Deck 15: Chemical Equilibrium
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/99
Play
Full screen (f)
Deck 15: Chemical Equilibrium
1
Consider the two gaseous equilibria: SO2(g)+ 1/2O2(g) 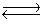 SO3(g)K1
SO3(g)K1
2SO3(g)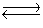 SO2(g)+ O2(g)K2
SO2(g)+ O2(g)K2
The values of the equilibrium constants K1 and K2 are related by
A)K2 = K12
B)K22 = K1
C)K2 = 1/K
D)K2 = 1/K1
E)none of these.
 SO3(g)K1
SO3(g)K12SO3(g)
 SO2(g)+ O2(g)K2
SO2(g)+ O2(g)K2The values of the equilibrium constants K1 and K2 are related by
A)K2 = K12
B)K22 = K1
C)K2 = 1/K
D)K2 = 1/K1
E)none of these.
K2 = 1/K 
2
The equilibrium constant expression for the reaction 2BrF5(g) ![<strong>The equilibrium constant expression for the reaction 2BrF<sub>5</sub>(g) Br<sub>2</sub>(g)+ 5F<sub>2</sub>(g)is</strong> A)K<sub>c</sub> = [Br<sub>2</sub>] [F<sub>2</sub>]<sup> </sup>/<sup> </sup>[BrF<sub>5</sub>] B)K<sub>c</sub> = [Br<sub>2</sub>] [F<sub>2</sub>]<sup>5 </sup>/<sup> </sup>[BrF<sub>5</sub>]<sup>2</sup> C)K<sub>c</sub> = [Br<sub>2</sub>] [F<sub>2</sub>]<sup>2 </sup>/<sup> </sup>[BrF<sub>5</sub>]<sup>5</sup> D)K<sub>c</sub> = [BrF<sub>5</sub>]<sup>2 </sup>/<sup> </sup>[Br<sub>2</sub>][F<sub>2</sub>]<sup>5</sup> E)K<sub>c</sub> = 2[BrF<sub>5</sub>]<sup>2 </sup>/<sup> </sup>([Br<sub>2</sub>] * 5[F<sub>2</sub>]<sup>5</sup>)](https://storage.examlex.com/TB3244/11ea7995_6655_854d_911b_4b21a08d71ee_TB3244_00.jpg) Br2(g)+ 5F2(g)is
Br2(g)+ 5F2(g)is
A)Kc = [Br2] [F2] / [BrF5]
B)Kc = [Br2] [F2]5 / [BrF5]2
C)Kc = [Br2] [F2]2 / [BrF5]5
D)Kc = [BrF5]2 / [Br2][F2]5
E)Kc = 2[BrF5]2 / ([Br2] * 5[F2]5)
![<strong>The equilibrium constant expression for the reaction 2BrF<sub>5</sub>(g) Br<sub>2</sub>(g)+ 5F<sub>2</sub>(g)is</strong> A)K<sub>c</sub> = [Br<sub>2</sub>] [F<sub>2</sub>]<sup> </sup>/<sup> </sup>[BrF<sub>5</sub>] B)K<sub>c</sub> = [Br<sub>2</sub>] [F<sub>2</sub>]<sup>5 </sup>/<sup> </sup>[BrF<sub>5</sub>]<sup>2</sup> C)K<sub>c</sub> = [Br<sub>2</sub>] [F<sub>2</sub>]<sup>2 </sup>/<sup> </sup>[BrF<sub>5</sub>]<sup>5</sup> D)K<sub>c</sub> = [BrF<sub>5</sub>]<sup>2 </sup>/<sup> </sup>[Br<sub>2</sub>][F<sub>2</sub>]<sup>5</sup> E)K<sub>c</sub> = 2[BrF<sub>5</sub>]<sup>2 </sup>/<sup> </sup>([Br<sub>2</sub>] * 5[F<sub>2</sub>]<sup>5</sup>)](https://storage.examlex.com/TB3244/11ea7995_6655_854d_911b_4b21a08d71ee_TB3244_00.jpg) Br2(g)+ 5F2(g)is
Br2(g)+ 5F2(g)isA)Kc = [Br2] [F2] / [BrF5]
B)Kc = [Br2] [F2]5 / [BrF5]2
C)Kc = [Br2] [F2]2 / [BrF5]5
D)Kc = [BrF5]2 / [Br2][F2]5
E)Kc = 2[BrF5]2 / ([Br2] * 5[F2]5)
Kc = [Br2] [F2]5 / [BrF5]2
3
Equilibrium is established for the reaction 2X(s)+ Y(g) 2Z(g)at 500K, Kc = 100. Determine the concentration of Z in equilibrium with 0.2 mol X and 0.50 M Y at 500K.
A)3.2 M
B)3.5 M
C)4.5 M
D)7.1 M
E)None of these.
A)3.2 M
B)3.5 M
C)4.5 M
D)7.1 M
E)None of these.
7.1 M
4
The following reactions occur at 500 K. Arrange them in order of increasing tendency to proceed to completion (least completion greatest completion).
1)2NOCl 2NO + Cl2 Kp = 1.7 * 10- 2
2NO + Cl2 Kp = 1.7 * 10- 2
2)N2O4 11ec7153_21b4_20f5_88eb_03ce8bf4f194_TB3244_11 2NO2 Kp = 1.5 * 103
3)2SO3 11ec7153_21b4_20f5_88eb_03ce8bf4f194_TB3244_11 2SO2 + O2 Kp = 1.3 * 10 -5
4)2NO2 11ec7153_21b4_20f5_88eb_03ce8bf4f194_TB3244_11 2NO + O2 Kp = 5.9 * 10 -5
A)2 < 1 < 3 < 4
B)3 < 1 < 4 < 2
C)3 < 4 < 1 < 2
D)4 < 3 < 2 < 1
E)4 < 3 < 1 < 2
1)2NOCl
 2NO + Cl2 Kp = 1.7 * 10- 2
2NO + Cl2 Kp = 1.7 * 10- 22)N2O4 11ec7153_21b4_20f5_88eb_03ce8bf4f194_TB3244_11 2NO2 Kp = 1.5 * 103
3)2SO3 11ec7153_21b4_20f5_88eb_03ce8bf4f194_TB3244_11 2SO2 + O2 Kp = 1.3 * 10 -5
4)2NO2 11ec7153_21b4_20f5_88eb_03ce8bf4f194_TB3244_11 2NO + O2 Kp = 5.9 * 10 -5
A)2 < 1 < 3 < 4
B)3 < 1 < 4 < 2
C)3 < 4 < 1 < 2
D)4 < 3 < 2 < 1
E)4 < 3 < 1 < 2

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
5
At 250ºC, the equilibrium constant Kp for the reaction PCl5(g)  PCl3(g)+ Cl2(g)is 1.80. Sufficient PCl5 is put into a reaction vessel to give an initial pressure of 2.74 atm at 250ºC. Calculate the pressure of PCl5 after the system has reached equilibrium.
PCl3(g)+ Cl2(g)is 1.80. Sufficient PCl5 is put into a reaction vessel to give an initial pressure of 2.74 atm at 250ºC. Calculate the pressure of PCl5 after the system has reached equilibrium.
A)1.50 atm
B)1.24 atm
C)4.24 atm
D)0.94 atm
E)1.12 atm
 PCl3(g)+ Cl2(g)is 1.80. Sufficient PCl5 is put into a reaction vessel to give an initial pressure of 2.74 atm at 250ºC. Calculate the pressure of PCl5 after the system has reached equilibrium.
PCl3(g)+ Cl2(g)is 1.80. Sufficient PCl5 is put into a reaction vessel to give an initial pressure of 2.74 atm at 250ºC. Calculate the pressure of PCl5 after the system has reached equilibrium.A)1.50 atm
B)1.24 atm
C)4.24 atm
D)0.94 atm
E)1.12 atm

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
6
If one starts with pure NO2(g)at a pressure of 0.500 atm, the total pressure inside the reaction vessel when 2NO2(g)  2NO(g)+ O2(g)reaches equilibrium is 0.674 atm. Calculate the equilibrium partial pressure of NO2.
2NO(g)+ O2(g)reaches equilibrium is 0.674 atm. Calculate the equilibrium partial pressure of NO2.
A)0.152 atm
B)0.174 atm
C)0.200 atm
D)0.326 atm
E)The total pressure cannot be calculated because Kp is not given
 2NO(g)+ O2(g)reaches equilibrium is 0.674 atm. Calculate the equilibrium partial pressure of NO2.
2NO(g)+ O2(g)reaches equilibrium is 0.674 atm. Calculate the equilibrium partial pressure of NO2.A)0.152 atm
B)0.174 atm
C)0.200 atm
D)0.326 atm
E)The total pressure cannot be calculated because Kp is not given

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
7
Calculate Kc for the reaction 2HI(g) ![<strong>Calculate K<sub>c</sub> for the reaction 2HI(g)<sub> </sub> <sub> </sub> <sub> </sub>H<sub>2</sub>(g)+ I<sub>2</sub>(g)given that the concentrations of each species at equilibrium are as follows: [HI] = 0.85 mol/L, [I<sub>2</sub>] = 0.60 mol/L, [H<sub>2</sub>] = 0.27 mol/L.</strong> A)5.25 B)0.22 C)4.5 D)0.19 E)1.6 * 10<sup>2</sup>](https://storage.examlex.com/TB3244/11ec7153_74a6_4277_88eb_656263c228fc_TB3244_11.jpg) H2(g)+ I2(g)given that the concentrations of each species at equilibrium are as follows: [HI] = 0.85 mol/L, [I2] = 0.60 mol/L, [H2] = 0.27 mol/L.
H2(g)+ I2(g)given that the concentrations of each species at equilibrium are as follows: [HI] = 0.85 mol/L, [I2] = 0.60 mol/L, [H2] = 0.27 mol/L.
A)5.25
B)0.22
C)4.5
D)0.19
E)1.6 * 102
![<strong>Calculate K<sub>c</sub> for the reaction 2HI(g)<sub> </sub> <sub> </sub> <sub> </sub>H<sub>2</sub>(g)+ I<sub>2</sub>(g)given that the concentrations of each species at equilibrium are as follows: [HI] = 0.85 mol/L, [I<sub>2</sub>] = 0.60 mol/L, [H<sub>2</sub>] = 0.27 mol/L.</strong> A)5.25 B)0.22 C)4.5 D)0.19 E)1.6 * 10<sup>2</sup>](https://storage.examlex.com/TB3244/11ec7153_74a6_4277_88eb_656263c228fc_TB3244_11.jpg) H2(g)+ I2(g)given that the concentrations of each species at equilibrium are as follows: [HI] = 0.85 mol/L, [I2] = 0.60 mol/L, [H2] = 0.27 mol/L.
H2(g)+ I2(g)given that the concentrations of each species at equilibrium are as follows: [HI] = 0.85 mol/L, [I2] = 0.60 mol/L, [H2] = 0.27 mol/L.A)5.25
B)0.22
C)4.5
D)0.19
E)1.6 * 102

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
8
When the following reaction is at equilibrium, which of these relationships is always true?
2NOCl(g)![<strong>When the following reaction is at equilibrium, which of these relationships is always true? 2NOCl(g) 2NO(g)+ Cl<sub>2</sub>(g)</strong> A)[NO] [Cl<sub>2</sub>] = [NOCl] B)[NO]<sup>2</sup> [Cl<sub>2</sub>] = [NOCl]<sup>2</sup> C)[NOCl] = [NO] D)2[NO] = [Cl<sub>2</sub>] E)[NO]<sup>2</sup> [Cl<sub>2</sub>] = K<sub>c</sub>[NOCl]<sup>2</sup>](https://storage.examlex.com/TB3244/11ec7153_639f_4806_88eb_5baa5f980637_TB3244_11.jpg) 2NO(g)+ Cl2(g)
2NO(g)+ Cl2(g)
A)[NO] [Cl2] = [NOCl]
B)[NO]2 [Cl2] = [NOCl]2
C)[NOCl] = [NO]
D)2[NO] = [Cl2]
E)[NO]2 [Cl2] = Kc[NOCl]2
2NOCl(g)
![<strong>When the following reaction is at equilibrium, which of these relationships is always true? 2NOCl(g) 2NO(g)+ Cl<sub>2</sub>(g)</strong> A)[NO] [Cl<sub>2</sub>] = [NOCl] B)[NO]<sup>2</sup> [Cl<sub>2</sub>] = [NOCl]<sup>2</sup> C)[NOCl] = [NO] D)2[NO] = [Cl<sub>2</sub>] E)[NO]<sup>2</sup> [Cl<sub>2</sub>] = K<sub>c</sub>[NOCl]<sup>2</sup>](https://storage.examlex.com/TB3244/11ec7153_639f_4806_88eb_5baa5f980637_TB3244_11.jpg) 2NO(g)+ Cl2(g)
2NO(g)+ Cl2(g)A)[NO] [Cl2] = [NOCl]
B)[NO]2 [Cl2] = [NOCl]2
C)[NOCl] = [NO]
D)2[NO] = [Cl2]
E)[NO]2 [Cl2] = Kc[NOCl]2

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
9
The equilibrium between carbon dioxide gas and carbonic acid is very important in biology and environmental science. CO2(aq)+ H2O(l) 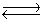 H2CO3(aq)
H2CO3(aq)
Which one of the following is the correct equilibrium constant expression (Kc)for this reaction?
A)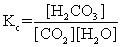
B)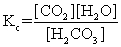
C)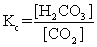
D)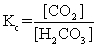
E)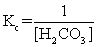
 H2CO3(aq)
H2CO3(aq)Which one of the following is the correct equilibrium constant expression (Kc)for this reaction?
A)
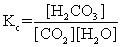
B)
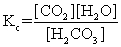
C)
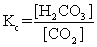
D)
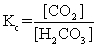
E)
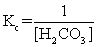

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
10
3. 2NO2  2NO + O2 Kp = 5.9 *10 -5
2NO + O2 Kp = 5.9 *10 -5
A)2 < 1 < 3
B)1 < 2 < 3
C)2 < 3 < 1
D)3 < 2 < 1
E)3 < 1 < 2
 2NO + O2 Kp = 5.9 *10 -5
2NO + O2 Kp = 5.9 *10 -5A)2 < 1 < 3
B)1 < 2 < 3
C)2 < 3 < 1
D)3 < 2 < 1
E)3 < 1 < 2

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
11
The reaction A(g)+ 2B(g)  C(g)was allowed to come to equilibrium. The initial amounts of reactants placed into a 5.00 L vessel were 1.0 mol A and 1.8 mol B. After the reaction reached equilibrium, 1.0 mol of B was found. Calculate Kc for this reaction.
C(g)was allowed to come to equilibrium. The initial amounts of reactants placed into a 5.00 L vessel were 1.0 mol A and 1.8 mol B. After the reaction reached equilibrium, 1.0 mol of B was found. Calculate Kc for this reaction.
A)0.060
B)5.1
C)17
D)19
E)25
 C(g)was allowed to come to equilibrium. The initial amounts of reactants placed into a 5.00 L vessel were 1.0 mol A and 1.8 mol B. After the reaction reached equilibrium, 1.0 mol of B was found. Calculate Kc for this reaction.
C(g)was allowed to come to equilibrium. The initial amounts of reactants placed into a 5.00 L vessel were 1.0 mol A and 1.8 mol B. After the reaction reached equilibrium, 1.0 mol of B was found. Calculate Kc for this reaction.A)0.060
B)5.1
C)17
D)19
E)25

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
12
The brown gas NO2 and the colorless gas N2O4 exist in equilibrium, 2NO2  N2O4. In an experiment, 0.625 mole of N2O4 was introduced into a 5.00 L vessel and was allowed to decompose until equilibrium was reached. The concentration of N2O4 at equilibrium was 0.0750 M. Calculate Kc for the reaction.
N2O4. In an experiment, 0.625 mole of N2O4 was introduced into a 5.00 L vessel and was allowed to decompose until equilibrium was reached. The concentration of N2O4 at equilibrium was 0.0750 M. Calculate Kc for the reaction.
A)7.5
B)0.125
C)0.0750
D)0.10
E)0.050
 N2O4. In an experiment, 0.625 mole of N2O4 was introduced into a 5.00 L vessel and was allowed to decompose until equilibrium was reached. The concentration of N2O4 at equilibrium was 0.0750 M. Calculate Kc for the reaction.
N2O4. In an experiment, 0.625 mole of N2O4 was introduced into a 5.00 L vessel and was allowed to decompose until equilibrium was reached. The concentration of N2O4 at equilibrium was 0.0750 M. Calculate Kc for the reaction.A)7.5
B)0.125
C)0.0750
D)0.10
E)0.050

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
13
Calculate Kp for the reaction 2NOCl(g)  2NO(g)+ Cl2(g)at 400°C if Kc at 400°C for this reaction is 2.1 *10-2.
2NO(g)+ Cl2(g)at 400°C if Kc at 400°C for this reaction is 2.1 *10-2.
A)2.1 * 10-2
B)1.7 * 10-3
C)0.70
D)1.2
E)3.8 * 10-4
 2NO(g)+ Cl2(g)at 400°C if Kc at 400°C for this reaction is 2.1 *10-2.
2NO(g)+ Cl2(g)at 400°C if Kc at 400°C for this reaction is 2.1 *10-2.A)2.1 * 10-2
B)1.7 * 10-3
C)0.70
D)1.2
E)3.8 * 10-4

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
14
At 35ºC, the equilibrium constant for the reaction 2NOCl(g) ![<strong>At 35ºC, the equilibrium constant for the reaction 2NOCl(g) 2NO(g)+ Cl<sub>2</sub>(g)is K<sub>c</sub> = 1.6 *10<sup>-5</sup>. An equilibrium mixture was found to have the following concentrations of Cl<sub>2</sub> and NOCl: [Cl<sub>2</sub>] = 1.2 * 10<sup>-2 </sup>M; [NOCl] = 2.8 * 10<sup>-1 </sup>M. Calculate the concentration of NO(g)at equilibrium.</strong> A)1.0 * 10<sup>-4</sup> M B)1.0 * 10<sup>-2</sup> M C)2.8 * 10<sup>-1</sup> M D)2.4 * 10<sup>-2</sup> M E)1.6 * 10<sup>-3</sup> M](https://storage.examlex.com/TB3244/11ec7153_74a6_4277_88eb_656263c228fc_TB3244_11.jpg) 2NO(g)+ Cl2(g)is Kc = 1.6 *10-5. An equilibrium mixture was found to have the following concentrations of Cl2 and NOCl: [Cl2] = 1.2 * 10-2 M; [NOCl] = 2.8 * 10-1 M. Calculate the concentration of NO(g)at equilibrium.
2NO(g)+ Cl2(g)is Kc = 1.6 *10-5. An equilibrium mixture was found to have the following concentrations of Cl2 and NOCl: [Cl2] = 1.2 * 10-2 M; [NOCl] = 2.8 * 10-1 M. Calculate the concentration of NO(g)at equilibrium.
A)1.0 * 10-4 M
B)1.0 * 10-2 M
C)2.8 * 10-1 M
D)2.4 * 10-2 M
E)1.6 * 10-3 M
![<strong>At 35ºC, the equilibrium constant for the reaction 2NOCl(g) 2NO(g)+ Cl<sub>2</sub>(g)is K<sub>c</sub> = 1.6 *10<sup>-5</sup>. An equilibrium mixture was found to have the following concentrations of Cl<sub>2</sub> and NOCl: [Cl<sub>2</sub>] = 1.2 * 10<sup>-2 </sup>M; [NOCl] = 2.8 * 10<sup>-1 </sup>M. Calculate the concentration of NO(g)at equilibrium.</strong> A)1.0 * 10<sup>-4</sup> M B)1.0 * 10<sup>-2</sup> M C)2.8 * 10<sup>-1</sup> M D)2.4 * 10<sup>-2</sup> M E)1.6 * 10<sup>-3</sup> M](https://storage.examlex.com/TB3244/11ec7153_74a6_4277_88eb_656263c228fc_TB3244_11.jpg) 2NO(g)+ Cl2(g)is Kc = 1.6 *10-5. An equilibrium mixture was found to have the following concentrations of Cl2 and NOCl: [Cl2] = 1.2 * 10-2 M; [NOCl] = 2.8 * 10-1 M. Calculate the concentration of NO(g)at equilibrium.
2NO(g)+ Cl2(g)is Kc = 1.6 *10-5. An equilibrium mixture was found to have the following concentrations of Cl2 and NOCl: [Cl2] = 1.2 * 10-2 M; [NOCl] = 2.8 * 10-1 M. Calculate the concentration of NO(g)at equilibrium.A)1.0 * 10-4 M
B)1.0 * 10-2 M
C)2.8 * 10-1 M
D)2.4 * 10-2 M
E)1.6 * 10-3 M

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
15
On analysis, an equilibrium mixture for the reaction 2H2S(g)  2H2(g)+ S2(g)was found to contain 1.0 mol H2S, 4.0 mol H2, and 0.80 mol S2 in a 4.0 L vessel. Calculate the equilibrium constant, Kc, for this reaction.
2H2(g)+ S2(g)was found to contain 1.0 mol H2S, 4.0 mol H2, and 0.80 mol S2 in a 4.0 L vessel. Calculate the equilibrium constant, Kc, for this reaction.
A)1.6
B)3.2
C)12.8
D)0.64
E)0.8
 2H2(g)+ S2(g)was found to contain 1.0 mol H2S, 4.0 mol H2, and 0.80 mol S2 in a 4.0 L vessel. Calculate the equilibrium constant, Kc, for this reaction.
2H2(g)+ S2(g)was found to contain 1.0 mol H2S, 4.0 mol H2, and 0.80 mol S2 in a 4.0 L vessel. Calculate the equilibrium constant, Kc, for this reaction.A)1.6
B)3.2
C)12.8
D)0.64
E)0.8

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
16
Phosgene, COCl2, a poisonous gas, decomposes according to the equation COCl2(g)  CO(g)+ Cl2(g). Calculate Kp for this reaction if Kc = 0.083 at 900ºC.
CO(g)+ Cl2(g). Calculate Kp for this reaction if Kc = 0.083 at 900ºC.
A)0.125
B)8.0
C)6.1
D)0.16
E)0.083
 CO(g)+ Cl2(g). Calculate Kp for this reaction if Kc = 0.083 at 900ºC.
CO(g)+ Cl2(g). Calculate Kp for this reaction if Kc = 0.083 at 900ºC.A)0.125
B)8.0
C)6.1
D)0.16
E)0.083

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
17
The equilibrium constant for the reaction Ni(s)+ 4CO(g) Ni(CO)4(g)is 5.0 * 104 at 25ºC. What is the equilibrium constant for the reaction Ni(CO)4(g)  Ni(s)+ 4CO(g)?
Ni(s)+ 4CO(g)?
A)2.0 * 10-5
B)2.5 * 109
C)5.0 * 104
D)5.0 * 10-4
E)2.0 * 10-3
 Ni(s)+ 4CO(g)?
Ni(s)+ 4CO(g)?A)2.0 * 10-5
B)2.5 * 109
C)5.0 * 104
D)5.0 * 10-4
E)2.0 * 10-3

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
18
Carbon tetrachloride reacts at high temperatures with oxygen to produce two toxic gases, phosgene and chlorine. CCl4(g)+ 1/2O2(g)  COCl2(g)+ Cl2(g), Kc = 4.4 * 109 at 1,000 K
COCl2(g)+ Cl2(g), Kc = 4.4 * 109 at 1,000 K
Calculate Kc for the reaction 2CCl4(g)+ O2(g) 2COCl2(g)+ 2Cl2(g).
2COCl2(g)+ 2Cl2(g).
A)4.4 * 109
B)8.8 * 109
C)1.9 * 1010
D)1.9 * 1019
E)2.3 * 10-10
 COCl2(g)+ Cl2(g), Kc = 4.4 * 109 at 1,000 K
COCl2(g)+ Cl2(g), Kc = 4.4 * 109 at 1,000 KCalculate Kc for the reaction 2CCl4(g)+ O2(g)
 2COCl2(g)+ 2Cl2(g).
2COCl2(g)+ 2Cl2(g).A)4.4 * 109
B)8.8 * 109
C)1.9 * 1010
D)1.9 * 1019
E)2.3 * 10-10

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
19
Kp for the reaction of SO2(g)with O2 to produce SO3(g)is 3 * 1024 . Calculate Kc for this equilibrium at 25ºC. (The relevant reaction is 2SO2(g)+ O2(g)  2SO3(g).)
2SO3(g).)
A)3 * 1024
B)5 * 1021
C)2 * 1020
D)5 * 1022
E)7 * 1025
 2SO3(g).)
2SO3(g).)A)3 * 1024
B)5 * 1021
C)2 * 1020
D)5 * 1022
E)7 * 1025

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
20
Which is the correct equilibrium constant expression for the following reaction? Fe2O3(s)+ 3H2(g) ![<strong>Which is the correct equilibrium constant expression for the following reaction? Fe<sub>2</sub>O<sub>3</sub>(s)+ 3H<sub>2</sub>(g) <sub> </sub>2Fe(s)+ 3H<sub>2</sub>O(g)</strong> A)K<sub>c</sub> = [Fe<sub>2</sub>O<sub>3</sub>] [H<sub>2</sub>]<sup>3 </sup>/<sup> </sup>[Fe]<sup>2</sup>[H<sub>2</sub>O]<sup>3</sup> B)K<sub>c</sub> = [H<sub>2</sub>]<sup> </sup>/<sup> </sup>[H<sub>2</sub>O] C)K<sub>c</sub> = [H<sub>2</sub>O]<sup>3 </sup>/ [H<sub>2</sub>]<sup>3</sup> D)K<sub>c</sub> = [Fe]<sup>2</sup>[H<sub>2</sub>O]<sup>3 </sup>/ [Fe<sub>2</sub>O<sub>3</sub>] [H<sub>2</sub>]<sup>3</sup> E)K<sub>c</sub> = [Fe] [H<sub>2</sub>O] / [Fe<sub>2</sub>O<sub>3</sub>] [H<sub>2</sub>]](https://storage.examlex.com/TB3244/11ea7995_6655_5e3c_911b_153776291ab7_TB3244_00.jpg) 2Fe(s)+ 3H2O(g)
2Fe(s)+ 3H2O(g)
A)Kc = [Fe2O3] [H2]3 / [Fe]2[H2O]3
B)Kc = [H2] / [H2O]
C)Kc = [H2O]3 / [H2]3
D)Kc = [Fe]2[H2O]3 / [Fe2O3] [H2]3
E)Kc = [Fe] [H2O] / [Fe2O3] [H2]
![<strong>Which is the correct equilibrium constant expression for the following reaction? Fe<sub>2</sub>O<sub>3</sub>(s)+ 3H<sub>2</sub>(g) <sub> </sub>2Fe(s)+ 3H<sub>2</sub>O(g)</strong> A)K<sub>c</sub> = [Fe<sub>2</sub>O<sub>3</sub>] [H<sub>2</sub>]<sup>3 </sup>/<sup> </sup>[Fe]<sup>2</sup>[H<sub>2</sub>O]<sup>3</sup> B)K<sub>c</sub> = [H<sub>2</sub>]<sup> </sup>/<sup> </sup>[H<sub>2</sub>O] C)K<sub>c</sub> = [H<sub>2</sub>O]<sup>3 </sup>/ [H<sub>2</sub>]<sup>3</sup> D)K<sub>c</sub> = [Fe]<sup>2</sup>[H<sub>2</sub>O]<sup>3 </sup>/ [Fe<sub>2</sub>O<sub>3</sub>] [H<sub>2</sub>]<sup>3</sup> E)K<sub>c</sub> = [Fe] [H<sub>2</sub>O] / [Fe<sub>2</sub>O<sub>3</sub>] [H<sub>2</sub>]](https://storage.examlex.com/TB3244/11ea7995_6655_5e3c_911b_153776291ab7_TB3244_00.jpg) 2Fe(s)+ 3H2O(g)
2Fe(s)+ 3H2O(g)A)Kc = [Fe2O3] [H2]3 / [Fe]2[H2O]3
B)Kc = [H2] / [H2O]
C)Kc = [H2O]3 / [H2]3
D)Kc = [Fe]2[H2O]3 / [Fe2O3] [H2]3
E)Kc = [Fe] [H2O] / [Fe2O3] [H2]

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
21
For the nitrogen fixation reaction 3H2(g)+ N2(g)  2NH3(g), Kc = 6.0 * 10-2 at 500°C. If 0.250 M H2 and 0.050 M NH3 are present at equilibrium, what is the equilibrium concentration of N2?
2NH3(g), Kc = 6.0 * 10-2 at 500°C. If 0.250 M H2 and 0.050 M NH3 are present at equilibrium, what is the equilibrium concentration of N2?
A)0.750 M
B)2.7 M
C)0.250 M
D)0.025 M
E)1.85 M
 2NH3(g), Kc = 6.0 * 10-2 at 500°C. If 0.250 M H2 and 0.050 M NH3 are present at equilibrium, what is the equilibrium concentration of N2?
2NH3(g), Kc = 6.0 * 10-2 at 500°C. If 0.250 M H2 and 0.050 M NH3 are present at equilibrium, what is the equilibrium concentration of N2?A)0.750 M
B)2.7 M
C)0.250 M
D)0.025 M
E)1.85 M

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
22
Sodium carbonate, Na2CO3(s), can be prepared by heating sodium bicarbonate, NaHCO3(s). 2NaHCO3(s)  Na2CO3(s)+ CO2(g)+ H2O(g)Kp = 0.23 at 100ºC
Na2CO3(s)+ CO2(g)+ H2O(g)Kp = 0.23 at 100ºC
If a sample of NaHCO3 is placed in an evacuated flask and allowed to achieve equilibrium at 100ºC, what will the total gas pressure be?
A)0.46 atm
B)0.96 atm
C)0.23 atm
D)0.48 atm
E)0.11 atm
 Na2CO3(s)+ CO2(g)+ H2O(g)Kp = 0.23 at 100ºC
Na2CO3(s)+ CO2(g)+ H2O(g)Kp = 0.23 at 100ºCIf a sample of NaHCO3 is placed in an evacuated flask and allowed to achieve equilibrium at 100ºC, what will the total gas pressure be?
A)0.46 atm
B)0.96 atm
C)0.23 atm
D)0.48 atm
E)0.11 atm

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
23
At 700 K, the reaction 2SO2(g)+ O2(g) ![<strong>At 700 K, the reaction 2SO<sub>2</sub>(g)+ O<sub>2</sub>(g) 2SO<sub>3</sub>(g)has the equilibrium constant K<sub>c</sub> = 4.3 * 10<sup>6</sup>, and the following concentrations are present: [SO<sub>2</sub>] = 0.010 M; [SO<sub>3</sub>] = 10. M; [O<sub>2</sub>] = 0.010 M. Is the mixture at equilibrium? If not at equilibrium, in which direction (as the equation is written), left to right or right to left, will the reaction proceed to reach equilibrium?</strong> A)Yes, the mixture is at equilibrium. B)No, left to right C)No, right to left D)There is not enough information to be able to predict the direction.](https://storage.examlex.com/TB3244/11ec7153_74a6_4277_88eb_656263c228fc_TB3244_11.jpg) 2SO3(g)has the equilibrium constant Kc = 4.3 * 106, and the following concentrations are present: [SO2] = 0.010 M; [SO3] = 10. M; [O2] = 0.010 M. Is the mixture at equilibrium? If not at equilibrium, in which direction (as the equation is written), left to right or right to left, will the reaction proceed to reach equilibrium?
2SO3(g)has the equilibrium constant Kc = 4.3 * 106, and the following concentrations are present: [SO2] = 0.010 M; [SO3] = 10. M; [O2] = 0.010 M. Is the mixture at equilibrium? If not at equilibrium, in which direction (as the equation is written), left to right or right to left, will the reaction proceed to reach equilibrium?
A)Yes, the mixture is at equilibrium.
B)No, left to right
C)No, right to left
D)There is not enough information to be able to predict the direction.
![<strong>At 700 K, the reaction 2SO<sub>2</sub>(g)+ O<sub>2</sub>(g) 2SO<sub>3</sub>(g)has the equilibrium constant K<sub>c</sub> = 4.3 * 10<sup>6</sup>, and the following concentrations are present: [SO<sub>2</sub>] = 0.010 M; [SO<sub>3</sub>] = 10. M; [O<sub>2</sub>] = 0.010 M. Is the mixture at equilibrium? If not at equilibrium, in which direction (as the equation is written), left to right or right to left, will the reaction proceed to reach equilibrium?</strong> A)Yes, the mixture is at equilibrium. B)No, left to right C)No, right to left D)There is not enough information to be able to predict the direction.](https://storage.examlex.com/TB3244/11ec7153_74a6_4277_88eb_656263c228fc_TB3244_11.jpg) 2SO3(g)has the equilibrium constant Kc = 4.3 * 106, and the following concentrations are present: [SO2] = 0.010 M; [SO3] = 10. M; [O2] = 0.010 M. Is the mixture at equilibrium? If not at equilibrium, in which direction (as the equation is written), left to right or right to left, will the reaction proceed to reach equilibrium?
2SO3(g)has the equilibrium constant Kc = 4.3 * 106, and the following concentrations are present: [SO2] = 0.010 M; [SO3] = 10. M; [O2] = 0.010 M. Is the mixture at equilibrium? If not at equilibrium, in which direction (as the equation is written), left to right or right to left, will the reaction proceed to reach equilibrium?A)Yes, the mixture is at equilibrium.
B)No, left to right
C)No, right to left
D)There is not enough information to be able to predict the direction.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
24
At 700 K, the reaction 2SO2(g)+ O2(g) ![<strong>At 700 K, the reaction 2SO<sub>2</sub>(g)+ O<sub>2</sub>(g) 2SO<sub>3</sub>(g)has the equilibrium constant K<sub>c</sub> = 4.3 * 10<sup>6</sup> , and the following concentrations are present: [SO<sub>2</sub>] = 0.10 M; [SO<sub>3</sub>] = 10. M; [O<sub>2</sub>] = 0.10 M. Is the mixture at equilibrium? If not at equilibrium, in which direction (as the equation is written), left to right or right to left, will the reaction proceed to reach equilibrium?</strong> A)Yes, the mixture is at equilibrium. B)No, left to right C)No, right to left D)There is not enough information to be able to predict the direction.](https://storage.examlex.com/TB3244/11ec7153_74a6_4277_88eb_656263c228fc_TB3244_11.jpg) 2SO3(g)has the equilibrium constant Kc = 4.3 * 106 , and the following concentrations are present: [SO2] = 0.10 M; [SO3] = 10. M; [O2] = 0.10 M. Is the mixture at equilibrium? If not at equilibrium, in which direction (as the equation is written), left to right or right to left, will the reaction proceed to reach equilibrium?
2SO3(g)has the equilibrium constant Kc = 4.3 * 106 , and the following concentrations are present: [SO2] = 0.10 M; [SO3] = 10. M; [O2] = 0.10 M. Is the mixture at equilibrium? If not at equilibrium, in which direction (as the equation is written), left to right or right to left, will the reaction proceed to reach equilibrium?
A)Yes, the mixture is at equilibrium.
B)No, left to right
C)No, right to left
D)There is not enough information to be able to predict the direction.
![<strong>At 700 K, the reaction 2SO<sub>2</sub>(g)+ O<sub>2</sub>(g) 2SO<sub>3</sub>(g)has the equilibrium constant K<sub>c</sub> = 4.3 * 10<sup>6</sup> , and the following concentrations are present: [SO<sub>2</sub>] = 0.10 M; [SO<sub>3</sub>] = 10. M; [O<sub>2</sub>] = 0.10 M. Is the mixture at equilibrium? If not at equilibrium, in which direction (as the equation is written), left to right or right to left, will the reaction proceed to reach equilibrium?</strong> A)Yes, the mixture is at equilibrium. B)No, left to right C)No, right to left D)There is not enough information to be able to predict the direction.](https://storage.examlex.com/TB3244/11ec7153_74a6_4277_88eb_656263c228fc_TB3244_11.jpg) 2SO3(g)has the equilibrium constant Kc = 4.3 * 106 , and the following concentrations are present: [SO2] = 0.10 M; [SO3] = 10. M; [O2] = 0.10 M. Is the mixture at equilibrium? If not at equilibrium, in which direction (as the equation is written), left to right or right to left, will the reaction proceed to reach equilibrium?
2SO3(g)has the equilibrium constant Kc = 4.3 * 106 , and the following concentrations are present: [SO2] = 0.10 M; [SO3] = 10. M; [O2] = 0.10 M. Is the mixture at equilibrium? If not at equilibrium, in which direction (as the equation is written), left to right or right to left, will the reaction proceed to reach equilibrium?A)Yes, the mixture is at equilibrium.
B)No, left to right
C)No, right to left
D)There is not enough information to be able to predict the direction.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
25
Hydrogen iodide decomposes according to the equation 2HI(g)  H2(g)+ I2(g), for which Kc = 0.0156 at 400ºC. 0.550 mol HI was injected into a 2.00 L reaction vessel at 400ºC. Calculate the concentration of HI at equilibrium.
H2(g)+ I2(g), for which Kc = 0.0156 at 400ºC. 0.550 mol HI was injected into a 2.00 L reaction vessel at 400ºC. Calculate the concentration of HI at equilibrium.
A)0.138 M
B)0.220 M
C)0.550 M
D)0.275 M
E)0.0275 M
 H2(g)+ I2(g), for which Kc = 0.0156 at 400ºC. 0.550 mol HI was injected into a 2.00 L reaction vessel at 400ºC. Calculate the concentration of HI at equilibrium.
H2(g)+ I2(g), for which Kc = 0.0156 at 400ºC. 0.550 mol HI was injected into a 2.00 L reaction vessel at 400ºC. Calculate the concentration of HI at equilibrium.A)0.138 M
B)0.220 M
C)0.550 M
D)0.275 M
E)0.0275 M

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
26
Consider the reaction N2(g)+ O2(g)  2NO(g), for which Kc = 0.10 at 2,000ºC. Starting with initial concentrations of 0.040 M of N2 and 0.040 M of O2, determine the equilibrium concentration of NO.
2NO(g), for which Kc = 0.10 at 2,000ºC. Starting with initial concentrations of 0.040 M of N2 and 0.040 M of O2, determine the equilibrium concentration of NO.
A)5.4 * 10-3 M
B)0.0096 M
C)0.011 M
D)0.080 M
E)0.10 M
 2NO(g), for which Kc = 0.10 at 2,000ºC. Starting with initial concentrations of 0.040 M of N2 and 0.040 M of O2, determine the equilibrium concentration of NO.
2NO(g), for which Kc = 0.10 at 2,000ºC. Starting with initial concentrations of 0.040 M of N2 and 0.040 M of O2, determine the equilibrium concentration of NO.A)5.4 * 10-3 M
B)0.0096 M
C)0.011 M
D)0.080 M
E)0.10 M

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
27
For the reaction PCl3(g)+ Cl2(g) ![<strong>For the reaction PCl<sub>3</sub>(g)+ Cl<sub>2</sub>(g) PCl<sub>5</sub>(g)at a particular temperature, K<sub>c</sub> = 24.3. Suppose a system at that temperature is prepared with [PCl<sub>3</sub>] = 0.10 M, [Cl<sub>2</sub>] = 0.15 M, and [PCl<sub>5</sub>] = 0.60 M. Which of these statements is true?</strong> A)The reaction is at equilibrium. B)The reaction will proceed in the direction of forming more PCl<sub>5</sub> until equilibrium is reached. C)The reaction will proceed in the direction of forming more PCl<sub>3</sub> and Cl<sub>2</sub> until equilibrium is reached. D)None of the above statements is true.](https://storage.examlex.com/TB3244/11ec7153_74a6_4277_88eb_656263c228fc_TB3244_11.jpg) PCl5(g)at a particular temperature, Kc = 24.3. Suppose a system at that temperature is prepared with [PCl3] = 0.10 M, [Cl2] = 0.15 M, and [PCl5] = 0.60 M. Which of these statements is true?
PCl5(g)at a particular temperature, Kc = 24.3. Suppose a system at that temperature is prepared with [PCl3] = 0.10 M, [Cl2] = 0.15 M, and [PCl5] = 0.60 M. Which of these statements is true?
A)The reaction is at equilibrium.
B)The reaction will proceed in the direction of forming more PCl5 until equilibrium is reached.
C)The reaction will proceed in the direction of forming more PCl3 and Cl2 until equilibrium is reached.
D)None of the above statements is true.
![<strong>For the reaction PCl<sub>3</sub>(g)+ Cl<sub>2</sub>(g) PCl<sub>5</sub>(g)at a particular temperature, K<sub>c</sub> = 24.3. Suppose a system at that temperature is prepared with [PCl<sub>3</sub>] = 0.10 M, [Cl<sub>2</sub>] = 0.15 M, and [PCl<sub>5</sub>] = 0.60 M. Which of these statements is true?</strong> A)The reaction is at equilibrium. B)The reaction will proceed in the direction of forming more PCl<sub>5</sub> until equilibrium is reached. C)The reaction will proceed in the direction of forming more PCl<sub>3</sub> and Cl<sub>2</sub> until equilibrium is reached. D)None of the above statements is true.](https://storage.examlex.com/TB3244/11ec7153_74a6_4277_88eb_656263c228fc_TB3244_11.jpg) PCl5(g)at a particular temperature, Kc = 24.3. Suppose a system at that temperature is prepared with [PCl3] = 0.10 M, [Cl2] = 0.15 M, and [PCl5] = 0.60 M. Which of these statements is true?
PCl5(g)at a particular temperature, Kc = 24.3. Suppose a system at that temperature is prepared with [PCl3] = 0.10 M, [Cl2] = 0.15 M, and [PCl5] = 0.60 M. Which of these statements is true?A)The reaction is at equilibrium.
B)The reaction will proceed in the direction of forming more PCl5 until equilibrium is reached.
C)The reaction will proceed in the direction of forming more PCl3 and Cl2 until equilibrium is reached.
D)None of the above statements is true.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
28
Consider the following reactions and their associated equilibrium constants: A + 2B  C K1
C K1
C 11ec7153_74a6_4277_88eb_656263c228fc_TB3244_11 D + E K2
For the reaction A + 2B D + E, having equilibrium constant Kc,
A)Kc = K1 + K2
B)Kc = K1/K2
C)Kc = K1 - K2
D)Kc = (K1)(K2)
E)Kc = K2/K1
 C K1
C K1C 11ec7153_74a6_4277_88eb_656263c228fc_TB3244_11 D + E K2
For the reaction A + 2B D + E, having equilibrium constant Kc,
A)Kc = K1 + K2
B)Kc = K1/K2
C)Kc = K1 - K2
D)Kc = (K1)(K2)
E)Kc = K2/K1

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
29
For the following reaction at equilibrium, which one of the changes below would cause the equilibrium to shift to the left? 2NOBr(g)  2NO(g)+ Br2(g), Hºrxn = 30 kJ/mol
2NO(g)+ Br2(g), Hºrxn = 30 kJ/mol
A)Increase the container volume.
B)Remove some NO.
C)Remove some Br2.
D)Add more NOBr.
E)Decrease the temperature.
 2NO(g)+ Br2(g), Hºrxn = 30 kJ/mol
2NO(g)+ Br2(g), Hºrxn = 30 kJ/molA)Increase the container volume.
B)Remove some NO.
C)Remove some Br2.
D)Add more NOBr.
E)Decrease the temperature.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
30
For the reaction H2(g)+ I2(g) ![<strong>For the reaction H<sub>2</sub>(g)+ I<sub>2</sub>(g) 2HI(g), K<sub>c</sub> = 50.2 at 445ºC. If [H<sub>2</sub>] = [I<sub>2</sub>] = [HI] = 1.75 * 10<sup>-3</sup> M at 445ºC, which one of these statements is true?</strong> A)The system is at equilibrium, thus no concentration changes will occur. B)The concentrations of HI and I<sub>2</sub> will increase as the system approaches equilibrium. C)The concentration of HI will increase as the system approaches equilibrium. D)The concentrations of H<sub>2</sub> and HI will fall as the system moves toward equilibrium. E)The concentrations of H<sub>2</sub> and I<sub>2</sub> will increase as the system approaches equilibrium.](https://storage.examlex.com/TB3244/11ec7153_74a6_4277_88eb_656263c228fc_TB3244_11.jpg) 2HI(g), Kc = 50.2 at 445ºC. If [H2] = [I2] = [HI] = 1.75 * 10-3 M at 445ºC, which one of these statements is true?
2HI(g), Kc = 50.2 at 445ºC. If [H2] = [I2] = [HI] = 1.75 * 10-3 M at 445ºC, which one of these statements is true?
A)The system is at equilibrium, thus no concentration changes will occur.
B)The concentrations of HI and I2 will increase as the system approaches equilibrium.
C)The concentration of HI will increase as the system approaches equilibrium.
D)The concentrations of H2 and HI will fall as the system moves toward equilibrium.
E)The concentrations of H2 and I2 will increase as the system approaches equilibrium.
![<strong>For the reaction H<sub>2</sub>(g)+ I<sub>2</sub>(g) 2HI(g), K<sub>c</sub> = 50.2 at 445ºC. If [H<sub>2</sub>] = [I<sub>2</sub>] = [HI] = 1.75 * 10<sup>-3</sup> M at 445ºC, which one of these statements is true?</strong> A)The system is at equilibrium, thus no concentration changes will occur. B)The concentrations of HI and I<sub>2</sub> will increase as the system approaches equilibrium. C)The concentration of HI will increase as the system approaches equilibrium. D)The concentrations of H<sub>2</sub> and HI will fall as the system moves toward equilibrium. E)The concentrations of H<sub>2</sub> and I<sub>2</sub> will increase as the system approaches equilibrium.](https://storage.examlex.com/TB3244/11ec7153_74a6_4277_88eb_656263c228fc_TB3244_11.jpg) 2HI(g), Kc = 50.2 at 445ºC. If [H2] = [I2] = [HI] = 1.75 * 10-3 M at 445ºC, which one of these statements is true?
2HI(g), Kc = 50.2 at 445ºC. If [H2] = [I2] = [HI] = 1.75 * 10-3 M at 445ºC, which one of these statements is true?A)The system is at equilibrium, thus no concentration changes will occur.
B)The concentrations of HI and I2 will increase as the system approaches equilibrium.
C)The concentration of HI will increase as the system approaches equilibrium.
D)The concentrations of H2 and HI will fall as the system moves toward equilibrium.
E)The concentrations of H2 and I2 will increase as the system approaches equilibrium.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
31
At 400ºC, Kc = 64 for the equilibrium H2(g)+ I2(g)  2HI(g). If 3.00 mol H2 and 3.00 mol I2 are introduced into an empty 4.0 L vessel, find the equilibrium concentration of HI at 400ºC.
2HI(g). If 3.00 mol H2 and 3.00 mol I2 are introduced into an empty 4.0 L vessel, find the equilibrium concentration of HI at 400ºC.
A)0.15 M
B)1.2 M
C)2.4 M
D)4.8 M
E)5.8 M
 2HI(g). If 3.00 mol H2 and 3.00 mol I2 are introduced into an empty 4.0 L vessel, find the equilibrium concentration of HI at 400ºC.
2HI(g). If 3.00 mol H2 and 3.00 mol I2 are introduced into an empty 4.0 L vessel, find the equilibrium concentration of HI at 400ºC.A)0.15 M
B)1.2 M
C)2.4 M
D)4.8 M
E)5.8 M

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
32
For the reaction SO2(g)+ NO2(g)  SO3(g)+ NO(g), the equilibrium constant is 18.0 at 1,200ºC. If 1.0 mole of SO2 and 2.0 moles of NO2 are placed in a 20. L container, what concentration of SO3 will be present at equilibrium?
SO3(g)+ NO(g), the equilibrium constant is 18.0 at 1,200ºC. If 1.0 mole of SO2 and 2.0 moles of NO2 are placed in a 20. L container, what concentration of SO3 will be present at equilibrium?
A)0.48 mol/L
B)0.11 mol/L
C)0.95 mol/L
D)2.22 mol/L
E)18 mol/L
 SO3(g)+ NO(g), the equilibrium constant is 18.0 at 1,200ºC. If 1.0 mole of SO2 and 2.0 moles of NO2 are placed in a 20. L container, what concentration of SO3 will be present at equilibrium?
SO3(g)+ NO(g), the equilibrium constant is 18.0 at 1,200ºC. If 1.0 mole of SO2 and 2.0 moles of NO2 are placed in a 20. L container, what concentration of SO3 will be present at equilibrium?A)0.48 mol/L
B)0.11 mol/L
C)0.95 mol/L
D)2.22 mol/L
E)18 mol/L

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
33
For the following reaction at equilibrium in a reaction vessel, which one of these changes would cause the Br2 concentration to decrease? 2NOBr(g)  2NO(g)+ Br2(g), Hºrxn= 30 kJ/mol
2NO(g)+ Br2(g), Hºrxn= 30 kJ/mol
A)Increase the temperature.
B)Remove some NO.
C)Add more NOBr.
D)Compress the gas mixture into a smaller volume.
 2NO(g)+ Br2(g), Hºrxn= 30 kJ/mol
2NO(g)+ Br2(g), Hºrxn= 30 kJ/molA)Increase the temperature.
B)Remove some NO.
C)Add more NOBr.
D)Compress the gas mixture into a smaller volume.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
34
For the equilibrium reaction 2SO2(g)+ O2(g)  2SO3(g), Hºrxn = -198 kJ/mol. Which one of these factors would cause the equilibrium constant to increase?
2SO3(g), Hºrxn = -198 kJ/mol. Which one of these factors would cause the equilibrium constant to increase?
A)Decrease the temperature.
B)Add SO2 gas.
C)Remove O2 gas.
D)Add a catalyst.
E)None of these.
 2SO3(g), Hºrxn = -198 kJ/mol. Which one of these factors would cause the equilibrium constant to increase?
2SO3(g), Hºrxn = -198 kJ/mol. Which one of these factors would cause the equilibrium constant to increase?A)Decrease the temperature.
B)Add SO2 gas.
C)Remove O2 gas.
D)Add a catalyst.
E)None of these.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
35
For the following reaction at equilibrium in a reaction vessel, which one of these changes would cause the Br2 concentration to increase? 2NOBr(g)  2NO(g)+ Br2(g), Hºrxn= 30 kJ/mol
2NO(g)+ Br2(g), Hºrxn= 30 kJ/mol
A)Lower the temperature.
B)Remove some NO.
C)Remove some NOBr.
D)Compress the gas mixture into a smaller volume.
 2NO(g)+ Br2(g), Hºrxn= 30 kJ/mol
2NO(g)+ Br2(g), Hºrxn= 30 kJ/molA)Lower the temperature.
B)Remove some NO.
C)Remove some NOBr.
D)Compress the gas mixture into a smaller volume.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
36
At 340 K, Kp = 69 for the reaction H2(g)+ I2(g)  2HI(g). 50.0 g of HI is injected into an evacuated 5.00-L rigid cylinder at 340 K. What is the total pressure inside the cylinder when the system comes to equilibrium?
2HI(g). 50.0 g of HI is injected into an evacuated 5.00-L rigid cylinder at 340 K. What is the total pressure inside the cylinder when the system comes to equilibrium?
A)2.60 atm
B)1.76 atm
C)0.424 atm
D)2.18 atm
E)10.9 atm
 2HI(g). 50.0 g of HI is injected into an evacuated 5.00-L rigid cylinder at 340 K. What is the total pressure inside the cylinder when the system comes to equilibrium?
2HI(g). 50.0 g of HI is injected into an evacuated 5.00-L rigid cylinder at 340 K. What is the total pressure inside the cylinder when the system comes to equilibrium?A)2.60 atm
B)1.76 atm
C)0.424 atm
D)2.18 atm
E)10.9 atm

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
37
Hydrogen iodide decomposes according to the equation 2HI(g)  H2(g)+ I2(g), for which Kc = 0.0156 at 400ºC. 0.550 mol HI was injected into a 2.00 L reaction vessel at 400ºC. Calculate the concentration of H2 at equilibrium.
H2(g)+ I2(g), for which Kc = 0.0156 at 400ºC. 0.550 mol HI was injected into a 2.00 L reaction vessel at 400ºC. Calculate the concentration of H2 at equilibrium.
A)0.275 M
B)0.138 M
C)0.0275 M
D)0.0550 M
E)0.220 M
 H2(g)+ I2(g), for which Kc = 0.0156 at 400ºC. 0.550 mol HI was injected into a 2.00 L reaction vessel at 400ºC. Calculate the concentration of H2 at equilibrium.
H2(g)+ I2(g), for which Kc = 0.0156 at 400ºC. 0.550 mol HI was injected into a 2.00 L reaction vessel at 400ºC. Calculate the concentration of H2 at equilibrium.A)0.275 M
B)0.138 M
C)0.0275 M
D)0.0550 M
E)0.220 M

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
38
Consider the following equilibria: 2SO3(g)  2SO2(g)+ O2(g)Kc = 2.3 * 10-7
2SO2(g)+ O2(g)Kc = 2.3 * 10-7
2NO3(g) 11ec7153_74a6_4277_88eb_656263c228fc_TB3244_11 2NO2(g)+ O2(g)Kc = 1.4 * 10-3
Calculate the equilibrium constant for the reaction
SO2(g)+ NO3(g) SO3(g)+ NO2(g)
A)78
B)1.3 * 10-2
C)1.6 * 10-4
D)3.2 * 10-10
E)6.1 * 103
 2SO2(g)+ O2(g)Kc = 2.3 * 10-7
2SO2(g)+ O2(g)Kc = 2.3 * 10-72NO3(g) 11ec7153_74a6_4277_88eb_656263c228fc_TB3244_11 2NO2(g)+ O2(g)Kc = 1.4 * 10-3
Calculate the equilibrium constant for the reaction
SO2(g)+ NO3(g) SO3(g)+ NO2(g)
A)78
B)1.3 * 10-2
C)1.6 * 10-4
D)3.2 * 10-10
E)6.1 * 103

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
39
The reaction 2SO3(g)  2SO2(g)+ O2(g)is endothermic. If the temperature is increased,
2SO2(g)+ O2(g)is endothermic. If the temperature is increased,
A)more SO3 will be produced.
B)Kc will decrease.
C)no change will occur in Kc .
D)Kc will increase.
E)the pressure will decrease.
 2SO2(g)+ O2(g)is endothermic. If the temperature is increased,
2SO2(g)+ O2(g)is endothermic. If the temperature is increased,A)more SO3 will be produced.
B)Kc will decrease.
C)no change will occur in Kc .
D)Kc will increase.
E)the pressure will decrease.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
40
For the following reaction at equilibrium, which choice gives a change that will shift the position of equilibrium to favor formation of more products? 2NOBr(g)  2NO(g)+ Br2(g), Hºrxn = 30 kJ/mol
2NO(g)+ Br2(g), Hºrxn = 30 kJ/mol
A)Increase the total pressure by decreasing the volume.
B)Add more NO.
C)Remove Br2.
D)Lower the temperature.
E)Remove NOBr selectively.
 2NO(g)+ Br2(g), Hºrxn = 30 kJ/mol
2NO(g)+ Br2(g), Hºrxn = 30 kJ/molA)Increase the total pressure by decreasing the volume.
B)Add more NO.
C)Remove Br2.
D)Lower the temperature.
E)Remove NOBr selectively.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
41
Consider this gas phase equilibrium system: PCl5(g)  PCl3(g)+ Cl2(g) Hºrxn = +87.8 kJ/mol.
PCl3(g)+ Cl2(g) Hºrxn = +87.8 kJ/mol.
Which of these statements is false?
A)Increasing the system volume shifts the equilibrium to the right.
B)Increasing the temperature shifts the equilibrium to the right.
C)A catalyst speeds up the approach to equilibrium and shifts the position of equilibrium to the right.
D)Decreasing the total pressure of the system shifts the equilibrium to the right.
E)Increasing the temperature causes the equilibrium constant to increase.
 PCl3(g)+ Cl2(g) Hºrxn = +87.8 kJ/mol.
PCl3(g)+ Cl2(g) Hºrxn = +87.8 kJ/mol.Which of these statements is false?
A)Increasing the system volume shifts the equilibrium to the right.
B)Increasing the temperature shifts the equilibrium to the right.
C)A catalyst speeds up the approach to equilibrium and shifts the position of equilibrium to the right.
D)Decreasing the total pressure of the system shifts the equilibrium to the right.
E)Increasing the temperature causes the equilibrium constant to increase.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
42
A solution was prepared such that the initial concentrations of Cu2+(aq)and CN-(aq)were 0.0120 M and 0.0400 M, respectively. These ions react according to the following chemical equation
Cu2+(aq)+ 4CN-(aq)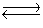 Cd(CN)42-(aq)Kc = 1.0 * 1025
Cd(CN)42-(aq)Kc = 1.0 * 1025
What will be the concentration of CN-(aq)at equilibrium?
Cu2+(aq)+ 4CN-(aq)
 Cd(CN)42-(aq)Kc = 1.0 * 1025
Cd(CN)42-(aq)Kc = 1.0 * 1025What will be the concentration of CN-(aq)at equilibrium?

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
43
Consider this reaction at equilibrium: 2SO2(g)+ O2(g)  2SO3(g), Hºrxn = -198 kJ/mol
2SO3(g), Hºrxn = -198 kJ/mol
If the volume of the system is compressed at constant temperature, what change will occur in the position of the equilibrium?
A)A shift to produce more SO2
B)A shift to produce more O2
C)No change
D)A shift to produce more SO3
 2SO3(g), Hºrxn = -198 kJ/mol
2SO3(g), Hºrxn = -198 kJ/molIf the volume of the system is compressed at constant temperature, what change will occur in the position of the equilibrium?
A)A shift to produce more SO2
B)A shift to produce more O2
C)No change
D)A shift to produce more SO3

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
44
For the reaction at equilibrium 2SO3 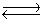 2SO2 + O2 ( Hºrxn= 198 kJ/mol), if we increase the reaction temperature, the equilibrium will
2SO2 + O2 ( Hºrxn= 198 kJ/mol), if we increase the reaction temperature, the equilibrium will
A)shift to the right.
B)shift to the left.
C)not shift.
D)The question cannot be answered because the equilibrium constant is not given.
 2SO2 + O2 ( Hºrxn= 198 kJ/mol), if we increase the reaction temperature, the equilibrium will
2SO2 + O2 ( Hºrxn= 198 kJ/mol), if we increase the reaction temperature, the equilibrium willA)shift to the right.
B)shift to the left.
C)not shift.
D)The question cannot be answered because the equilibrium constant is not given.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
45
When the substances in the equation below are at equilibrium, at pressure P and temperature T, the equilibrium can be shifted to favor the products by CuO(s)+ H2(g)  H2O(g)+ Cu(s) Hºrxn = -2.0 kJ/mol
H2O(g)+ Cu(s) Hºrxn = -2.0 kJ/mol
A)increasing the pressure by means of a moving piston at constant T.
B)increasing the pressure by adding an inert gas such as nitrogen.
C)decreasing the temperature.
D)allowing some gases to escape at constant P and T.
E)adding a catalyst.
 H2O(g)+ Cu(s) Hºrxn = -2.0 kJ/mol
H2O(g)+ Cu(s) Hºrxn = -2.0 kJ/molA)increasing the pressure by means of a moving piston at constant T.
B)increasing the pressure by adding an inert gas such as nitrogen.
C)decreasing the temperature.
D)allowing some gases to escape at constant P and T.
E)adding a catalyst.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
46
Consider this reaction at equilibrium at a total pressure P1: 2SO2(g)+ O2(g)  2SO3(g)
2SO3(g)
Suppose the volume of this system is compressed to one-half its initial volume and then equilibrium is reestablished. The new equilibrium total pressure will be
A)twice P1.
B)three times P1.
C)3.5 P1.
D)less than twice P1.
E)unchanged.
 2SO3(g)
2SO3(g)Suppose the volume of this system is compressed to one-half its initial volume and then equilibrium is reestablished. The new equilibrium total pressure will be
A)twice P1.
B)three times P1.
C)3.5 P1.
D)less than twice P1.
E)unchanged.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
47
Consider the reaction N2(g)+ 3H2(g) 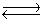 2NH3(g). If nitrogen is added to the system at equilibrium, what will happen to the ammonia concentration?
2NH3(g). If nitrogen is added to the system at equilibrium, what will happen to the ammonia concentration?
 2NH3(g). If nitrogen is added to the system at equilibrium, what will happen to the ammonia concentration?
2NH3(g). If nitrogen is added to the system at equilibrium, what will happen to the ammonia concentration?
Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
48
When the reaction 2H2S(g)  2H2(g)+ S2(g)is carried out at 1065°C, Kp = 0.012. Starting with pure H2S at 1065°, what must the initial pressure of H2S be if the equilibrated mixture at this temperature is to contain 0.250 atm of H2(g)?
2H2(g)+ S2(g)is carried out at 1065°C, Kp = 0.012. Starting with pure H2S at 1065°, what must the initial pressure of H2S be if the equilibrated mixture at this temperature is to contain 0.250 atm of H2(g)?
A)1.06 atm
B)1.86 atm
C)0.94 atm
D)0.90 atm
E)1.52 atm
 2H2(g)+ S2(g)is carried out at 1065°C, Kp = 0.012. Starting with pure H2S at 1065°, what must the initial pressure of H2S be if the equilibrated mixture at this temperature is to contain 0.250 atm of H2(g)?
2H2(g)+ S2(g)is carried out at 1065°C, Kp = 0.012. Starting with pure H2S at 1065°, what must the initial pressure of H2S be if the equilibrated mixture at this temperature is to contain 0.250 atm of H2(g)?A)1.06 atm
B)1.86 atm
C)0.94 atm
D)0.90 atm
E)1.52 atm

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
49
The equilibrium constants for the chemical reaction N2(g)+ O2(g)  2NO(g)are KP = 1.1 * 10-3 and 3.6 *10-3 at 2,200 K and 2,500 K, respectively. Which one of these statements is true?
2NO(g)are KP = 1.1 * 10-3 and 3.6 *10-3 at 2,200 K and 2,500 K, respectively. Which one of these statements is true?
A)The reaction is exothermic, Hº < 0.
B)The partial pressure of NO(g)is less at 2,200 K than at 2,500 K.
C)KP is less than Kc by a factor of (RT).
D)The total pressure at 2,200 K is the same as at 2,500 K.
E)Higher total pressure shifts the equilibrium to the left.
 2NO(g)are KP = 1.1 * 10-3 and 3.6 *10-3 at 2,200 K and 2,500 K, respectively. Which one of these statements is true?
2NO(g)are KP = 1.1 * 10-3 and 3.6 *10-3 at 2,200 K and 2,500 K, respectively. Which one of these statements is true?A)The reaction is exothermic, Hº < 0.
B)The partial pressure of NO(g)is less at 2,200 K than at 2,500 K.
C)KP is less than Kc by a factor of (RT).
D)The total pressure at 2,200 K is the same as at 2,500 K.
E)Higher total pressure shifts the equilibrium to the left.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
50
A quantity of liquid methanol, CH3OH, is introduced into a rigid 3.00-L vessel, the vessel is sealed, and the temperature is raised to 500K. At this temperature, the methanol vaporizes and decomposes according to the reaction CH3OH(g)  CO(g)+ 2 H2(g), Kc= 6.90 * 10-2.
CO(g)+ 2 H2(g), Kc= 6.90 * 10-2.
If the concentration of H2 in the equilibrium mixture is 0.426M, what mass of methanol was initially introduced into the vessel?
A)147 g
B)74.3 g
C)33.9 g
D)49.0 g
E)24.8 g
 CO(g)+ 2 H2(g), Kc= 6.90 * 10-2.
CO(g)+ 2 H2(g), Kc= 6.90 * 10-2.If the concentration of H2 in the equilibrium mixture is 0.426M, what mass of methanol was initially introduced into the vessel?
A)147 g
B)74.3 g
C)33.9 g
D)49.0 g
E)24.8 g

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
51
If the reaction 2H2S(g)  2H2(g)+ S2(g)is carried out at 1065°C, Kp = 0.0120. Starting from pure H2S introduced into an evacuated vessel at 1065°C, what will the total pressure in the vessel be at equilibrium if the equilibrated mixture contains 0.300 atm of H2(g)?
2H2(g)+ S2(g)is carried out at 1065°C, Kp = 0.0120. Starting from pure H2S introduced into an evacuated vessel at 1065°C, what will the total pressure in the vessel be at equilibrium if the equilibrated mixture contains 0.300 atm of H2(g)?
A)1.06 atm
B)1.36 atm
C)2.39 atm
D)4.20 atm
E)1.51 atm
 2H2(g)+ S2(g)is carried out at 1065°C, Kp = 0.0120. Starting from pure H2S introduced into an evacuated vessel at 1065°C, what will the total pressure in the vessel be at equilibrium if the equilibrated mixture contains 0.300 atm of H2(g)?
2H2(g)+ S2(g)is carried out at 1065°C, Kp = 0.0120. Starting from pure H2S introduced into an evacuated vessel at 1065°C, what will the total pressure in the vessel be at equilibrium if the equilibrated mixture contains 0.300 atm of H2(g)?A)1.06 atm
B)1.36 atm
C)2.39 atm
D)4.20 atm
E)1.51 atm

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
52
For the reaction 2NOCl(g)  2NO(g)+ Cl2(g), Kc = 8.0 at a certain temperature. What concentration of NOCl must be put into an empty 4.00 L reaction vessel in order that the equilibrium concentration of NOCl be 1.00 M?
2NO(g)+ Cl2(g), Kc = 8.0 at a certain temperature. What concentration of NOCl must be put into an empty 4.00 L reaction vessel in order that the equilibrium concentration of NOCl be 1.00 M?
A)1.26 M
B)2.25 M
C)2.50 M
D)3.52 M
E)11.0 M
 2NO(g)+ Cl2(g), Kc = 8.0 at a certain temperature. What concentration of NOCl must be put into an empty 4.00 L reaction vessel in order that the equilibrium concentration of NOCl be 1.00 M?
2NO(g)+ Cl2(g), Kc = 8.0 at a certain temperature. What concentration of NOCl must be put into an empty 4.00 L reaction vessel in order that the equilibrium concentration of NOCl be 1.00 M?A)1.26 M
B)2.25 M
C)2.50 M
D)3.52 M
E)11.0 M

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
53
Describe why addition of a catalyst does not affect the equilibrium constant for a reaction.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
54
Which of these situations will result if some CH4(g)is removed from the reaction CO(g)+ 3H2(g)  CH4(g)+ H2O(g)at equilibrium?
CH4(g)+ H2O(g)at equilibrium?
A)H2O will be consumed.
B)More CH4 and H2O will be produced.
C)Kp will decrease.
D)More CO will be produced.
E)No change will occur.
 CH4(g)+ H2O(g)at equilibrium?
CH4(g)+ H2O(g)at equilibrium?A)H2O will be consumed.
B)More CH4 and H2O will be produced.
C)Kp will decrease.
D)More CO will be produced.
E)No change will occur.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
55
The reaction 2NO(g)  N2(g)+ O2(g)is exothermic, Hºrxn = -180 kJ/mol. Which one of these statements is true?
N2(g)+ O2(g)is exothermic, Hºrxn = -180 kJ/mol. Which one of these statements is true?
A)Kp at 1,000 K is less than Kp at 2,000 K.
B)Kp at 1,000 K is larger than Kp at 2,000 K.
C)The Kp's at 1000 K and 2000 K are the same.
D)Kp depends on total pressure as well as temperature.
 N2(g)+ O2(g)is exothermic, Hºrxn = -180 kJ/mol. Which one of these statements is true?
N2(g)+ O2(g)is exothermic, Hºrxn = -180 kJ/mol. Which one of these statements is true?A)Kp at 1,000 K is less than Kp at 2,000 K.
B)Kp at 1,000 K is larger than Kp at 2,000 K.
C)The Kp's at 1000 K and 2000 K are the same.
D)Kp depends on total pressure as well as temperature.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
56
Consider the reaction N2(g)+ 3H2(g) 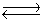 2NH3(g). If hydrogen gas is added to this system at equilibrium, which direction will the reaction shift?
2NH3(g). If hydrogen gas is added to this system at equilibrium, which direction will the reaction shift?
 2NH3(g). If hydrogen gas is added to this system at equilibrium, which direction will the reaction shift?
2NH3(g). If hydrogen gas is added to this system at equilibrium, which direction will the reaction shift?
Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
57
A solution was prepared such that the initial concentrations of Cu2+(aq)and CN-(aq)were 0.0120 M and 0.0400 M, respectively. These ions react according to the following chemical equation
Cu2+(aq)+ 4CN-(aq)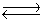 Cu(CN)42-(aq)Kc = 1.0 * 1025
Cu(CN)42-(aq)Kc = 1.0 * 1025
What will be the concentration of Cu2+(aq)at equilibrium?
Cu2+(aq)+ 4CN-(aq)
 Cu(CN)42-(aq)Kc = 1.0 * 1025
Cu(CN)42-(aq)Kc = 1.0 * 1025What will be the concentration of Cu2+(aq)at equilibrium?

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
58
In which of these gas-phase equilibria is the yield of products increased by increasing the total pressure on the reaction mixture?
A)CO(g)+ H2O(g) CO2(g)+ H2(g)
CO2(g)+ H2(g)
B)2NO(g)+ Cl2(g) 11ec7153_74a6_4277_88eb_656263c228fc_TB3244_11 2NOCl(g)
C)2SO3(g) 11ec7153_74a6_4277_88eb_656263c228fc_TB3244_11 2SO2(g)+ O2(g)
D)PCl5(g) 11ec7153_74a6_4277_88eb_656263c228fc_TB3244_11 PCl3(g)+ Cl2(g)
A)CO(g)+ H2O(g)
 CO2(g)+ H2(g)
CO2(g)+ H2(g)B)2NO(g)+ Cl2(g) 11ec7153_74a6_4277_88eb_656263c228fc_TB3244_11 2NOCl(g)
C)2SO3(g) 11ec7153_74a6_4277_88eb_656263c228fc_TB3244_11 2SO2(g)+ O2(g)
D)PCl5(g) 11ec7153_74a6_4277_88eb_656263c228fc_TB3244_11 PCl3(g)+ Cl2(g)

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
59
Solid ammonium hydrogen sulfide is introduced into a 2.00-L flask, and the flask is sealed. If this solid decomposes according to the equation
NH4HS(s)
 NH3(g)+ H2S(g), Kp = 0.108 at 25°C,
NH3(g)+ H2S(g), Kp = 0.108 at 25°C,
What is the minimum mass of ammonium hydrogen sulfide that must be present in the flask initially if equilibrium is to be established at 25°C?
A)0.917 g
B)1.37 g
C)2.74 g
D)0.581 g
E)0.452 g
NH4HS(s)
 NH3(g)+ H2S(g), Kp = 0.108 at 25°C,
NH3(g)+ H2S(g), Kp = 0.108 at 25°C,What is the minimum mass of ammonium hydrogen sulfide that must be present in the flask initially if equilibrium is to be established at 25°C?
A)0.917 g
B)1.37 g
C)2.74 g
D)0.581 g
E)0.452 g

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
60
For the common allotropes of carbon (graphite and diamond), C(gr) C(dia)with equilibrium constant K = 0.32. The molar volumes of graphite and diamond are, respectively, 5.30 cm3/mol and 3.42 cm3/mol; Hf of diamond is 1.90 kJ/mol. This data suggests that the formation of diamond is favored at
A)low temperatures and low pressures.
B)high temperatures and low pressures.
C)low temperatures and high pressures.
D)high temperatures and high pressures.
A)low temperatures and low pressures.
B)high temperatures and low pressures.
C)low temperatures and high pressures.
D)high temperatures and high pressures.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
61
The data below refer to the following reaction:
2NO(g)+ Br2(g)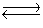 2NOBr(g)
2NOBr(g)  Find the concentration of NOBr when the system reaches equilibrium.
Find the concentration of NOBr when the system reaches equilibrium.
2NO(g)+ Br2(g)
 2NOBr(g)
2NOBr(g)  Find the concentration of NOBr when the system reaches equilibrium.
Find the concentration of NOBr when the system reaches equilibrium.
Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
62
Hydrogen iodide decomposes according to the equation:
2HI(g)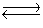 H2(g)+ I2(g), Kc = 0.0156 at 400ºC
H2(g)+ I2(g), Kc = 0.0156 at 400ºC
A 0.660 mol sample of HI was injected into a 2.00 L reaction vessel held at 400ºC.
Calculate the concentration of HI at equilibrium.
2HI(g)
 H2(g)+ I2(g), Kc = 0.0156 at 400ºC
H2(g)+ I2(g), Kc = 0.0156 at 400ºCA 0.660 mol sample of HI was injected into a 2.00 L reaction vessel held at 400ºC.
Calculate the concentration of HI at equilibrium.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
63
The dissociation of solid silver chloride in water to produce silver ions and chloride ions has an equilibrium constant of 1.8 * 10-18. Based on the magnitude of the equilibrium constant, is silver chloride very soluble in water? Why?

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
64
Calcium carbonate decomposes at high temperatures to give calcium oxide and carbon dioxide.
CaCO3(s)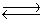 CaO(s)+ CO2(g)
CaO(s)+ CO2(g)
KP for this reaction is 1.16 at 800°C. A 5.00 L vessel containing 10.0 g of CaCO3(s)was evacuated to remove the air, sealed, and then heated to 800°C. Ignoring the volume occupied by the solid, what will be the mass of the solid in the vessel once equilibrium is reached?
CaCO3(s)
 CaO(s)+ CO2(g)
CaO(s)+ CO2(g)KP for this reaction is 1.16 at 800°C. A 5.00 L vessel containing 10.0 g of CaCO3(s)was evacuated to remove the air, sealed, and then heated to 800°C. Ignoring the volume occupied by the solid, what will be the mass of the solid in the vessel once equilibrium is reached?

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
65
Consider the chemical reaction 2NH3(g) 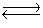 N2(g)+ 3H2(g). The equilibrium is to be established in a 1.0 L container at 1,000 K, where Kc = 4.0 * 10-2. Initially, 1,220 moles of NH3(g)are present. Estimate the equilibrium concentration of N2(g).
N2(g)+ 3H2(g). The equilibrium is to be established in a 1.0 L container at 1,000 K, where Kc = 4.0 * 10-2. Initially, 1,220 moles of NH3(g)are present. Estimate the equilibrium concentration of N2(g).
 N2(g)+ 3H2(g). The equilibrium is to be established in a 1.0 L container at 1,000 K, where Kc = 4.0 * 10-2. Initially, 1,220 moles of NH3(g)are present. Estimate the equilibrium concentration of N2(g).
N2(g)+ 3H2(g). The equilibrium is to be established in a 1.0 L container at 1,000 K, where Kc = 4.0 * 10-2. Initially, 1,220 moles of NH3(g)are present. Estimate the equilibrium concentration of N2(g).
Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
66
Consider the equilibrium equation C(s)+ H2O(g)+ 2296 J  CO(g)+ H2(g). If additional gaseous water is added to this reaction mixture, what will happen to the temperature of the mixture?
CO(g)+ H2(g). If additional gaseous water is added to this reaction mixture, what will happen to the temperature of the mixture?
 CO(g)+ H2(g). If additional gaseous water is added to this reaction mixture, what will happen to the temperature of the mixture?
CO(g)+ H2(g). If additional gaseous water is added to this reaction mixture, what will happen to the temperature of the mixture?
Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
67
Consider the equilibrium equation C(s)+ H2O(g)+ 2296 J  CO(g)+ H2(g). Which way will the reaction shift if the pressure on the system is increased?
CO(g)+ H2(g). Which way will the reaction shift if the pressure on the system is increased?
 CO(g)+ H2(g). Which way will the reaction shift if the pressure on the system is increased?
CO(g)+ H2(g). Which way will the reaction shift if the pressure on the system is increased?
Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
68
Hydrogen iodide decomposes according to the equation:
2HI(g)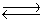 H2(g)+ I2(g), Kc = 0.0156 at 400ºC
H2(g)+ I2(g), Kc = 0.0156 at 400ºC
A 0.660 mol sample of HI was injected into a 2.00 L reaction vessel held at 400ºC.
Calculate the concentration of H2 equilibrium.
2HI(g)
 H2(g)+ I2(g), Kc = 0.0156 at 400ºC
H2(g)+ I2(g), Kc = 0.0156 at 400ºCA 0.660 mol sample of HI was injected into a 2.00 L reaction vessel held at 400ºC.
Calculate the concentration of H2 equilibrium.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
69
The data below refer to the following reaction:
2NO(g)+ Br2(g)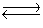 2NOBr(g)
2NOBr(g)  Calculate Kc.
Calculate Kc.
2NO(g)+ Br2(g)
 2NOBr(g)
2NOBr(g)  Calculate Kc.
Calculate Kc.
Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
70
Ethanol and acetic acid react to form ethyl acetate and water according to the following chemical equation:
C2H5OH(l)+ CH3COOH(l)![Ethanol and acetic acid react to form ethyl acetate and water according to the following chemical equation: C<sub>2</sub>H<sub>5</sub>OH(l)+ CH<sub>3</sub>COOH(l) CH<sub>3</sub>COOC<sub>2</sub>H<sub>5</sub>(l)+ H<sub>2</sub>O(l) When two moles each of ethanol and acetic acid are combined, equilibrium is reached when two-thirds of a mole of each of the reactants remains. Calculate the equilibrium constant for this reaction assuming total volume does not change during the course of the reaction. (Note: since water is not the solvent, but rather a product of the reaction, [H<sub>2</sub>O] changes during the course of the reaction, so it should be included in the equilibrium constant expression.)](https://storage.examlex.com/TB3244/11ea7995_6663_6851_911b_1f4b8d143e02_TB3244_00.jpg) CH3COOC2H5(l)+ H2O(l)
CH3COOC2H5(l)+ H2O(l)
When two moles each of ethanol and acetic acid are combined, equilibrium is reached when two-thirds of a mole of each of the reactants remains. Calculate the equilibrium constant for this reaction assuming total volume does not change during the course of the reaction. (Note: since water is not the solvent, but rather a product of the reaction, [H2O] changes during the course of the reaction, so it should be included in the equilibrium constant expression.)
C2H5OH(l)+ CH3COOH(l)
![Ethanol and acetic acid react to form ethyl acetate and water according to the following chemical equation: C<sub>2</sub>H<sub>5</sub>OH(l)+ CH<sub>3</sub>COOH(l) CH<sub>3</sub>COOC<sub>2</sub>H<sub>5</sub>(l)+ H<sub>2</sub>O(l) When two moles each of ethanol and acetic acid are combined, equilibrium is reached when two-thirds of a mole of each of the reactants remains. Calculate the equilibrium constant for this reaction assuming total volume does not change during the course of the reaction. (Note: since water is not the solvent, but rather a product of the reaction, [H<sub>2</sub>O] changes during the course of the reaction, so it should be included in the equilibrium constant expression.)](https://storage.examlex.com/TB3244/11ea7995_6663_6851_911b_1f4b8d143e02_TB3244_00.jpg) CH3COOC2H5(l)+ H2O(l)
CH3COOC2H5(l)+ H2O(l)When two moles each of ethanol and acetic acid are combined, equilibrium is reached when two-thirds of a mole of each of the reactants remains. Calculate the equilibrium constant for this reaction assuming total volume does not change during the course of the reaction. (Note: since water is not the solvent, but rather a product of the reaction, [H2O] changes during the course of the reaction, so it should be included in the equilibrium constant expression.)

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
71
Consider the chemical reaction 2NH3(g) 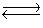 N2(g)+ 3H2(g). The equilibrium is to be established in a 1.0 L container at 1,000 K, where Kc = 4.0 * 10-2. Initially, 1,220 moles of NH3(g)are present. Estimate the equilibrium concentration of H2(g).
N2(g)+ 3H2(g). The equilibrium is to be established in a 1.0 L container at 1,000 K, where Kc = 4.0 * 10-2. Initially, 1,220 moles of NH3(g)are present. Estimate the equilibrium concentration of H2(g).
 N2(g)+ 3H2(g). The equilibrium is to be established in a 1.0 L container at 1,000 K, where Kc = 4.0 * 10-2. Initially, 1,220 moles of NH3(g)are present. Estimate the equilibrium concentration of H2(g).
N2(g)+ 3H2(g). The equilibrium is to be established in a 1.0 L container at 1,000 K, where Kc = 4.0 * 10-2. Initially, 1,220 moles of NH3(g)are present. Estimate the equilibrium concentration of H2(g).
Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
72
The data below refer to the following reaction:
2NO(g)+ Br2(g)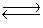 2NOBr(g)
2NOBr(g)  Find the concentration of Br2 when the system reaches equilibrium.
Find the concentration of Br2 when the system reaches equilibrium.
2NO(g)+ Br2(g)
 2NOBr(g)
2NOBr(g)  Find the concentration of Br2 when the system reaches equilibrium.
Find the concentration of Br2 when the system reaches equilibrium.
Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
73
Consider the reaction N2(g)+ 3H2(g) 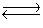 2NH3(g). If we use a catalyst, which way will the reaction shift?
2NH3(g). If we use a catalyst, which way will the reaction shift?
 2NH3(g). If we use a catalyst, which way will the reaction shift?
2NH3(g). If we use a catalyst, which way will the reaction shift?
Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
74
Consider the reaction N2(g)+ 3H2(g) 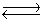 2NH3(g). If nitrogen is removed from the system at equilibrium, what will happen to the hydrogen (H2)concentration?
2NH3(g). If nitrogen is removed from the system at equilibrium, what will happen to the hydrogen (H2)concentration?
 2NH3(g). If nitrogen is removed from the system at equilibrium, what will happen to the hydrogen (H2)concentration?
2NH3(g). If nitrogen is removed from the system at equilibrium, what will happen to the hydrogen (H2)concentration?
Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
75
Consider the equilibrium equation C(s)+ H2O(g)+ 2296 J  CO(g)+ H2(g). What will happen to the concentration of carbon monoxide if the temperature of this system is raised?
CO(g)+ H2(g). What will happen to the concentration of carbon monoxide if the temperature of this system is raised?
 CO(g)+ H2(g). What will happen to the concentration of carbon monoxide if the temperature of this system is raised?
CO(g)+ H2(g). What will happen to the concentration of carbon monoxide if the temperature of this system is raised?
Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
76
What conditions are used in the Haber process to enhance the yield of ammonia? Explain why each condition affects the yield in terms of the Le Châtelier principle.

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
77
Consider the chemical reaction 2NH3(g) 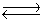 N2(g)+ 3H2(g). The equilibrium is to be established in a 1.0 L container at 1,000 K, where Kc = 4.0 * 10-2. Initially, 1,220 moles of NH3(g)are present. Calculate Kp for the reaction.
N2(g)+ 3H2(g). The equilibrium is to be established in a 1.0 L container at 1,000 K, where Kc = 4.0 * 10-2. Initially, 1,220 moles of NH3(g)are present. Calculate Kp for the reaction.
 N2(g)+ 3H2(g). The equilibrium is to be established in a 1.0 L container at 1,000 K, where Kc = 4.0 * 10-2. Initially, 1,220 moles of NH3(g)are present. Calculate Kp for the reaction.
N2(g)+ 3H2(g). The equilibrium is to be established in a 1.0 L container at 1,000 K, where Kc = 4.0 * 10-2. Initially, 1,220 moles of NH3(g)are present. Calculate Kp for the reaction.
Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
78
Consider the reaction N2(g)+ 3H2(g)  2NH3(g). The production of ammonia is an endothermic reaction. Will heating the equilibrium system increase or decrease the amount of ammonia produced?
2NH3(g). The production of ammonia is an endothermic reaction. Will heating the equilibrium system increase or decrease the amount of ammonia produced?
 2NH3(g). The production of ammonia is an endothermic reaction. Will heating the equilibrium system increase or decrease the amount of ammonia produced?
2NH3(g). The production of ammonia is an endothermic reaction. Will heating the equilibrium system increase or decrease the amount of ammonia produced?
Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
79
Consider the equilibrium equation C(s)+ H2O(g)+ 2296 J  CO(g)+ H2(g). What will happen to the mass of carbon if we add gaseous water to the system?
CO(g)+ H2(g). What will happen to the mass of carbon if we add gaseous water to the system?
 CO(g)+ H2(g). What will happen to the mass of carbon if we add gaseous water to the system?
CO(g)+ H2(g). What will happen to the mass of carbon if we add gaseous water to the system?
Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck
80
Calcium carbonate decomposes at high temperatures to give calcium oxide and carbon dioxide.
CaCO3(s) CaO(s)+ CO2(g)
CaO(s)+ CO2(g)
KP for this reaction is 1.16 at 800°C. A 5.00 L vessel containing 10.0 g of CaCO3(s)was evacuated to remove the air, sealed, and then heated to 800°C. Ignoring the volume occupied by the solid, what will be the overall mass percent of carbon in the solid once equilibrium is reached?
CaCO3(s)
 CaO(s)+ CO2(g)
CaO(s)+ CO2(g)KP for this reaction is 1.16 at 800°C. A 5.00 L vessel containing 10.0 g of CaCO3(s)was evacuated to remove the air, sealed, and then heated to 800°C. Ignoring the volume occupied by the solid, what will be the overall mass percent of carbon in the solid once equilibrium is reached?

Unlock Deck
Unlock for access to all 99 flashcards in this deck.
Unlock Deck
k this deck


