+ 2H2O(l) 2NaOH(aq)+ H2(g)
C)CaBr2(aq)+ H2SO4(aq) CaSO4(s)+ 2HBr(g)
D)2KNO3(s) 2KNO2(s)+ O2(g)
E)2LiOH(aq)+ H2SO4(aq) Li2SO4(aq)+ 2H2O(l)
A)2KBr(aq)+ Cl2(g) 2KCl(aq)+ Br2(l)
B)2Na(s)+ 2H2O(l) 2NaOH(aq)+ H2(g)
C)CaBr2(aq)+ H2SO4(aq) CaSO4(s)+ 2HBr(g)
D)2KNO3(s) 2KNO2(s)+ O2(g)
E)2LiOH(aq)+ H2SO4(aq) Li2SO4(aq)+ 2H2O(l)

Unlock Deck
Unlock for access to all 156 flashcards in this deck.
Unlock Deck
k this deck
77
Which of the following represents a metal displacement reaction?
A)2NaN3(s) 2Na(s)+ 3N2(g)
B)Fe2O3(s)+ 2Al(s) 2Fe(s)+ Al2O3(s)
C)3NO2(g)+ H2O(l) 2HNO3(aq)+ NO(g)
D)2P(s)+ 3Cl2(g) 2PCl3(g)
E)2ZnS(s)+ 3O2(g) 2ZnO(s)+ 2SO2(g)
A)2NaN3(s) 2Na(s)+ 3N2(g)
B)Fe2O3(s)+ 2Al(s) 2Fe(s)+ Al2O3(s)
C)3NO2(g)+ H2O(l) 2HNO3(aq)+ NO(g)
D)2P(s)+ 3Cl2(g) 2PCl3(g)
E)2ZnS(s)+ 3O2(g) 2ZnO(s)+ 2SO2(g)

Unlock Deck
Unlock for access to all 156 flashcards in this deck.
Unlock Deck
k this deck
78
What mass of Li3PO4 is needed to prepare 500. mL of a solution having a lithium ion concentration of 0.175 M?
A)6.75 g
B)10.1 g
C)19.3 g
D)30.4 g
E)3.38 g
A)6.75 g
B)10.1 g
C)19.3 g
D)30.4 g
E)3.38 g

Unlock Deck
Unlock for access to all 156 flashcards in this deck.
Unlock Deck
k this deck
79
When 38.0 mL of 0.1250 M H2SO4 is added to 100. mL of a solution of PbI2, a precipitate of PbSO4 forms. The PbSO4 is then filtered from the solution, dried, and weighed. If the recovered PbSO4 is found to have a mass of 0.0471 g, what was the concentration of iodide ions in the original solution?
A)3.10 * 10-4 M
B)1.55* 10-4 M
C)6.20 * 10-3 M
D)3.11 * 10-3 M
E)1.55 * 10-3 M
A)3.10 * 10-4 M
B)1.55* 10-4 M
C)6.20 * 10-3 M
D)3.11 * 10-3 M
E)1.55 * 10-3 M

Unlock Deck
Unlock for access to all 156 flashcards in this deck.
Unlock Deck
k this deck
80
A 0.9182 g sample of CaBr2 is dissolved in enough water to give 500. mL of solution. What is the calcium ion concentration in this solution?
A)9.19 * 10-3 M
B)2.30 * 10-3 M
C)2.72 * 10-3 M
D)4.59 * 10-3 M
E)1.25 * 10-3 M
A)9.19 * 10-3 M
B)2.30 * 10-3 M
C)2.72 * 10-3 M
D)4.59 * 10-3 M
E)1.25 * 10-3 M

Unlock Deck
Unlock for access to all 156 flashcards in this deck.
Unlock Deck
k this deck


