Deck 10: Chemical Bonding Ii: Molecular Geometry and Hybridization of Atomic Orbitals
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/120
Play
Full screen (f)
Deck 10: Chemical Bonding Ii: Molecular Geometry and Hybridization of Atomic Orbitals
1
Give the number of lone pairs around the central atom and the molecular geometry of XeF2.
A)0 lone pairs, linear
B)1 lone pair, bent
C)2 lone pairs, bent
D)3 lone pairs, bent
E)3 lone pairs, linear
A)0 lone pairs, linear
B)1 lone pair, bent
C)2 lone pairs, bent
D)3 lone pairs, bent
E)3 lone pairs, linear
3 lone pairs, linear
2
Give the number of lone pairs around the central atom and the geometry of the ion SeO42-.
A)0 lone pairs, square planar
B)0 lone pairs, tetrahedral
C)1 lone pair, distorted tetrahedron (seesaw)
D)1 lone pair, square pyramidal
E)2 lone pairs, square planar
A)0 lone pairs, square planar
B)0 lone pairs, tetrahedral
C)1 lone pair, distorted tetrahedron (seesaw)
D)1 lone pair, square pyramidal
E)2 lone pairs, square planar
0 lone pairs, tetrahedral
3
Give the number of lone pairs around the central atom and the geometry of the ion NO2-.
A)0 lone pairs, linear
B)1 lone pair, bent
C)2 lone pair, bent
D)3 lone pairs, bent
E)3 lone pairs, linear
A)0 lone pairs, linear
B)1 lone pair, bent
C)2 lone pair, bent
D)3 lone pairs, bent
E)3 lone pairs, linear
1 lone pair, bent
4
The geometry of the ClF3 molecule is best described as
A)distorted tetrahedron.
B)trigonal planar.
C)tetrahedral.
D)T-shaped.
E)trigonal pyramidal.
A)distorted tetrahedron.
B)trigonal planar.
C)tetrahedral.
D)T-shaped.
E)trigonal pyramidal.

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
5
Give the number of lone pairs around the central atom and the molecular geometry of SCl2.
A)0 lone pairs, linear
B)1 lone pair, bent
C)2 lone pairs, bent
D)3 lone pairs, bent
E)3 lone pairs, linear
A)0 lone pairs, linear
B)1 lone pair, bent
C)2 lone pairs, bent
D)3 lone pairs, bent
E)3 lone pairs, linear

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
6
Give the number of lone pairs around the central atom and the geometry of the ion ClO3-.
A)0 lone pairs, trigonal
B)1 lone pair, bent
C)1 lone pair, trigonal pyramidal
D)2 lone pairs, T-shaped
E)2 lone pairs, trigonal
A)0 lone pairs, trigonal
B)1 lone pair, bent
C)1 lone pair, trigonal pyramidal
D)2 lone pairs, T-shaped
E)2 lone pairs, trigonal

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
7
Give the number of lone pairs around the central atom and the geometry of the ion IBr2-.
A)0 lone pairs, linear
B)1 lone pair, bent
C)2 lone pairs, bent
D)3 lone pairs, bent
E)3 lone pairs, linear
A)0 lone pairs, linear
B)1 lone pair, bent
C)2 lone pairs, bent
D)3 lone pairs, bent
E)3 lone pairs, linear

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
8
According to VSEPR theory, the geometry of the PH3 molecule is best described as
A)linear.
B)trigonal planar.
C)tetrahedral.
D)bent.
E)trigonal pyramidal.
A)linear.
B)trigonal planar.
C)tetrahedral.
D)bent.
E)trigonal pyramidal.

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
9
Give the number of lone pairs around the central atom and the molecular geometry of XeF4.
A)0 lone pairs, tetrahedral
B)1 lone pair, distorted tetrahedron (seesaw)
C)1 lone pair, square pyramidal
D)1 lone pair, tetrahedral
E)2 lone pairs, square planar
A)0 lone pairs, tetrahedral
B)1 lone pair, distorted tetrahedron (seesaw)
C)1 lone pair, square pyramidal
D)1 lone pair, tetrahedral
E)2 lone pairs, square planar

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
10
Give the number of lone pairs around the central atom and the molecular geometry of IF5.
A)0 lone pairs, square pyramidal
B)0 lone pairs, trigonal bipyramidal
C)1 lone pair, octahedral
D)1 lone pair, square pyramidal
E)2 lone pairs, pentagonal
A)0 lone pairs, square pyramidal
B)0 lone pairs, trigonal bipyramidal
C)1 lone pair, octahedral
D)1 lone pair, square pyramidal
E)2 lone pairs, pentagonal

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
11
According to the VSEPR theory, the geometry of the SO3 molecule is
A)pyramidal.
B)tetrahedral.
C)trigonal planar.
D)distorted tetrahedron (seesaw).
E)square planar.
A)pyramidal.
B)tetrahedral.
C)trigonal planar.
D)distorted tetrahedron (seesaw).
E)square planar.

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
12
Give the number of lone pairs around the central atom and the geometry of the ion ClO2-.
A)0 lone pairs, linear
B)1 lone pair, bent
C)2 lone pairs, bent
D)3 lone pairs, bent
E)3 lone pairs, linear
A)0 lone pairs, linear
B)1 lone pair, bent
C)2 lone pairs, bent
D)3 lone pairs, bent
E)3 lone pairs, linear

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
13
According to the VSEPR theory, the molecular geometry of the carbonate ion, CO32 -, is
A)square planar.
B)tetrahedral.
C)pyramidal.
D)trigonal planar.
E)octahedral.
A)square planar.
B)tetrahedral.
C)pyramidal.
D)trigonal planar.
E)octahedral.

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
14
Give the number of lone pairs around the central atom and the molecular geometry of SeF4.
A)0 lone pairs, tetrahedral
B)1 lone pair, distorted tetrahedron (seesaw)
C)1 lone pair, square pyramidal
D)1 lone pair, tetrahedral
E)2 lone pairs, square planar
A)0 lone pairs, tetrahedral
B)1 lone pair, distorted tetrahedron (seesaw)
C)1 lone pair, square pyramidal
D)1 lone pair, tetrahedral
E)2 lone pairs, square planar

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
15
Give the number of lone pairs around the central atom and the geometry of the ion PCl4-.
A)0 lone pairs, tetrahedral
B)1 lone pair, distorted tetrahedron (seesaw)
C)1 lone pair, square pyramidal
D)1 lone pair, tetrahedral
E)2 lone pairs, square planar
A)0 lone pairs, tetrahedral
B)1 lone pair, distorted tetrahedron (seesaw)
C)1 lone pair, square pyramidal
D)1 lone pair, tetrahedral
E)2 lone pairs, square planar

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
16
The geometry of the CS2 molecule is best described as
A)linear.
B)trigonal planar.
C)tetrahedral.
D)bent.
E)trigonal pyramidal.
A)linear.
B)trigonal planar.
C)tetrahedral.
D)bent.
E)trigonal pyramidal.

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
17
The geometry of the SF4 molecule is
A)tetrahedral.
B)trigonal pyramidal.
C)trigonal planar.
D)square planar.
E)distorted tetrahedron (seesaw).
A)tetrahedral.
B)trigonal pyramidal.
C)trigonal planar.
D)square planar.
E)distorted tetrahedron (seesaw).

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
18
According to the VSEPR theory, the molecular geometry of beryllium chloride is
A)linear.
B)trigonal planar.
C)bent.
D)tetrahedral.
E)trigonal pyramidal.
A)linear.
B)trigonal planar.
C)bent.
D)tetrahedral.
E)trigonal pyramidal.

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
19
Give the number of lone pairs around the central atom and the molecular geometry of CBr4.
A)0 lone pairs, square planar
B)0 lone pairs, tetahedral
C)1 lone pair, square pyramidal
D)1 lone pair, trigonal bipyramidal
E)2 lone pairs, square planar
A)0 lone pairs, square planar
B)0 lone pairs, tetahedral
C)1 lone pair, square pyramidal
D)1 lone pair, trigonal bipyramidal
E)2 lone pairs, square planar

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
20
Use VSEPR theory to predict the geometry of the PCl3 molecule.
A)linear
B)bent
C)trigonal planar
D)trigonal pyramidal
E)tetrahedral
A)linear
B)bent
C)trigonal planar
D)trigonal pyramidal
E)tetrahedral

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
21
Which one of the following molecules has tetrahedral geometry?
A)XeF4
B)BF3
C)AsF5
D)CF4
E)NH3
A)XeF4
B)BF3
C)AsF5
D)CF4
E)NH3

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
22
According to VSEPR theory, which one of the following molecules should be nonlinear?
A)CO2
B)C2H2
C)SO2
D)BeCl2
E)KrF2
A)CO2
B)C2H2
C)SO2
D)BeCl2
E)KrF2

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
23
According to VSEPR theory, which one of the following molecules has tetrahedral geometry?
A)NH3
B)CCl4
C)CO2
D)SF4
E)PCl5
A)NH3
B)CCl4
C)CO2
D)SF4
E)PCl5

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
24
According to the VSEPR theory, the molecular geometry of boron trichloride is
A)linear.
B)trigonal planar.
C)bent.
D)tetrahedral.
E)trigonal pyramidal.
A)linear.
B)trigonal planar.
C)bent.
D)tetrahedral.
E)trigonal pyramidal.

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
25
The bond angle in Cl2O is expected to be approximately
A)90°.
B)109.5°.
C)120°.
D)145°.
E)180°.
A)90°.
B)109.5°.
C)120°.
D)145°.
E)180°.

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
26
The bond angles in SF5+ are expected to be
A)90°.
B)120°.
C)90° and 120°.
D)90° and 180°.
E)90°, 120°, and 180°.
A)90°.
B)120°.
C)90° and 120°.
D)90° and 180°.
E)90°, 120°, and 180°.

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
27
Which of the following substance is/are planar? (i)SO3 (ii)SO32- (iii)NO3- (iv)PF3 (v)BF3
A)only (i)and (ii)
B)only (i), (iii), and (v)
C)only (iv)
D)all are planar except (iv)
E)all are planar except (ii)
A)only (i)and (ii)
B)only (i), (iii), and (v)
C)only (iv)
D)all are planar except (iv)
E)all are planar except (ii)

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
28
Predict the geometry around the central atom in PO43-.
A)trigonal planar
B)trigonal pyramidal
C)tetrahedral
D)trigonal bipyramidal
E)octahedral
A)trigonal planar
B)trigonal pyramidal
C)tetrahedral
D)trigonal bipyramidal
E)octahedral

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
29
The bond angle in SCl2 is expected to be
A)a little less than 109.5°.
B)109.5°.
C)a little more than 109.5°.
D)120°.
E)180°.
A)a little less than 109.5°.
B)109.5°.
C)a little more than 109.5°.
D)120°.
E)180°.

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
30
The F - S - F bond angles in SF6 are
A)90° and 180°.
B)109.5°.
C)120°.
D)180°.
E)90° and 120°.
A)90° and 180°.
B)109.5°.
C)120°.
D)180°.
E)90° and 120°.

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
31
The bond angles in CO32- are expected to be
A)a little less than 109.5°.
B)109.5°.
C)a little less than 120°.
D)120°.
E)a little more than 120°.
A)a little less than 109.5°.
B)109.5°.
C)a little less than 120°.
D)120°.
E)a little more than 120°.

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
32
According to the VSEPR theory, the molecular geometry of ammonia is
A)linear.
B)trigonal planar.
C)bent.
D)tetrahedral.
E)trigonal pyramidal.
A)linear.
B)trigonal planar.
C)bent.
D)tetrahedral.
E)trigonal pyramidal.

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
33
According to VSEPR theory, which one of the following species has a tetrahedral geometry?
A)IF4+
B)IF4-
C)PCl4+
D)PCl4-
E)SeF4
A)IF4+
B)IF4-
C)PCl4+
D)PCl4-
E)SeF4

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
34
Predict the geometry around the central atom in XeO4.
A)trigonal planar
B)trigonal pyramidal
C)tetrahedral
D)trigonal bipyramidal
E)octahedral
A)trigonal planar
B)trigonal pyramidal
C)tetrahedral
D)trigonal bipyramidal
E)octahedral

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
35
According to the VSEPR theory, which one of the following species should be linear?
A)H2S
B)HCN
C)BF3
D)H2CO
E)SO2
A)H2S
B)HCN
C)BF3
D)H2CO
E)SO2

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
36
Which of the following substances is/are bent?
(i)H2S
(ii)CO2
(iii)ClNO
(iv)NH2-
(v)O3
A)only (iii)
B)only (i)and (v)
C)only (i), (iii), and (v)
D)all are bent except for (iv)
E)all are bent except for (ii)
(i)H2S
(ii)CO2
(iii)ClNO
(iv)NH2-
(v)O3
A)only (iii)
B)only (i)and (v)
C)only (i), (iii), and (v)
D)all are bent except for (iv)
E)all are bent except for (ii)

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
37
According to the VSEPR theory, the molecular geometry of SiCl4 is
A)linear.
B)trigonal planar.
C)bent.
D)tetrahedral.
E)trigonal pyramidal.
A)linear.
B)trigonal planar.
C)bent.
D)tetrahedral.
E)trigonal pyramidal.

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
38
The bond angle in ICl2- is expected to be
A)a little less than 109.5°.
B)109.5°.
C)a little more than 109.5°.
D)120°.
E)180°.
A)a little less than 109.5°.
B)109.5°.
C)a little more than 109.5°.
D)120°.
E)180°.

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
39
According to VSEPR theory, which one of the following molecules should have a geometry that is trigonal bipyramidal?
A)SF4
B)XeF4
C)NF3
D)SF6
E)PF5
A)SF4
B)XeF4
C)NF3
D)SF6
E)PF5

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
40
Predict the geometry around the central atom in SO42-.
A)trigonal planar
B)trigonal pyramidal
C)tetrahedral
D)trigonal bipyramidal
E)octahedral
A)trigonal planar
B)trigonal pyramidal
C)tetrahedral
D)trigonal bipyramidal
E)octahedral

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
41
Predict the molecular geometry and polarity of the SO2 molecule.
A)linear, polar
B)linear, nonpolar
C)bent, polar
D)bent, nonpolar
E)None of the above.
A)linear, polar
B)linear, nonpolar
C)bent, polar
D)bent, nonpolar
E)None of the above.

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
42
Which of the following species has the largest dipole moment (i.e., is the most polar)?
A)CH4
B)CH3Br
C)CH3Cl
D)CH3F
E)CH3I
A)CH4
B)CH3Br
C)CH3Cl
D)CH3F
E)CH3I

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
43
N,N-diethyl-m-tolumide (DEET)is the active ingredient in many mosquito repellents. What is the hybridization state of carbon indicated by the arrow in the structure of DEET shown below? 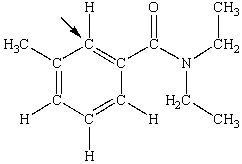
A)sp
B)sp2
C)sp3
D)sp3d
E)sp3d2

A)sp
B)sp2
C)sp3
D)sp3d
E)sp3d2

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
44
Ibuprofen is used as an analgesic for the relief of pain, and also to help reduce fever. What is the hybridization state of carbon indicated by the arrow in the structure of ibuprofen shown below? 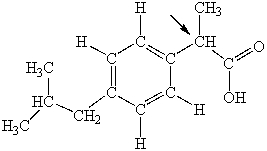
A)sp
B)sp2
C)sp3
D)sp3d
E)sp3d2

A)sp
B)sp2
C)sp3
D)sp3d
E)sp3d2

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
45
The F -Cl -F bond angles in ClF3 are expected to be approximately
A)90° only.
B)109.5° only.
C)120° only.
D)180° only.
E)90° and 180°.
A)90° only.
B)109.5° only.
C)120° only.
D)180° only.
E)90° and 180°.

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
46
N,N-diethyl-m-tolumide (DEET)is the active ingredient in many mosquito repellents. What is the hybridization state of the nitrogen atom in the structure of DEET shown below? 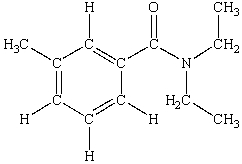
A)sp
B)sp2
C)sp3
D)sp3d
E)sp3d2

A)sp
B)sp2
C)sp3
D)sp3d
E)sp3d2

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
47
Predict the geometry and polarity of the CS2 molecule.
A)linear, polar
B)linear, nonpolar
C)tetrahedral, nonpolar
D)bent, nonpolar
E)bent, polar
A)linear, polar
B)linear, nonpolar
C)tetrahedral, nonpolar
D)bent, nonpolar
E)bent, polar

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
48
Which one of the following molecules has a non-zero dipole moment?
A)BeCl2
B)Br2
C)BF3
D)IBr
E)CO2
A)BeCl2
B)Br2
C)BF3
D)IBr
E)CO2

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
49
Which one of the following molecules has a zero dipole moment?
A)CO
B)CH2Cl2
C)SO3
D)SO2
E)NH3
A)CO
B)CH2Cl2
C)SO3
D)SO2
E)NH3

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
50
The C-N-O bond angle in nitromethane, CH3NO2, is expected to be approximately
A)60°.
B)90°.
C)109.5°.
D)120°.
E)180°.
A)60°.
B)90°.
C)109.5°.
D)120°.
E)180°.

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
51
Indicate the type of hybrid orbitals used by the central atom in PCl3.
A)sp
B)sp2
C)sp3
D)sp3d
E)sp3d2
A)sp
B)sp2
C)sp3
D)sp3d
E)sp3d2

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
52
Ibuprofen is used as an analgesic for the relief of pain, and also to help reduce fever. What is the hybridization state of carbon indicated by the arrow in the structure of ibuprofen shown below? 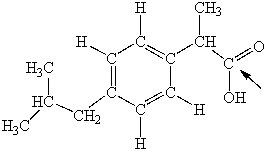
A)sp
B)sp2
C)sp3
D)sp3d
E)sp3d2

A)sp
B)sp2
C)sp3
D)sp3d
E)sp3d2

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
53
Ibuprofen is used as an analgesic for the relief of pain, and also to help reduce fever. What is the hybridization state of oxygen indicated by the arrow in the structure of ibuprofen shown below? 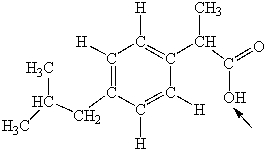
A)sp
B)sp2
C)sp3
D)sp3d
E)sp3d2

A)sp
B)sp2
C)sp3
D)sp3d
E)sp3d2

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
54
N,N-diethyl-m-tolumide (DEET)is the active ingredient in many mosquito repellents. What is the hybridization state of carbon indicated by the arrow in the structure of DEET shown below? 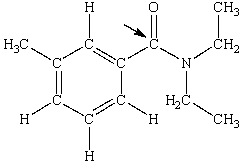
A)sp
B)sp2
C)sp3
D)sp3d
E)sp3d2

A)sp
B)sp2
C)sp3
D)sp3d
E)sp3d2

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
55
Which one of the following molecules is nonpolar?
A)NH3
B)OF2
C)CH3Cl
D)H2O
E)BeCl2
A)NH3
B)OF2
C)CH3Cl
D)H2O
E)BeCl2

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
56
According to the VSEPR theory, the actual F -As -F bond angles in the AsF4- ion are predicted to be
A)109.5°.
B)90° and 120°.
C)180°.
D)< 109.5°.
E)< 90° and < 120°.
A)109.5°.
B)90° and 120°.
C)180°.
D)< 109.5°.
E)< 90° and < 120°.

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
57
Complete this sentence: The PCl5 molecule has
A)nonpolar bonds, and is a nonpolar molecule.
B)nonpolar bonds, but is a polar molecule.
C)polar bonds, and is a polar molecule.
D)polar bonds, but is a nonpolar molecule.
A)nonpolar bonds, and is a nonpolar molecule.
B)nonpolar bonds, but is a polar molecule.
C)polar bonds, and is a polar molecule.
D)polar bonds, but is a nonpolar molecule.

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
58
Ibuprofen is used as an analgesic for the relief of pain, and also to help reduce fever. What is the hybridization state of carbon indicated by the arrow in the structure of ibuprofen shown below? 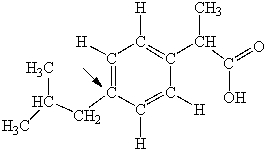
A)sp
B)sp2
C)sp3
D)sp3d
E)sp3d2

A)sp
B)sp2
C)sp3
D)sp3d
E)sp3d2

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
59
Which one of the following molecules is polar?
A)PBr5
B)CCl4
C)BrF5
D)XeF2
E)XeF4
A)PBr5
B)CCl4
C)BrF5
D)XeF2
E)XeF4

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
60
N,N-diethyl-m-tolumide (DEET)is the active ingredient in many mosquito repellents. What is the hybridization state of carbon indicated by the arrow in the structure of DEET shown below? 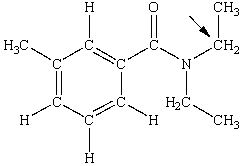
A)sp
B)sp2
C)sp3
D)sp3d
E)sp3d2

A)sp
B)sp2
C)sp3
D)sp3d
E)sp3d2

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
61
What is the hybridization of the As atom in the AsF5 molecule?
A)sp
B)sp2
C)sp3
D)sp3d
E)sp3d2
A)sp
B)sp2
C)sp3
D)sp3d
E)sp3d2

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
62
What is the hybridization of As in the AsF4- ion?
A)sp
B)sp2
C)sp3
D)sp3d
E)sp3d2
A)sp
B)sp2
C)sp3
D)sp3d
E)sp3d2

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
63
Consider the species Cl2+, Cl2, and Cl2-. Which of these species will be paramagnetic?
A)only Cl2
B)Cl2+ and Cl2
C)Cl2 and Cl2-
D)Cl2+ and Cl2-
E)all three are paramagnetic
A)only Cl2
B)Cl2+ and Cl2
C)Cl2 and Cl2-
D)Cl2+ and Cl2-
E)all three are paramagnetic

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
64
The number of pi bonds in the molecule below is 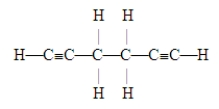
A)2.
B)4.
C)6.
D)10.
E)15.

A)2.
B)4.
C)6.
D)10.
E)15.

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
65
In which of these molecules do the two nitrogen atoms have different hybridizations?
A)NH4NO3
B)N2H4
C)N2O4
D)N2O5
E)none of these
A)NH4NO3
B)N2H4
C)N2O4
D)N2O5
E)none of these

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
66
Which of the following molecules have the same geometries?
A)SF4 and CH4
B)CO2 and H2O
C)CO2 and BeH2
D)N2O and NO2
A)SF4 and CH4
B)CO2 and H2O
C)CO2 and BeH2
D)N2O and NO2

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
67
What is the hybridization of the iodine atom in the IF5 molecule?
A)sp
B)sp2
C)sp3
D)sp3d
E)sp3d2
A)sp
B)sp2
C)sp3
D)sp3d
E)sp3d2

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
68
Indicate the type of hybrid orbitals used by the central atom in CCl4.
A)sp
B)sp2
C)sp3
D)sp3d
E)sp3d2
A)sp
B)sp2
C)sp3
D)sp3d
E)sp3d2

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
69
Indicate the type of hybrid orbitals used by the central atom in BrF3.
A)sp
B)sp2
C)sp3
D)sp3d
E)sp3d2
A)sp
B)sp2
C)sp3
D)sp3d
E)sp3d2

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
70
What is the hybridization of the central atom in ClO3- ?
A)sp
B)sp2
C)sp3
D)sp3d
E)sp3d2
A)sp
B)sp2
C)sp3
D)sp3d
E)sp3d2

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
71
Indicate the type of hybrid orbitals used by the central atom in SF6.
A)sp
B)sp2
C)sp3
D)sp3d
E)sp3d2
A)sp
B)sp2
C)sp3
D)sp3d
E)sp3d2

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
72
Consider the species O2-, O2, and O2+. Which of these species will be paramagnetic?
A)O2 and O2-
B)O2+ and O2
C)O2+ and O2-
D)only O2
E)all three are paramagnetic
A)O2 and O2-
B)O2+ and O2
C)O2+ and O2-
D)only O2
E)all three are paramagnetic

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
73
Indicate the type of hybrid orbitals used by the central atom in TeF4.
A)sp
B)sp2
C)sp3
D)sp3d
E)sp3d2
A)sp
B)sp2
C)sp3
D)sp3d
E)sp3d2

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
74
Which of the following species have the same geometries?
A)NH2- and H2O
B)NH2- and BeH2
C)H2O and BeH2
D)NH2-, H2O, and BeH2
A)NH2- and H2O
B)NH2- and BeH2
C)H2O and BeH2
D)NH2-, H2O, and BeH2

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
75
If a triatomic molecule is linear, then the hybridization of the central atom will be
A)sp.
B)sp2.
C)sp or sp3.
D)sp or sp3d.
E)sp2 or sp3d2.
A)sp.
B)sp2.
C)sp or sp3.
D)sp or sp3d.
E)sp2 or sp3d2.

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
76
The hybridization of the central nitrogen atom in the molecule N2O is
A)sp.
B)sp2.
C)sp3.
D)sp3d.
E)sp3d2.
A)sp.
B)sp2.
C)sp3.
D)sp3d.
E)sp3d2.

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
77
The number of pi bonds in the molecule below is 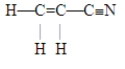
A)1.
B)2.
C)3.
D)5.
E)9.
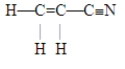
A)1.
B)2.
C)3.
D)5.
E)9.

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
78
In which one of the following molecules is the central atom sp2 hybridized?
A)SO2
B)N2O
C)BeCl2
D)NF3
E)PF5
A)SO2
B)N2O
C)BeCl2
D)NF3
E)PF5

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
79
What is the hybridization on the central atom in NO3- ?
A)sp
B)sp2
C)sp3
D)sp3d
E)sp3d2
A)sp
B)sp2
C)sp3
D)sp3d
E)sp3d2

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck
80
The number of pi bonds in the oxalate ion (C2O42-)is
A)1.
B)2.
C)3.
D)4.
E)5.
A)1.
B)2.
C)3.
D)4.
E)5.

Unlock Deck
Unlock for access to all 120 flashcards in this deck.
Unlock Deck
k this deck


