Deck 9: Chemical Bonding I: the Covalent Bond
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/118
Play
Full screen (f)
Deck 9: Chemical Bonding I: the Covalent Bond
1
Which one of the following is most likely to be an ionic compound?
A)ClF3
B)FeCl3
C)NH3
D)PF3
E)SO3
A)ClF3
B)FeCl3
C)NH3
D)PF3
E)SO3
FeCl3
2
Which of the following solids would have the lowest melting point?
A)KI
B)KBr
C)KCl
D)KF
A)KI
B)KBr
C)KCl
D)KF
KI
3
Which one of the following is most likely to be a covalent compound?
A)Rb2O
B)BaO
C)SrO
D)SeO2
E)MnO2
A)Rb2O
B)BaO
C)SrO
D)SeO2
E)MnO2
SeO2
4
Which one of the following is most likely to be a covalent compound?
A)KF
B)CaCl2
C)SF4
D)Al2O3
E)CaSO4
A)KF
B)CaCl2
C)SF4
D)Al2O3
E)CaSO4

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
5
The Lewis dot symbol for the chloride ion is
A)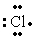
B)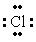 -
-
C)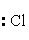 -
-
D)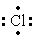 -
-
E)Cl-
A)
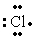
B)
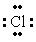 -
-C)
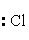 -
-D)
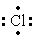 -
-E)Cl-

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
6
Calculate the energy change for the reaction K(g)+ Br(g) K+(g)+ Br- (g)
Given the following ionization energy (IE)and electron affinity (EA)values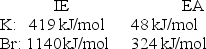
A)-1,092 kJ/mol
B)-95 kJ/mol
C)95 kJ/mol
D)1,092 kJ/mol
E)1,187 kJ/mol
Given the following ionization energy (IE)and electron affinity (EA)values
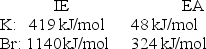
A)-1,092 kJ/mol
B)-95 kJ/mol
C)95 kJ/mol
D)1,092 kJ/mol
E)1,187 kJ/mol

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
7
The Lewis dot symbol for the calcium ion is
A)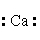 2+
2+
B)(-Ca-)
C)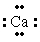 2+
2+
D)Ca2+
E)Ca
A)
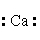 2+
2+B)(-Ca-)
C)
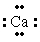 2+
2+D)Ca2+
E)Ca

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
8
Which of the following pairs of elements would be most likely to form an ionic compound?
A)Cl and I
B)Al and K
C)Cl and Mg
D)C and S
E)Al and Mg
A)Cl and I
B)Al and K
C)Cl and Mg
D)C and S
E)Al and Mg

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
9
Which of the following solids would have the highest melting point?
A)NaF
B)NaCl
C)NaBr
D)NaI
A)NaF
B)NaCl
C)NaBr
D)NaI

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
10
Which one of the following is most likely to be an ionic compound?
A)NCl3
B)BaCl2
C)CO
D)SO2
E)SF4
A)NCl3
B)BaCl2
C)CO
D)SO2
E)SF4

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
11
Which of the following ionic solids would have the largest lattice energy?
A)KF
B)KI
C)LiF
D)LiI
E)NaF
A)KF
B)KI
C)LiF
D)LiI
E)NaF

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
12
Which one of the following ionic solids would have the largest lattice energy?
A)NaCl
B)NaF
C)CaBr2
D)CsI
E)CaCl2
A)NaCl
B)NaF
C)CaBr2
D)CsI
E)CaCl2

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
13
Which of the following ionic solids would have the largest lattice energy?
A)SrO
B)NaF
C)CaBr2
D)CsI
E)BaSO4
A)SrO
B)NaF
C)CaBr2
D)CsI
E)BaSO4

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
14
Which of the following solids would have the highest melting point?
A)NaI
B)NaF
C)MgO
D)MgCl2
E)KF
A)NaI
B)NaF
C)MgO
D)MgCl2
E)KF

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
15
The Lewis dot symbol for the a lead atom is
A)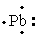
B)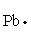
C)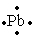
D)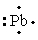
E)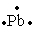
A)
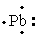
B)
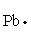
C)
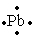
D)
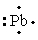
E)
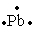

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
16
The Lewis dot symbol for the S 2- ion is
A)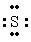
B)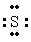 2-
2-
C)(S2-)
D)
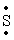 -2-
-2-
E)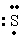 -
-
A)
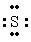
B)
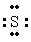 2-
2-C)(S2-)
D)
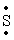 -2-
-2-E)
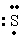 -
-
Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
17
Calculate the energy change for the reaction K(g)+ I(g) K+(g)+ I - (g)
Given the following ionization energy (IE)and electron affinity (EA)values.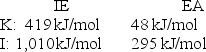
A)-124 kJ/mol
B)-715 kJ/mol
C)715 kJ/mol
D)1429 kJ/mol
E)None of these
Given the following ionization energy (IE)and electron affinity (EA)values.
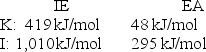
A)-124 kJ/mol
B)-715 kJ/mol
C)715 kJ/mol
D)1429 kJ/mol
E)None of these

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
18
Complete this statement: Coulomb's law states that the magnitude of the force of interaction between two charged bodies is
A)directly proportional to the product of the charges on the bodies and directly proportional to the distance separating them.
B)directly proportional to the product of the charges on the bodies, and inversely proportional to the square of the distance separating them.
C)inversely proportional to the product of the charges on the bodies, and directly proportional to the square of the distance separating them.
D)directly proportional to the sum of the charges on the bodies, and inversely proportional to the square of the distance separating them.
A)directly proportional to the product of the charges on the bodies and directly proportional to the distance separating them.
B)directly proportional to the product of the charges on the bodies, and inversely proportional to the square of the distance separating them.
C)inversely proportional to the product of the charges on the bodies, and directly proportional to the square of the distance separating them.
D)directly proportional to the sum of the charges on the bodies, and inversely proportional to the square of the distance separating them.

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
19
Which one of the following is most likely to be a covalent compound?
A)CsOH
B)NF3
C)Sr(NO3)2
D)CaO
E)LiF
A)CsOH
B)NF3
C)Sr(NO3)2
D)CaO
E)LiF

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
20
Which one of the following is most likely to be an ionic compound?
A)CaCl2
B)CO2
C)CS2
D)SO2
E)OF2
A)CaCl2
B)CO2
C)CS2
D)SO2
E)OF2

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
21
Which of the elements listed below is the least electronegative?
A)Sr
B)V
C)Ni
D)P
E)I
A)Sr
B)V
C)Ni
D)P
E)I

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
22
Which of the following covalent bonds is the most polar (i.e., highest percent ionic character)?
A)Al - I
B)Si - I
C)Al - Cl
D)Si - Cl
E)Si - P
A)Al - I
B)Si - I
C)Al - Cl
D)Si - Cl
E)Si - P

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
23
Use the Born-Haber cycle to calculate the lattice energy of KCl(s)given the following data: H(sublimation)K = 79.2 kJ/mol
I1 (K)= 418.7 kJ/mol
Bond energy (Cl-Cl)= 242.8 kJ/mol
EA (Cl)= 348 kJ/mol
H (KCl(s))= -435.7 kJ/mol
(KCl(s))= -435.7 kJ/mol
A)-165 kJ/mol
B)288 kJ/mol
C)629 kJ/mol
D)707 kJ/mol
E)828 kJ/mol
I1 (K)= 418.7 kJ/mol
Bond energy (Cl-Cl)= 242.8 kJ/mol
EA (Cl)= 348 kJ/mol
H
 (KCl(s))= -435.7 kJ/mol
(KCl(s))= -435.7 kJ/molA)-165 kJ/mol
B)288 kJ/mol
C)629 kJ/mol
D)707 kJ/mol
E)828 kJ/mol

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
24
Which one of these polar covalent bonds would have the greatest percent ionic character?
A)H - Br
B)H - Cl
C)H - F
D)H - I
A)H - Br
B)H - Cl
C)H - F
D)H - I

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
25
A polar covalent bond would form in which one of the following pairs of atoms?
A)Cl - Cl
B)Si - Si
C)Ca - Cl
D)Cr - Br
E)P - Cl
A)Cl - Cl
B)Si - Si
C)Ca - Cl
D)Cr - Br
E)P - Cl

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
26
The covalent bond with the greatest polarity would form in which of the atom pairs below?
A)Br - Br
B)S - O
C)C - P
D)C - O
E)B - O
A)Br - Br
B)S - O
C)C - P
D)C - O
E)B - O

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
27
Which of the elements listed below has the greatest electronegativity?
A)Se
B)Sb
C)K
D)Ga
E)Fe
A)Se
B)Sb
C)K
D)Ga
E)Fe

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
28
The bond in which of the following pairs of atoms would have the greatest percent ionic character (i.e., most polar)?
A)C - O
B)S - O
C)Na - I
D)Na - Br
E)F - F
A)C - O
B)S - O
C)Na - I
D)Na - Br
E)F - F

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
29
A nonpolar covalent bond (i.e., pure covalent)would form in which one of the following pairs of atoms?
A)Na - Cl
B)H - Cl
C)Li - Br
D)Se - Br
E)Br - Br
A)Na - Cl
B)H - Cl
C)Li - Br
D)Se - Br
E)Br - Br

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
30
Which of the elements listed below has the greatest electronegativity?
A)Na
B)As
C)Ga
D)Cs
E)Sb
A)Na
B)As
C)Ga
D)Cs
E)Sb

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
31
The bond in which of the following pairs of atoms would be the least polar (i.e., lowest percent ionic character)?
A)C - Cl
B)C - C
C)C - H
D)O - C
E)N - C
A)C - Cl
B)C - C
C)C - H
D)O - C
E)N - C

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
32
Classify the C - Cl bond in CCl4 as ionic, polar covalent, or nonpolar covalent.
A)ionic
B)polar covalent
C)nonpolar covalent
A)ionic
B)polar covalent
C)nonpolar covalent

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
33
The bond in which one of the following pairs of atoms would be the most polar?
A)B - C
B)C - N
C)C - O
D)Si - O
E)C - C
A)B - C
B)C - N
C)C - O
D)Si - O
E)C - C

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
34
What type of chemical bond holds the atoms together within a water molecule?
A)Ionic bond
B)Nonpolar covalent bond
C)Polar covalent bond
D)Coordinate covalent bond
A)Ionic bond
B)Nonpolar covalent bond
C)Polar covalent bond
D)Coordinate covalent bond

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
35
Classify the O - H bond in CH3OH as ionic, polar covalent, or nonpolar covalent.
A)ionic
B)polar covalent
C)nonpolar covalent
A)ionic
B)polar covalent
C)nonpolar covalent

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
36
Use the Born-Haber cycle to calculate the lattice energy of MgO (s)given the following data: H(sublimation)Mg = 130 kJ/mol
I1 (Mg)= 738.1 kJ/mol
I2 (Mg)= 1450 kJ/mol
Bond energy (O=O)= 498.7 kJ/mol
EA (O)= 141 kJ/mol
EA (O-)= -780 kJ/mol
H (MgO(s))= -601.8 kJ/mol
(MgO(s))= -601.8 kJ/mol
A)2200 kJ/mol
B)2800 kJ/mol
C)3200 kJ/mol
D)3800 kJ/mol
E)4100 kJ/mol
I1 (Mg)= 738.1 kJ/mol
I2 (Mg)= 1450 kJ/mol
Bond energy (O=O)= 498.7 kJ/mol
EA (O)= 141 kJ/mol
EA (O-)= -780 kJ/mol
H
 (MgO(s))= -601.8 kJ/mol
(MgO(s))= -601.8 kJ/molA)2200 kJ/mol
B)2800 kJ/mol
C)3200 kJ/mol
D)3800 kJ/mol
E)4100 kJ/mol

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
37
Use the Born-Haber cycle to calculate the standard enthalpy of formation ( H  )for LiCl(s)given the following data: H(sublimation)Li = 155.2 kJ/mol
)for LiCl(s)given the following data: H(sublimation)Li = 155.2 kJ/mol
I1 (Li)= 520 kJ/mol
Bond energy (Cl-Cl)= 242.7 kJ/mol
EA (Cl)= 349 kJ/mol
Lattice energy (LiCl(s))= 828 kJ/mol
A)440 kJ/mol
B)320 kJ/mol
C)-260 kJ/mol
D)-380 kJ/mol
E)-1420 kJ/mol
 )for LiCl(s)given the following data: H(sublimation)Li = 155.2 kJ/mol
)for LiCl(s)given the following data: H(sublimation)Li = 155.2 kJ/molI1 (Li)= 520 kJ/mol
Bond energy (Cl-Cl)= 242.7 kJ/mol
EA (Cl)= 349 kJ/mol
Lattice energy (LiCl(s))= 828 kJ/mol
A)440 kJ/mol
B)320 kJ/mol
C)-260 kJ/mol
D)-380 kJ/mol
E)-1420 kJ/mol

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
38
Which of the atoms listed below is the most electronegative?
A)Li
B)Cs
C)P
D)As
E)Ge
A)Li
B)Cs
C)P
D)As
E)Ge

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
39
Which of the elements listed below has the greatest electronegativity?
A)Mg
B)Ga
C)Si
D)Ba
E)Pb
A)Mg
B)Ga
C)Si
D)Ba
E)Pb

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
40
Which of the bonds below would have the greatest polarity (i.e., highest percent ionic character)?
A)Si - P
B)Si - S
C)Si - Se
D)Si - Cl
E)Si - I
A)Si - P
B)Si - S
C)Si - Se
D)Si - Cl
E)Si - I

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
41
Assuming the octet rule is obeyed, how many covalent bonds will a nitrogen atom form to give a formal charge of zero?
A)0
B)1
C)2
D)3
E)4
A)0
B)1
C)2
D)3
E)4

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
42
The number of lone electron pairs in the CO32- ion is ___.
A)4
B)5
C)6
D)7
E)8
A)4
B)5
C)6
D)7
E)8

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
43
The Lewis structure for CS2 is:
A)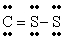
B)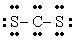
C)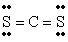
D)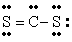
A)
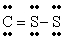
B)
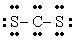
C)
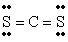
D)
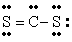

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
44
The total number of bonding electrons in a molecule of formaldehyde (H2CO)is
A)3.
B)4.
C)6.
D)8.
E)18.
A)3.
B)4.
C)6.
D)8.
E)18.

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
45
Assuming the octet rule is obeyed, how many covalent bonds will a carbon atom form to give a formal charge of zero?
A)0
B)1
C)2
D)3
E)4
A)0
B)1
C)2
D)3
E)4

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
46
The number of lone electron pairs in the N2 molecule is ___.
A)1
B)2
C)3
D)4
E)5
A)1
B)2
C)3
D)4
E)5

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
47
Assuming the octet rule is obeyed, how many covalent bonds will a neon atom form to give a formal charge of zero?
A)0
B)1
C)2
D)3
E)4
A)0
B)1
C)2
D)3
E)4

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
48
The formal charge on the bromine atom in BrO3- drawn with three single bonds is
A)-2.
B)-1.
C)0.
D)+1.
E)+2.
A)-2.
B)-1.
C)0.
D)+1.
E)+2.

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
49
Classify the Ca - Cl bond in CaCl2 as ionic, polar covalent, or nonpolar covalent.
A)ionic
B)polar covalent
C)nonpolar covalent
A)ionic
B)polar covalent
C)nonpolar covalent

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
50
The total number of lone pairs in NCl3 is
A)6.
B)8.
C)9.
D)10.
E)13.
A)6.
B)8.
C)9.
D)10.
E)13.

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
51
Which of the following is a useful guideline for the application of formal charges in neutral molecules?
A)A Lewis structure in which there are no formal charges is preferred.
B)Lewis structures with large formal charges are preferred.
C)The preferred Lewis structure is one in which positive formal charges are on the most electronegative atoms.
A)A Lewis structure in which there are no formal charges is preferred.
B)Lewis structures with large formal charges are preferred.
C)The preferred Lewis structure is one in which positive formal charges are on the most electronegative atoms.

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
52
Which of the following Lewis structures is incorrect?
A)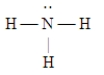
B)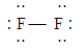
C)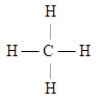
D)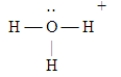
E)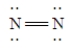
A)
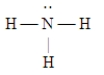
B)
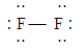
C)

D)
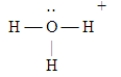
E)
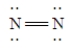

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
53
The electron dot formula for O2 shows
A)a single covalent bond.
B)a double covalent bond.
C)an ionic bond.
D)a total of 8 x 2 = 16 electron dots.
E)a total of 32 electron dots.
A)a single covalent bond.
B)a double covalent bond.
C)an ionic bond.
D)a total of 8 x 2 = 16 electron dots.
E)a total of 32 electron dots.

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
54
Assuming the octet rule is obeyed, how many covalent bonds will an oxygen atom form to give a formal charge of zero?
A)0
B)1
C)2
D)3
E)4
A)0
B)1
C)2
D)3
E)4

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
55
The number of resonance structures for the sulfur dioxide molecule that satisfy the octet rule is
A)1.
B)2.
C)3.
D)4.
E)None of these.
A)1.
B)2.
C)3.
D)4.
E)None of these.

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
56
The number of lone electron pairs in the NO2- ion is ___.
A)4
B)5
C)6
D)7
E)8
A)4
B)5
C)6
D)7
E)8

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
57
What is the formal charge on the oxygen atom in N2O (the atomic order is N-N-O)?
A)0
B)+1
C)-1
D)-2
E)+2
A)0
B)+1
C)-1
D)-2
E)+2

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
58
The azide ion, N3-, is very reactive although it is isoelectronic with the very stable CO2 molecule. This reactivity is reasonable inasmuch as
A)a Lewis structure cannot be written for the azide ion that has nitrogen formal charges of zero.
B)there is no valid Lewis structure possible for the azide ion.
C)there are resonance structures for azide ion but not for carbon dioxide.
D)nitrogen cannot form multiple bonds.
E)charged species always decompose in solution.
A)a Lewis structure cannot be written for the azide ion that has nitrogen formal charges of zero.
B)there is no valid Lewis structure possible for the azide ion.
C)there are resonance structures for azide ion but not for carbon dioxide.
D)nitrogen cannot form multiple bonds.
E)charged species always decompose in solution.

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
59
The electron dot structure for AsCl3 shows
A)a total of 84 electron dots.
B)three single bonds and 10 lone pairs.
C)two single bonds, one double bond, and 9 lone pairs.
D)one single bond, two double bonds, and 8 lone pairs.
E)three single bonds and one lone pair.
A)a total of 84 electron dots.
B)three single bonds and 10 lone pairs.
C)two single bonds, one double bond, and 9 lone pairs.
D)one single bond, two double bonds, and 8 lone pairs.
E)three single bonds and one lone pair.

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
60
The number of resonance structures for the nitrate ion that satisfy the octet rule is
A)1.
B)2.
C)3.
D)4.
E)None of these.
A)1.
B)2.
C)3.
D)4.
E)None of these.

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
61
What is the formal charge on phosphorus in a Lewis structure for the phosphate ion that satisfies the octet rule?
A)-2
B)-1
C)0
D)+1
E)+2
A)-2
B)-1
C)0
D)+1
E)+2

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
62
In the best Lewis structure for the fulminate ion, CNO-, what is the formal charge on the central nitrogen atom?
A)+2
B)+1
C)0
D)-1
E)-2
A)+2
B)+1
C)0
D)-1
E)-2

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
63
Which one of the following molecules has an atom with an expanded octet?
A)HCl
B)AsCl5
C)ICl
D)NCl3
E)Cl2
A)HCl
B)AsCl5
C)ICl
D)NCl3
E)Cl2

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
64
Which one of the following molecules has an atom with an incomplete octet?
A)NF3
B)H2O
C)AsCl3
D)GeH4
E)BF3
A)NF3
B)H2O
C)AsCl3
D)GeH4
E)BF3

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
65
Which response includes all the molecules below that do not follow the octet rule? (1)H2S (2)BCl3 (3)PH3 (4)SF4
A)(2)and (4)
B)(2)and (3)
C)(1)and (2)
D)(3)and (4)
E)(1)and (4)
A)(2)and (4)
B)(2)and (3)
C)(1)and (2)
D)(3)and (4)
E)(1)and (4)

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
66
What is total number of lone pairs in the best Lewis structure for SOF4 that exceeds the octet rule (S is the central atom)?
A)0
B)2
C)14
D)16
E)18
A)0
B)2
C)14
D)16
E)18

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
67
What is the formal charge on sulfur in the best Lewis structure for the SCN- (thiocyanate)ion?
A)+2
B)-2
C)+1
D)-1
E)0
A)+2
B)-2
C)+1
D)-1
E)0

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
68
Which of the elements listed below is most likely to exhibit an expanded octet in its compounds?
A)O
B)S
C)Na
D)C
E)N
A)O
B)S
C)Na
D)C
E)N

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
69
The formal charge on the sulfur atom in the resonance structure of sulfur dioxide which has one single bond and one double bond is
A)0.
B)+1.
C)-1.
D)+2.
E)-2.
A)0.
B)+1.
C)-1.
D)+2.
E)-2.

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
70
Each of the three resonance structures of NO3- has how many lone pairs of electrons?
A)7
B)8
C)9
D)10
E)13
A)7
B)8
C)9
D)10
E)13

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
71
Which one of the following compounds does not follow the octet rule?
A)NF3
B)CO2
C)CF4
D)Br2
E)NO
A)NF3
B)CO2
C)CF4
D)Br2
E)NO

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
72
For which of these species does the best Lewis structure have two or more equivalent resonance structures?
A)HCO2-
B)SCN-
C)CNO-
D)N3-
E)CO2
A)HCO2-
B)SCN-
C)CNO-
D)N3-
E)CO2

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
73
Which of the following substances will display an incomplete octet in its Lewis structure?
A)CO2
B)Cl2
C)ICl
D)NO
E)SO2
A)CO2
B)Cl2
C)ICl
D)NO
E)SO2

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
74
BeF42- is called the fluoberyllate ion. The formal charge on the beryllium atom in this ion is
A)-2.
B)-1.
C)0.
D)+1.
E)+2.
A)-2.
B)-1.
C)0.
D)+1.
E)+2.

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
75
Which one of the following compounds does not follow the octet rule?
A)NF3
B)CF4
C)PF5
D)AsH3
E)HCl
A)NF3
B)CF4
C)PF5
D)AsH3
E)HCl

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
76
In the Lewis structure of the iodate ion, IO3-, that satisfies the octet rule, the formal charge on the central iodine atom is
A)+2.
B)+1.
C)0.
D)-1.
E)-2.
A)+2.
B)+1.
C)0.
D)-1.
E)-2.

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
77
How many covalent bonds will be drawn to bromine in BrO3- for the dot structure that expands the octet to minimize formal charge and if necessary places negative formal charges on the most electronegative atom(s)?
A)3
B)4
C)5
D)6
E)7
A)3
B)4
C)5
D)6
E)7

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
78
What is the formal charge on the singly bonded oxygens in the Lewis structure for the carbonate ion?
A)-2
B)-1
C)0
D)+1
E)+2
A)-2
B)-1
C)0
D)+1
E)+2

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
79
Nitrous oxide, N2O, is sometimes called "laughing gas". What is the formal charge on the central nitrogen atom in the best Lewis structure for nitrous oxide? (The atom connectivity is N-N-O.)
A)-2
B)-1
C)0
D)+1
E)+2
A)-2
B)-1
C)0
D)+1
E)+2

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck
80
How many covalent bonds will be drawn to phosphorous in PO43- for the dot structure that expands the octet to minimize formal charge and if necessary places negative formal charges on the most electronegative atom(s)?
A)4
B)5
C)6
D)7
E)8
A)4
B)5
C)6
D)7
E)8

Unlock Deck
Unlock for access to all 118 flashcards in this deck.
Unlock Deck
k this deck


