Deck 6: Energy Relationships in Chemical Reactions
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/105
Play
Full screen (f)
Deck 6: Energy Relationships in Chemical Reactions
1
Heat is
A)a measure of temperature.
B)a measure of the change in temperature.
C)a measure of thermal energy.
D)a measure of thermal energy transferred between two bodies at different temperature.
A)a measure of temperature.
B)a measure of the change in temperature.
C)a measure of thermal energy.
D)a measure of thermal energy transferred between two bodies at different temperature.
a measure of thermal energy transferred between two bodies at different temperature.
2
When 0.7521 g of benzoic acid was burned in a calorimeter containing 1,000. g of water, a temperature rise of 3.60°C was observed. What is the heat capacity of the bomb calorimeter, excluding the water? The heat of combustion of benzoic acid is -26.42 kJ/g.
A)15.87 kJ/°C
B)4.18 kJ/°C
C)5.52 kJ/°C
D)1.34 kJ/°C
E)752.1 kJ/°C
A)15.87 kJ/°C
B)4.18 kJ/°C
C)5.52 kJ/°C
D)1.34 kJ/°C
E)752.1 kJ/°C
1.34 kJ/°C
3
If 325 g of water at 4.2°C absorbs 12.28 kJ, what is the final temperature of the water? The specific heat of water is 4.184 J/g·°C.
A)4.21°C
B)4.8°C
C)9.0°C
D)13.2°C
E)2,938°C
A)4.21°C
B)4.8°C
C)9.0°C
D)13.2°C
E)2,938°C
13.2°C
4
Thermal energy is
A)the energy stored within the structural units of chemical substances.
B)the energy associated with the random motion of atoms and molecules.
C)solar energy, i.e. energy that comes from the sun.
D)energy available by virtue of an object's position.
A)the energy stored within the structural units of chemical substances.
B)the energy associated with the random motion of atoms and molecules.
C)solar energy, i.e. energy that comes from the sun.
D)energy available by virtue of an object's position.

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
5
Potential energy is
A)the energy stored within the structural units of chemical substances.
B)the energy associated with the random motion of atoms and molecules.
C)solar energy, i.e. energy that comes from the sun.
D)energy available by virtue of an object's position.
A)the energy stored within the structural units of chemical substances.
B)the energy associated with the random motion of atoms and molecules.
C)solar energy, i.e. energy that comes from the sun.
D)energy available by virtue of an object's position.

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
6
Radiant energy is
A)the energy stored within the structural units of chemical substances.
B)the energy associated with the random motion of atoms and molecules.
C)solar energy, i.e. energy that comes from the sun.
D)energy available by virtue of an object's position.
A)the energy stored within the structural units of chemical substances.
B)the energy associated with the random motion of atoms and molecules.
C)solar energy, i.e. energy that comes from the sun.
D)energy available by virtue of an object's position.

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
7
A beaker contains 115 g of ethanol at 18.2°C. If the ethanol absorbs 1125 J of heat without losing heat to the surroundings, what will be the final temperature of the ethanol? The specific heat of ethanol is 2.46 J/g.°C.
A)4.08°C
B)14.1°C
C)18.4°C
D)22.2°C
E)36.4°C
A)4.08°C
B)14.1°C
C)18.4°C
D)22.2°C
E)36.4°C

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
8
How many degrees of temperature rise will occur when a 25.0 g block of aluminum absorbs 10.0 kJ of heat? The specific heat of Al is 0.900 J/g·°C.
A)0.44°C
B)22.5°C
C)225°C
D)360°C
E)444°C
A)0.44°C
B)22.5°C
C)225°C
D)360°C
E)444°C

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
9
Chemical energy is
A)the energy stored within the structural units of chemical substances.
B)the energy associated with the random motion of atoms and molecules.
C)solar energy, i.e. energy that comes from the sun.
D)energy available by virtue of an object's position.
A)the energy stored within the structural units of chemical substances.
B)the energy associated with the random motion of atoms and molecules.
C)solar energy, i.e. energy that comes from the sun.
D)energy available by virtue of an object's position.

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
10
Suppose a 50.0 g block of silver (specific heat = 0.2350 J/g·°C)at 100°C is placed in contact with a 50.0 g block of iron (specific heat = 0.4494 J/g·°C)at 0°C, and the two blocks are insulated from the rest of the universe. The final temperature of the two blocks
A)will be higher than 50°C.
B)will be lower than 50°C.
C)will be exactly 50°C.
D)is unrelated to the composition of the blocks.
E)cannot be predicted.
A)will be higher than 50°C.
B)will be lower than 50°C.
C)will be exactly 50°C.
D)is unrelated to the composition of the blocks.
E)cannot be predicted.

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
11
How much heat is required to raise the temperature of 2,500 g of water from 27°C to 72°C? The specific heat of water is 4.184 J/g·°C.
A)0.19 kJ
B)10. kJ
C)280 kJ
D)470 kJ
E)750 kJ
A)0.19 kJ
B)10. kJ
C)280 kJ
D)470 kJ
E)750 kJ

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
12
The specific heat of gold is 0.129 J/g·°C. What is the molar heat capacity of gold?
A)0.039 J/mol·°C
B)0.129 J/mol·°C
C)25.4 J/mol·°C
D)39.0 kJ/mol·°C
E)197 J/mol·°C
A)0.039 J/mol·°C
B)0.129 J/mol·°C
C)25.4 J/mol·°C
D)39.0 kJ/mol·°C
E)197 J/mol·°C

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
13
An endothermic reaction causes the surroundings to
A)warm up.
B)become acidic.
C)condense.
D)decrease in temperature.
E)release CO2 .
A)warm up.
B)become acidic.
C)condense.
D)decrease in temperature.
E)release CO2 .

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
14
Copper metal has a specific heat of 0.385 J/g·°C. Calculate the amount of heat required to raise the temperature of 22.8 g of Cu from 20.0°C to 875°C.
A)1.97 * 10-5 J
B)1.0* 10-2 J
C)329 J
D)7.51 kJ
E)10.5 kJ
A)1.97 * 10-5 J
B)1.0* 10-2 J
C)329 J
D)7.51 kJ
E)10.5 kJ

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
15
Calculate the amount of heat necessary to raise the temperature of 12.0 g of water from 15.4°C to 93.0°C. The specific heat of water = 4.18 J/g·°C.
A)0.027 J
B)324 J
C)389 J
D)931 J
E)3,890 J
A)0.027 J
B)324 J
C)389 J
D)931 J
E)3,890 J

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
16
Naphthalene combustion can be used to calibrate the heat capacity of a bomb calorimeter. The heat of combustion of naphthalene is -40.1 kJ/g. When 0.8210 g of naphthalene was burned in a calorimeter containing 1,000. g of water, a temperature rise of 4.21°C was observed. What is the heat capacity of the bomb calorimeter excluding the water?
A)32.9 kJ/°C
B)7.8 kJ/°C
C)3.64 kJ/°C
D)1.76 kJ/°C
E)15.3 kJ/°C
A)32.9 kJ/°C
B)7.8 kJ/°C
C)3.64 kJ/°C
D)1.76 kJ/°C
E)15.3 kJ/°C

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
17
Which of the following processes is endothermic?
A)O2(g)+ 2H2(g) 2H2O(g)
B)H2O(g) H2O(l)
C)3O2(g)+ 2CH3OH(g) 2CO2(g)+ 2H2O(g)
D)H2O(s) H2O(l)
A)O2(g)+ 2H2(g) 2H2O(g)
B)H2O(g) H2O(l)
C)3O2(g)+ 2CH3OH(g) 2CO2(g)+ 2H2O(g)
D)H2O(s) H2O(l)

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
18
A piece of copper with a mass of 218 g has a heat capacity of 83.9 J/°C. What is the specific heat of copper?
A)0.385 J/g·°C
B)1.83 * 104 J/g·°C
C)2.60 J/g·°C
D)1.32 J/g·°C
E)24.5 J/g·°C
A)0.385 J/g·°C
B)1.83 * 104 J/g·°C
C)2.60 J/g·°C
D)1.32 J/g·°C
E)24.5 J/g·°C

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
19
An exothermic reaction causes the surroundings to
A)warm up.
B)become acidic.
C)expand.
D)decrease its temperature.
E)release CO2.
A)warm up.
B)become acidic.
C)expand.
D)decrease its temperature.
E)release CO2.

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
20
A glass containing 200. g of H2O at 20°C was placed in a refrigerator. The water loses 11.7 kJ as it cools to a constant temperature. What is its new temperature? The specific heat of water is 4.184 J/g·°C.
A)0.013°C
B)4°C
C)6°C
D)14°C
E)34°C
A)0.013°C
B)4°C
C)6°C
D)14°C
E)34°C

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
21
Calculate the standard enthalpy change for the reaction 2C8H18(l)+ 17O2(g) 16CO(g)+ 18H2O(l).
Given:
2C8H18(l)+ 25O2(g) 16CO2(g)+ 18H2O(l) H° = -11,020 kJ/mol
2CO(g)+ O2(g) 2CO2(g) H° = -566.0 kJ/mol
A)10,450 kJ/mol
B)6,492 kJ/mol
C)15,550 kJ/mol
D)-6,492 kJ/mol
E)-10.450 kJ/mol
Given:
2C8H18(l)+ 25O2(g) 16CO2(g)+ 18H2O(l) H° = -11,020 kJ/mol
2CO(g)+ O2(g) 2CO2(g) H° = -566.0 kJ/mol
A)10,450 kJ/mol
B)6,492 kJ/mol
C)15,550 kJ/mol
D)-6,492 kJ/mol
E)-10.450 kJ/mol

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
22
Given H2(g)+ (1/2)O2(g) H2O(l), H° = -286 kJ/mol, determine the standard enthalpy change for the reaction 2H2O(l) 2H2(g)+ O2(g).
A)( H°) = -286 kJ/mol
B)( H°) = +286 kJ/mol
C)( H°) = -572 kJ/mol
D)( H°) = +572 kJ/mol
E)( H°) = -143 kJ/mol
A)( H°) = -286 kJ/mol
B)( H°) = +286 kJ/mol
C)( H°) = -572 kJ/mol
D)( H°) = +572 kJ/mol
E)( H°) = -143 kJ/mol

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
23
Calculate the standard enthalpy of formation of liquid methanol, CH3OH(l), using the following information: C(graph)+ O2 CO2(g) H° = -393.5 kJ/mol
H2(g)+ (1/2)O2 H2O(l) H° = -285.8 kJ/mol
CH3OH(l)+ (3/2)O2(g) CO2(g)+ 2H2O(l) H° = -726.4 kJ/mol
A)-1,691.5 kJ/mol
B)-238.7 kJ/mol
C)1691.5 kJ/mol
D)47.1 kJ/mol
E)-47.1 kJ/mol
H2(g)+ (1/2)O2 H2O(l) H° = -285.8 kJ/mol
CH3OH(l)+ (3/2)O2(g) CO2(g)+ 2H2O(l) H° = -726.4 kJ/mol
A)-1,691.5 kJ/mol
B)-238.7 kJ/mol
C)1691.5 kJ/mol
D)47.1 kJ/mol
E)-47.1 kJ/mol

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
24
For the reaction C(graphite)+ O2(g) CO2(g) H° = -393 kJ/mol
How many grams of C(graphite)must be burned to release 275 kJ of heat?
A)22.3 g
B)0.70 g
C)12.0 g
D)17.1 g
E)8.40 g
How many grams of C(graphite)must be burned to release 275 kJ of heat?
A)22.3 g
B)0.70 g
C)12.0 g
D)17.1 g
E)8.40 g

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
25
A 0.1326 g sample of magnesium was burned in an oxygen bomb calorimeter. The total heat capacity of the calorimeter plus water was 5,760 J/°C. If the temperature rise of the calorimeter with water was 0.570°C, calculate the enthalpy of combustion of magnesium. Mg(s)+ 1/2O2(g) MgO(s)
A)-3280 kJ/mol
B)-24.8 kJ/mol
C)435 kJ/mol
D)106 kJ/mol
E)-602 kJ/mol
A)-3280 kJ/mol
B)-24.8 kJ/mol
C)435 kJ/mol
D)106 kJ/mol
E)-602 kJ/mol

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
26
Styrene, C8H8, is one of the substances used in the production of synthetic rubber. When styrene burns in oxygen to form carbon dioxide and liquid water under standard-state conditions at 25°C, 42.62 kJ are released per gram of styrene. Find the standard enthalpy of formation of styrene at 25°C. (Given: H°f[CO2(g)] = -393.5 kJ/mol, H°f[H2O(l)] = -285.8 kJ/mol, H°f[H2O(g)] = -241.8 kJ/mol)
A)323.8 kJ/mol
B)-4249 kJ/mol
C)-8730 kJ/mol
D)-636.7 kJ/mol
E)147.8 kJ/mol
A)323.8 kJ/mol
B)-4249 kJ/mol
C)-8730 kJ/mol
D)-636.7 kJ/mol
E)147.8 kJ/mol

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
27
Glycine, C2H5O2N, is important for biological energy. The combustion reaction of glycine is given by the equation
4C2H5O2N(s)+ 9O2(g) 8CO2(g)+ 10H2O(l)+ 2N2(g) H°rxn = -3857 kJ/mol
Given that H°f[CO2(g)] = -393.5 kJ/mol and H°f[H2O(l)] = -285.8 kJ/mol, calculate the enthalpy of formation of glycine.
A)-537.2 kJ/mol
B)-268.2 kJ/mol
C)2,149 kJ/mol
D)-3,178 kJ/mol
E)-964 kJ/mol
4C2H5O2N(s)+ 9O2(g) 8CO2(g)+ 10H2O(l)+ 2N2(g) H°rxn = -3857 kJ/mol
Given that H°f[CO2(g)] = -393.5 kJ/mol and H°f[H2O(l)] = -285.8 kJ/mol, calculate the enthalpy of formation of glycine.
A)-537.2 kJ/mol
B)-268.2 kJ/mol
C)2,149 kJ/mol
D)-3,178 kJ/mol
E)-964 kJ/mol

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
28
When 0.560 g of Na(s)reacts with excess F2(g)to form NaF(s), 13.8 kJ of heat is evolved at standard-state conditions. What is the standard enthalpy of formation ( H°f)of NaF(s)?
A)24.8 kJ/mol
B)570 kJ/mol
C)-24.8 kJ/mol
D)-7.8 kJ/mol
E)-570 kJ/mol
A)24.8 kJ/mol
B)570 kJ/mol
C)-24.8 kJ/mol
D)-7.8 kJ/mol
E)-570 kJ/mol

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
29
To which one of the following reactions occurring at 25°C does the symbol H°f[HNO3(l)] refer?
A)H(g)+ N(g)+ O3(g) HNO3(l)
B)(1/2)H2(g)+ (1/2)N2(g)+ (3/2)O2(g) HNO3(l)
C)HNO3(l) (1/2)H2(g)+ (1/2)N2(g)+ (3/2)O2(g)
D)HNO3(l) H(g)+ N(g)+ 3O(g)
E)H2(g)+ N2(g)+ O3(g) HNO3(l)
A)H(g)+ N(g)+ O3(g) HNO3(l)
B)(1/2)H2(g)+ (1/2)N2(g)+ (3/2)O2(g) HNO3(l)
C)HNO3(l) (1/2)H2(g)+ (1/2)N2(g)+ (3/2)O2(g)
D)HNO3(l) H(g)+ N(g)+ 3O(g)
E)H2(g)+ N2(g)+ O3(g) HNO3(l)

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
30
When 18.5 g of HgO(s)is decomposed to form Hg(l)and O2(g), 7.75 kJ of heat is absorbed at standard-state conditions. What is the standard enthalpy of formation ( H°f)of HgO(s)?
A)-90.7 kJ/mol
B)-7.75 kJ/mol
C)0.419 kJ/mol
D)27.9 kJ/mol
E)143 kJ/mol
A)-90.7 kJ/mol
B)-7.75 kJ/mol
C)0.419 kJ/mol
D)27.9 kJ/mol
E)143 kJ/mol

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
31
Find the standard enthalpy of formation of ethylene, C2H4(g), given the following data: heat of combustion of C2H4(g) = -1411 kJ/mol; H°f[CO2(g)] = -393.5 kJ/mol; H°f[H2O(l)] = -285.8 kJ/mol.
A)52 kJ/mol
B)87 kJ/mol
C)731 kJ/mol
D)1.41 * 103 kJ/mol
E)2.77 * 103 kJ/mol
A)52 kJ/mol
B)87 kJ/mol
C)731 kJ/mol
D)1.41 * 103 kJ/mol
E)2.77 * 103 kJ/mol

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
32
During volcanic eruptions, hydrogen sulfide gas is given off and oxidized by air according to the following chemical equation: 2H2S(g)+ 3O2(g) 2SO2(g)+ 2H2O(g)
Calculate the standard enthalpy change for the above reaction given:
3S(s)+ 2H2O(g) 2H2S(g)+ SO2(g) H° = 146.9 kJ/mol
S(s)+ O2(g) SO2(g) H° = -296.4 kJ/mol
A)-1036.1 kJ/mol
B)-742.3 kJ/mol
C)-149.5 kJ/mol
D)443.3 kJ/mol
E)742.3 kJ/mol
Calculate the standard enthalpy change for the above reaction given:
3S(s)+ 2H2O(g) 2H2S(g)+ SO2(g) H° = 146.9 kJ/mol
S(s)+ O2(g) SO2(g) H° = -296.4 kJ/mol
A)-1036.1 kJ/mol
B)-742.3 kJ/mol
C)-149.5 kJ/mol
D)443.3 kJ/mol
E)742.3 kJ/mol

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
33
Pentaborane B5H9(s)burns vigorously in O2 to give B2O3(s)and H2O(l). Calculate H°rxn for the combustion of 1 mol of B5H9. 0H°f[B2O3(s)] = -1,273.5 kJ/mol
H°f[B5H9(s)] = 73.2 kJ/mol
H°f[H2O(l)] = -285.8 kJ/mol
A)-1,2735 kJ/mol
B)-4,543 kJ/mol
C)-18,170 kJ/mol
D)-9,086 kJ/mol
E)-8,448 kJ/mol
H°f[B5H9(s)] = 73.2 kJ/mol
H°f[H2O(l)] = -285.8 kJ/mol
A)-1,2735 kJ/mol
B)-4,543 kJ/mol
C)-18,170 kJ/mol
D)-9,086 kJ/mol
E)-8,448 kJ/mol

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
34
Given 2Al(s)+ (3/2)O2(g) Al2O3(s), H°f = -1,670 kJ/mol for Al2O3 (s). Determine H° for the reaction 2Al2O3(s) 4Al(s)+ 3O2(g).
A)3,340 kJ/mol
B)1,670 kJ/mol
C)-3,340 kJ/mol
D)-1,670 kJ/mol
E)-835 kJ/mol
A)3,340 kJ/mol
B)1,670 kJ/mol
C)-3,340 kJ/mol
D)-1,670 kJ/mol
E)-835 kJ/mol

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
35
Octane (C8H18)undergoes combustion according to the following thermochemical equation: 2C8H18(l)+ 25O2(g) 16CO2(g)+ 18H2O(l) H°rxn = -11,020 kJ/mol.
Given that H°f[CO2(g)] = -393.5 kJ/mol and H°f[H2O(l)] = -285.8 kJ/mol, calculate the standard enthalpy of formation of octane.
A)-210 kJ/mol
B)-11,230 kJ/mol
C)22,040 kJ/mol
D)-420 kJ/mol
E)420 kJ/mol
Given that H°f[CO2(g)] = -393.5 kJ/mol and H°f[H2O(l)] = -285.8 kJ/mol, calculate the standard enthalpy of formation of octane.
A)-210 kJ/mol
B)-11,230 kJ/mol
C)22,040 kJ/mol
D)-420 kJ/mol
E)420 kJ/mol

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
36
Ethanol undergoes combustion in oxygen to produce carbon dioxide gas and liquid water. The standard heat of combustion of ethanol, C2H5OH(l), is -1366.8 kJ/mol. Given that H°f[CO2(g)] = -393.5 kJ/mol and H°f[H2O(l)] = -285.8 kJ/mol, what is the standard enthalpy of formation of ethanol?
A)3,010 kJ/mol
B)-687.6 kJ/mol
C)-277.6 kJ/mol
D)687.6 kJ/mol
E)1,367 kJ/mol
A)3,010 kJ/mol
B)-687.6 kJ/mol
C)-277.6 kJ/mol
D)687.6 kJ/mol
E)1,367 kJ/mol

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
37
Given the thermochemical equation 2SO2 + O2 2SO3, H°rxn = -198 kJ/mol, what is the standard enthalpy change for the decomposition of one mole of SO3?
A)198 kJ/mol
B)-99 kJ/mol
C)99 kJ/mol
D)396 kJ/mol
E)-198 kJ/mol
A)198 kJ/mol
B)-99 kJ/mol
C)99 kJ/mol
D)396 kJ/mol
E)-198 kJ/mol

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
38
Calculate the standard enthalpy change for the reaction 2C8H18(l)+ 21O2(g) 8CO(g)+ 8CO2(g)+ 18H2O(l).
Given:
2C8H18(l)+ 25O2(g) 16CO2(g)+ 18H2O(l) H° = -11,020 kJ/mol
2CO(g)+ O2(g) 2CO2(g) H° = -566.0 kJ/mol
A)1.0454 * 104 kJ/mol
B)-8,756 kJ/mol
C)1.1586 * 104 kJ/mol
D)-6,492 kJ/mol
E)-1.0454 * 104 kJ/mol
Given:
2C8H18(l)+ 25O2(g) 16CO2(g)+ 18H2O(l) H° = -11,020 kJ/mol
2CO(g)+ O2(g) 2CO2(g) H° = -566.0 kJ/mol
A)1.0454 * 104 kJ/mol
B)-8,756 kJ/mol
C)1.1586 * 104 kJ/mol
D)-6,492 kJ/mol
E)-1.0454 * 104 kJ/mol

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
39
A 100. mL sample of 0.200 M aqueous hydrochloric acid is added to 100. mL of 0.200 M aqueous ammonia in a calorimeter whose heat capacity (excluding any water)is 480. J/K. The following reaction occurs when the two solutions are mixed. HCl(aq)+ NH3(aq) NH4Cl(aq)
The temperature increase is 2.34°C. Calculate H per mole of HCl and NH3 reacted.
A)154 kJ/mol
B)1.96 kJ/mol
C)485 kJ/mol
D)-1.96 kJ/mol
E)-154 kJ/mol
The temperature increase is 2.34°C. Calculate H per mole of HCl and NH3 reacted.
A)154 kJ/mol
B)1.96 kJ/mol
C)485 kJ/mol
D)-1.96 kJ/mol
E)-154 kJ/mol

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
40
To which one of the following reactions occurring at 25°C does the symbol H°f[H2SO4(l)] refer?
A)2H(g)+ S(g)+ 4O(g) H2SO4(l)
B)H2(g)+ S(g)+ 2O2(g) H2SO4(l)
C)H2SO4(l) H2(g)+ S(s)+ 2O2(g)
D)H2SO4(l) 2H(g)+ S(s)+ 4O(g)
E)H2(g)+ S(s)+ 2O2(g) H2SO4(l)
A)2H(g)+ S(g)+ 4O(g) H2SO4(l)
B)H2(g)+ S(g)+ 2O2(g) H2SO4(l)
C)H2SO4(l) H2(g)+ S(s)+ 2O2(g)
D)H2SO4(l) 2H(g)+ S(s)+ 4O(g)
E)H2(g)+ S(s)+ 2O2(g) H2SO4(l)

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
41
Given that CaO(s)+ H2O(l) Ca(OH)2(s), H°rxn = -64.8 kJ/mol, how many grams of CaO must react in order to liberate 525 kJ of heat?
A)6.92 g
B)56.1 g
C)455 g
D)606 g
E)3.40 * 104 g
A)6.92 g
B)56.1 g
C)455 g
D)606 g
E)3.40 * 104 g

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
42
Find the heat absorbed from the surroundings when 15 g of O2 reacts according to the equation O + O2 O3, H°rxn= -103 kJ/mol.
A)4.6 * 10-3 kJ
B)48 kJ
C)96 kJ
D)32 kJ
E)110 kJ
A)4.6 * 10-3 kJ
B)48 kJ
C)96 kJ
D)32 kJ
E)110 kJ

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
43
At 25°C, the standard enthalpy of formation of KCl(s)is -435.87 kJ/mol. When one mole of KCl(s)is formed by reacting potassium vapor and chlorine gas at 25°C, the standard enthalpy of reaction is -525.86 kJ/mol. Find H° for the sublimation of potassium, K(s) K(g), at 25°C.
A)-345.88 kJ/mol
B)45.00 kJ/mol
C)345.88 kJ/mol
D)89.99 kJ/mol
E)-525.86 kJ/mol
A)-345.88 kJ/mol
B)45.00 kJ/mol
C)345.88 kJ/mol
D)89.99 kJ/mol
E)-525.86 kJ/mol

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
44
The combustion of octane produces heat according to the equation 2C8H18(l)+ 25O2(g) 16CO2(g)+ 18H2O(l) H°rxn= -11,020 kJ/mol
What is the heat of combustion per gram of octane?
A)-5,510 kJ/g
B)-96.5 kJ/g
C)-48.2 kJ/g
D)-193 kJ/g
E)-6.292 * 105 kJ/g
What is the heat of combustion per gram of octane?
A)-5,510 kJ/g
B)-96.5 kJ/g
C)-48.2 kJ/g
D)-193 kJ/g
E)-6.292 * 105 kJ/g

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
45
Determine the heat given off to the surroundings when 9.0 g of aluminum reacts according to the equation 2Al + Fe2O3 Al2O3 + 2Fe, H°rxn= -849 kJ/mol.
A)7.6 * 103 kJ
B)2.8 * 102 kJ
C)1.4 * 102 kJ
D)5.6 * 102 kJ
E)2.5 * 103 kJ
A)7.6 * 103 kJ
B)2.8 * 102 kJ
C)1.4 * 102 kJ
D)5.6 * 102 kJ
E)2.5 * 103 kJ

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
46
The combustion of pentane produces heat according to the equation C5H12(l)+ 8O2(g) 5CO2(g)+ 6H2O(l) H°rxn= -3,510 kJ/mol
How many grams of CO2 are produced per 2.50 * 103 kJ of heat released?
A)0.0809 g
B)3.56 g
C)31.3 g
D)157 g
E)309 g
How many grams of CO2 are produced per 2.50 * 103 kJ of heat released?
A)0.0809 g
B)3.56 g
C)31.3 g
D)157 g
E)309 g

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
47
At 25°C, the standard enthalpy of formation of anhydrous sodium carbonate is -1130.9 kJ/mol, whereas the standard enthalpy of formation of sodium carbonate monohydrate is -1430.1 kJ/mol. Determine H° at 25°C for the reaction Na2CO3(s)+ H2O(l) Na2CO3·H2O(s).
(Given: H°f[H2O(l)] = -285.8 kJ/mol)
A)-13.4 kJ/mol
B)-285.8 kJ/mol
C)-585.0 kJ/mol
D)-299.2 kJ/mol
E)-156.3 kJ/mol
(Given: H°f[H2O(l)] = -285.8 kJ/mol)
A)-13.4 kJ/mol
B)-285.8 kJ/mol
C)-585.0 kJ/mol
D)-299.2 kJ/mol
E)-156.3 kJ/mol

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
48
An average home in Colorado requires 20. GJ of heat per month. How many grams of natural gas (methane)must be burned to supply this energy? CH4(g)+ 2O2(g) CO2(g)+ 2H2O(l) H°rxn= -890.4 kJ/mol
A)1.4 * 103 g
B)3.6 * 105 g
C)7.1 * 10-4 g
D)2.2 * 104 g
E)1.4 * 104 g
A)1.4 * 103 g
B)3.6 * 105 g
C)7.1 * 10-4 g
D)2.2 * 104 g
E)1.4 * 104 g

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
49
Methanol (CH3OH)burns according to the equation 2CH3OH(l)+ 3O2(g) 2CO2(g)+ 4H2O(l), H°rxn = -1454 kJ/mol.
How much heat, in kilojoules, is given off when 75.0 g of methanol is burned?
A)727 kJ
B)3.22 * 103 kJ
C)1.45 * 103 kJ
D)1.70 * 10-3 kJ
E)3.41 * 103 kJ
How much heat, in kilojoules, is given off when 75.0 g of methanol is burned?
A)727 kJ
B)3.22 * 103 kJ
C)1.45 * 103 kJ
D)1.70 * 10-3 kJ
E)3.41 * 103 kJ

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
50
The enthalpy change when a strong acid is neutralized by strong base is -56.1 kJ/mol. If 12.0 mL of 6.00 M HBr at 21.30°C is mixed with 300. mL of 0.250 M NaOH, also at 21.30°C, what will be the maximum temperature reached by the resulting solution? (Assume that there is no heat loss to the container, that the specific heat of the final solution is 4.18 J/g·°C, and that the density of the final solution is that of water.)
A)18.20°C
B)24.53°C
C)101.8°C
D)24.40°C
E)34.25°C
A)18.20°C
B)24.53°C
C)101.8°C
D)24.40°C
E)34.25°C

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
51
Calcium oxide and water react in an exothermic reaction: CaO(s)+ H2O(l) Ca(OH)2(s) H°rxn = -64.8 kJ/mol
How much heat would be liberated when 7.15 g CaO(s)is dropped into a beaker containing 152g H2O?
A)1.97 * 10-3 kJ
B)8.26 kJ
C)508 kJ
D)547 kJ
E)555 kJ
How much heat would be liberated when 7.15 g CaO(s)is dropped into a beaker containing 152g H2O?
A)1.97 * 10-3 kJ
B)8.26 kJ
C)508 kJ
D)547 kJ
E)555 kJ

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
52
According to the first law of thermodynamics:
A)Energy is neither lost nor gained in any energy transformations.
B)Perpetual motion is possible.
C)Energy is conserved in quality but not in quantity.
D)Energy is being created as time passes. We have more energy in the universe now than when time began.
A)Energy is neither lost nor gained in any energy transformations.
B)Perpetual motion is possible.
C)Energy is conserved in quality but not in quantity.
D)Energy is being created as time passes. We have more energy in the universe now than when time began.

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
53
The combustion of butane produces heat according to the equation 2C4H10(g)+ 13O2(g) 8CO2(g)+ 10H2O(l) H°rxn = -5,314 kJ/mol
What is the heat of combustion per gram of butane?
A)-32.5 kJ/g
B)-45.7 kJ/g
C)-91.5 kJ/g
D)-2,656 kJ/g
E)-15,440 kJ/g
What is the heat of combustion per gram of butane?
A)-32.5 kJ/g
B)-45.7 kJ/g
C)-91.5 kJ/g
D)-2,656 kJ/g
E)-15,440 kJ/g

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
54
Calculate the amount of work done, in joules, when 2.5 mole of H2O vaporizes at 1.0 atm and 25°C. Assume the volume of liquid H2O is negligible compared to that of vapor. (1 L·atm = 101.3 J)
A)6,190 kJ
B)6.19 kJ
C)61.1 J
D)5.66 kJ
E)518 J
A)6,190 kJ
B)6.19 kJ
C)61.1 J
D)5.66 kJ
E)518 J

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
55
The enthalpy change when a strong acid is neutralized by strong base is -56.1 kJ/mol. If 135 mL of 0.450 M HI at 23.15°C is mixed with 145 mL of 0.500 M NaOH, also at 23.15°C, what will be the maximum temperature reached by the resulting solution? (Assume that there is no heat loss to the container, that the specific heat of the final solution is 4.18 J/g·°C, and that the density of the final solution is that of water.)
A)26.06°C
B)29.19°C
C)32.35°C
D)20.24°C
E)36.57°C
A)26.06°C
B)29.19°C
C)32.35°C
D)20.24°C
E)36.57°C

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
56
Solid sodium peroxide (Na2O2)reacts with liquid water yielding aqueous sodium hydroxide and oxygen gas. How much heat is released when 250.0 L of oxygen gas is produced from the reaction of sodium peroxide and water if the reaction is carried out in an open container at 1.000 atm pressure and 25°C? (Given: H°f[Na2O2(s)] = -510.9 kJ/mol; H°f[NaOH(aq)] = -469.2 kJ/mol; H°f[H2O(l)] = -285.8 kJ/mol)
A)35,400 kJ
B)1740 kJ
C)141.7 kJ
D)3330 kJ
E)2900 kJ
A)35,400 kJ
B)1740 kJ
C)141.7 kJ
D)3330 kJ
E)2900 kJ

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
57
The combustion of butane produces heat according to the equation 2C4H10(g)+ 13O2(g) 0 8CO2(g)+ 10H2O(l) H°rxn= -5,314 kJ/mol
How many grams of butane must be burned to release 1.00 * 104 kJ of heat?
A)30.9 g
B)61.8 g
C)109 g
D)153 g
E)219 g
How many grams of butane must be burned to release 1.00 * 104 kJ of heat?
A)30.9 g
B)61.8 g
C)109 g
D)153 g
E)219 g

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
58
The combustion of butane produces heat according to the equation 2C4H10(g)+ 13O2(g) 8CO2(g)+ 10H2O(l) H°rxn= -5,314 kJ/mol
How many grams of CO2 are produced per 1.00 * 104 kJ of heat released?
A)23.4 g
B)44.0 g
C)82.3 g
D)187 g
E)662 g
How many grams of CO2 are produced per 1.00 * 104 kJ of heat released?
A)23.4 g
B)44.0 g
C)82.3 g
D)187 g
E)662 g

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
59
Ethanol (C2H5OH)burns according to the equation C2H5OH(l)+ 3O2(g) 2CO2(g)+ 3H2O(l), H°rxn = -1367 kJ/mol.
How much heat is released when 35.0 g of ethanol is burned?
A)1,797 kJ
B)1,367 kJ
C)9.61 * 10-4 kJ
D)4.78 * 104 kJ
E)1,040 kJ
How much heat is released when 35.0 g of ethanol is burned?
A)1,797 kJ
B)1,367 kJ
C)9.61 * 10-4 kJ
D)4.78 * 104 kJ
E)1,040 kJ

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
60
Given the thermochemical equation 2SO2(g)+ O2(g) 2SO3(g), H°rxn= -198 kJ/mol, how much heat is evolved when 600. g of SO2 is burned?
A)5.46 * 10-2 kJ
B)928 kJ
C)1.85 * 103 kJ
D)59,400 kJ
E)3.71 * 103 kJ
A)5.46 * 10-2 kJ
B)928 kJ
C)1.85 * 103 kJ
D)59,400 kJ
E)3.71 * 103 kJ

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
61
Aluminum oxide can be reduced to aluminum metal using carbon, the other reaction product being carbon monoxide. Determine the enthalpy change when 12.5 g of aluminum is produced by this method. [ H°f(carbon monoxide)= -110.5 kJ/mol; H°f(aluminum oxide)= -1669.8 kJ/mol]
A)725 kJ
B)697 kJ
C)310 kJ
D)361 kJ
E)1504 kJ
A)725 kJ
B)697 kJ
C)310 kJ
D)361 kJ
E)1504 kJ

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
62
For which of these reactions will the difference between H° and E° be the greatest?
A)2H2O2(l) 2H2O(l)+ O2(g)
B)CaCO3(s) CaO(s)+ CO2(g)
C)NO(g)+ O3(g) NO2(g)+ O2(g)
D)2C2H6(g)+ 7O2(g) 4CO2(g)+ 6H2O(l)
E)4NH3(g)+ 5O2(g) 4NO(g)+ 6H2O(g)
A)2H2O2(l) 2H2O(l)+ O2(g)
B)CaCO3(s) CaO(s)+ CO2(g)
C)NO(g)+ O3(g) NO2(g)+ O2(g)
D)2C2H6(g)+ 7O2(g) 4CO2(g)+ 6H2O(l)
E)4NH3(g)+ 5O2(g) 4NO(g)+ 6H2O(g)

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
63
Which of the following processes always results in an increase in the energy of a system?
A)The system loses heat and does work on the surroundings.
B)The system gains heat and does work on the surroundings.
C)The system loses heat and has work done on it by the surroundings.
D)The system gains heat and has work done on it by the surroundings.
E)None of these is always true.
A)The system loses heat and does work on the surroundings.
B)The system gains heat and does work on the surroundings.
C)The system loses heat and has work done on it by the surroundings.
D)The system gains heat and has work done on it by the surroundings.
E)None of these is always true.

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
64
Ozone (O3)in the atmosphere can be converted to oxygen gas by reaction with nitric oxide (NO). Nitrogen dioxide is also produced in the reaction. What is the enthalpy change when 8.50L of ozone at a pressure of 1.00 atm and 25°C reacts with 12.00 L of nitric oxide at the same initial pressure and temperature? [ H°f(NO)= 90.4 kJ/mol; H°f(NO2)= 33.85 kJ/mol; H°f(O3)= 142.2 kJ/mol]
A)-69.2 kJ
B)-19.7 kJ
C)-1690 kJ
D)-97.6 kJ
E)-167 kJ
A)-69.2 kJ
B)-19.7 kJ
C)-1690 kJ
D)-97.6 kJ
E)-167 kJ

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
65
The heat of solution of ammonium chloride is 15.2 kJ/mol. If a 6.134 g sample of NH4Cl is added to 65.0 mL of water in a calorimeter at 24.5°C, what is the minimum temperature reached by the solution? (The specific heat of water = 4.18 J/g·°C; the heat capacity of the calorimeter = 365. J/°C.)
A)27.1°C
B)18.6°C
C)19.7°C
D)21.9°C
E)30.4°C
A)27.1°C
B)18.6°C
C)19.7°C
D)21.9°C
E)30.4°C

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
66
A 26.2 g piece of copper metal is heated from 21.5°C to 201.6°C. Calculate the amount of heat absorbed by the metal. The specific heat of Cu is 0.385 J/g·°C.

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
67
A gas is allowed to expand, at constant temperature, from a volume of 1.0 L to 10.1 L against an external pressure of 0.50 atm. If the gas absorbs 250 J of heat from the surroundings, what are the values of q, w, and E? 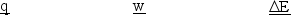
A)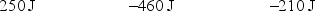
B)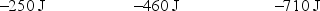
C)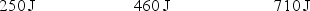
D)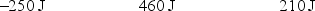
E)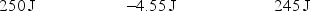
A)
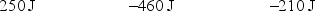
B)
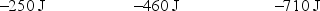
C)
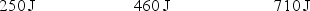
D)
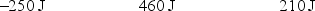
E)
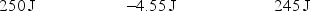

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
68
Given the following H° values,
H2(g)+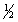 O2(g) H2O(l) H°f = -285.8 kJ/mol
O2(g) H2O(l) H°f = -285.8 kJ/mol
H2O2(l) H2(g)+ O2(g) H°rxn = 187.6 kJ/mol
calculate H°rxn for the reaction H2O2(l) H2O(l)+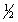 O2(g),
O2(g),
H2(g)+
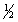 O2(g) H2O(l) H°f = -285.8 kJ/mol
O2(g) H2O(l) H°f = -285.8 kJ/molH2O2(l) H2(g)+ O2(g) H°rxn = 187.6 kJ/mol
calculate H°rxn for the reaction H2O2(l) H2O(l)+
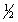 O2(g),
O2(g), 
Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
69
The heat of solution of ammonium nitrate is 26.2 kJ/mol. If a 5.368 g sample of NH4NO3 is added to 40.0 mL of water in a calorimeter at 23.5°C, what is the minimum temperature reached by the solution? (The specific heat of water = 4.18 J/g·°C; the heat capacity of the calorimeter = 650. J/°C.)
A)14.3°C
B)20.8°C
C)-7.7°C
D)25.6°C
E)21.4°C
A)14.3°C
B)20.8°C
C)-7.7°C
D)25.6°C
E)21.4°C

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
70
The enthalpy of combustion of acetylene C2H2 is described by
C2H2(g)+ (5/2)O2(g) 2CO2(g)+ H2O(l) H°rxn= -1299 kJ/mol
Calculate the enthalpy of formation of acetylene, given the following enthalpies of formation
H°f[CO2(g)] = -393.5 kJ/mol
H°f[H2O(l)] = -285.8 kJ/mol
C2H2(g)+ (5/2)O2(g) 2CO2(g)+ H2O(l) H°rxn= -1299 kJ/mol
Calculate the enthalpy of formation of acetylene, given the following enthalpies of formation
H°f[CO2(g)] = -393.5 kJ/mol
H°f[H2O(l)] = -285.8 kJ/mol

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
71
A 0.1946 g piece of magnesium metal is burned in a constant-volume calorimeter that has a heat capacity of 1349 J/°C. The calorimeter contains 500. g of water and the temperature rise is 1.40°C. Calculate the heat of combustion of magnesium metal in kJ/g, given that the specific heat of water = 4.184 J/g·°C.

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
72
For which of these reactions will the difference between H° and E° be the smallest?
A)N2(g)+ 3H2(g) 2NH3(g)
B)4PH3(g) P4(g)+ 6H2(g)
C)H2(g)+ Cl2(g) 2HCl(g)
D)CO2(g)+ 2H2O(l) CH4(g)+ 2O2(g)
E)P4(s)+ 10Cl2(g) 4PCl5(s)
A)N2(g)+ 3H2(g) 2NH3(g)
B)4PH3(g) P4(g)+ 6H2(g)
C)H2(g)+ Cl2(g) 2HCl(g)
D)CO2(g)+ 2H2O(l) CH4(g)+ 2O2(g)
E)P4(s)+ 10Cl2(g) 4PCl5(s)

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
73
The bond enthalpy of the Br-Cl bond is equal to H° for the reaction BrCl(g) Br(g)+ Cl(g).
Use the following data to find the bond enthalpy of the Br-Cl bond.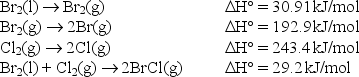
A)219.0 kJ/mol
B)203.5 kJ/mol
C)14.6 kJ/mol
D)438.0 kJ/mol
E)407.0 kJ/mol
Use the following data to find the bond enthalpy of the Br-Cl bond.
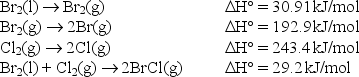
A)219.0 kJ/mol
B)203.5 kJ/mol
C)14.6 kJ/mol
D)438.0 kJ/mol
E)407.0 kJ/mol

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
74
At 25°C, the following heats of reaction are known: 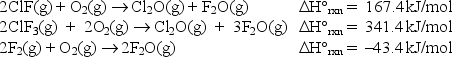 At the same temperature, use Hess's law to calculate H°rxn for the reaction: ClF(g)+ F2(g) ClF3(g)
At the same temperature, use Hess's law to calculate H°rxn for the reaction: ClF(g)+ F2(g) ClF3(g)
A)-217.5 kJ/mol
B)-130.2 kJ/mol
C)217.5 kJ/mol
D)-108.7 kJ/mol
E)465.4 kJ/mol
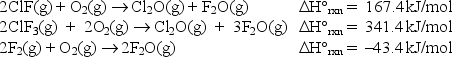 At the same temperature, use Hess's law to calculate H°rxn for the reaction: ClF(g)+ F2(g) ClF3(g)
At the same temperature, use Hess's law to calculate H°rxn for the reaction: ClF(g)+ F2(g) ClF3(g)A)-217.5 kJ/mol
B)-130.2 kJ/mol
C)217.5 kJ/mol
D)-108.7 kJ/mol
E)465.4 kJ/mol

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
75
Define specific heat.

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
76
A 0.3423 g sample of pentane, C5H12, was burned in a bomb calorimeter. The temperature of the calorimeter and the 1.000 kg of water contained therein rose from 20.22°C to 22.82°C. The heat capacity of the calorimeter is 2.21 kJ/°C. The heat capacity of water = 4.184 J/g·°C. How much heat was given off during combustion of the sample of pentane?

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
77
A gas is compressed in a cylinder from a volume of 20.0 L to 2.0 L by a constant pressure of 10.0 atm. Calculate the amount of work done on the system.
A)1.01 * 104 J
B)-180 J
C)1.81 * 104 J
D)-1.81 * 104 J
E)180 J
A)1.01 * 104 J
B)-180 J
C)1.81 * 104 J
D)-1.81 * 104 J
E)180 J

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
78
Calculate the enthalpy of reaction for H2(g)+ C2H4(g) C2H6(g).
[ H°f(C2H4(g))= 52.3 kJ/mol; H°f(C2H6(g))= -84.7 kJ/mol]
[ H°f(C2H4(g))= 52.3 kJ/mol; H°f(C2H6(g))= -84.7 kJ/mol]

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
79
How many grams of ethylene (C2H4)would have to be burned to produce 450 kJ of heat?
C2H4(g)+ 3O2(g) 2CO2(g)+ H2O(l) H°rxn= -1411 kJ/mol
C2H4(g)+ 3O2(g) 2CO2(g)+ H2O(l) H°rxn= -1411 kJ/mol

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck
80
Calculate the amount of work done against an atmospheric pressure of 1.00 atm when 500.0 g of zinc dissolves in excess acid at 30.0°C. Zn(s)+ 2H+(aq) Zn2+(aq)+ H2(g)
A)w = +22.4 kJ
B)w = +24.9 kJ
C)w = 0
D)w = -2.52 kJ
E)w = -19.3 kJ
A)w = +22.4 kJ
B)w = +24.9 kJ
C)w = 0
D)w = -2.52 kJ
E)w = -19.3 kJ

Unlock Deck
Unlock for access to all 105 flashcards in this deck.
Unlock Deck
k this deck


