Deck 18: Thermodynamics
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/112
Play
Full screen (f)
Deck 18: Thermodynamics
1
Aluminum forms a layer of aluminum oxide when exposed to air which protects the bulk metal from further corrosion. 4Al(s)+ 3O2(g) 2Al2O3(s)
Using the thermodynamic data provided below, calculate S° for this reaction.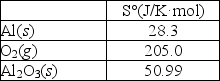
A)182.3 J/K·mol
B)131.5 J/K·mol
C)-182.3 J/K·mol
D)-626.2 J/K·mol
E)-802.9 J/K·mol
Using the thermodynamic data provided below, calculate S° for this reaction.

A)182.3 J/K·mol
B)131.5 J/K·mol
C)-182.3 J/K·mol
D)-626.2 J/K·mol
E)-802.9 J/K·mol
-626.2 J/K·mol
2
Which one of the following reactions would you expect to have highest S°?
A)CH4(g)+ 2O2(g) CO2(g)+ 2H2O(g)
B)C2H2(g)+ 5/2O2(g) 2CO2(g)+ H2O(g)
C)C2H4(g)+ 3O2(g) 2CO2(g)+ 2H2O(g)
D)C2H6(g)+ 7/2O2(g) 2CO2(g)+ 3H2O(g)
A)CH4(g)+ 2O2(g) CO2(g)+ 2H2O(g)
B)C2H2(g)+ 5/2O2(g) 2CO2(g)+ H2O(g)
C)C2H4(g)+ 3O2(g) 2CO2(g)+ 2H2O(g)
D)C2H6(g)+ 7/2O2(g) 2CO2(g)+ 3H2O(g)
C2H6(g)+ 7/2O2(g) 2CO2(g)+ 3H2O(g)
3
Arrange these compounds in order of increasing standard molar entropy at 25°C: C3H8(g), C2H4(g), ZnS(s), and H2O(l).
A)ZnS(s)< H2O(l)< C3H8(g)< C2H4(g)
B)C2H4(g)< H2O(l)< C3H8(g)< NaCl(s)
C)ZnS(s)< C3H8(g)< C2H4(g)< H2O(l)
D)C3H8(g)< C2H4(g)< H2O(l)< ZnS(s)
E)ZnS(s)< H2O(l)< C2H4(g)< C3H8(g)
A)ZnS(s)< H2O(l)< C3H8(g)< C2H4(g)
B)C2H4(g)< H2O(l)< C3H8(g)< NaCl(s)
C)ZnS(s)< C3H8(g)< C2H4(g)< H2O(l)
D)C3H8(g)< C2H4(g)< H2O(l)< ZnS(s)
E)ZnS(s)< H2O(l)< C2H4(g)< C3H8(g)
ZnS(s)< H2O(l)< C2H4(g)< C3H8(g)
4
Calculate S° at 25°C for the reduction of PbO(s), 2PbO(s)+ C(s) 2Pb(s)+ CO2(g)given these absolute entropies: 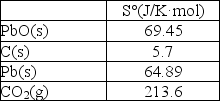
A)+198.8 J/K·mol
B)+488.0 J/K·mol
C)+353.6 J/K·mol
D)-203.3 J/K·mol
E)+203.3 J/K·mol

A)+198.8 J/K·mol
B)+488.0 J/K·mol
C)+353.6 J/K·mol
D)-203.3 J/K·mol
E)+203.3 J/K·mol

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
5
Which of these species would you expect to have the highest standard entropy (S°)?
A)CH4(g)
B)C2H2(g)
C)C2H4(g)
D)C2H6(g)
E)C3H8(g)
A)CH4(g)
B)C2H2(g)
C)C2H4(g)
D)C2H6(g)
E)C3H8(g)

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
6
Arrange the following substances in the order of increasing entropy at 25°C. HF(g), NaF(s), SiF4(g), SiH4(g), Al(s)
Lowest highest
A)SiF4(g)< SiH4(g)< NaF(s)< HF(g)< Al(s)
B)HF(g)< Al(s)< NaF(s)< SiF4(g)< SiH4(g)
C)Al(s)< NaF(s)< HF(g)< SiH4(g)< SiF4(g)
D)Al(s)< HF(g)< NaF(s)< SiF4(g)< SiH4(g)
E)NaF(s)< Al(s)< HF(g)< SiF4(g)< SiH4(g)
Lowest highest
A)SiF4(g)< SiH4(g)< NaF(s)< HF(g)< Al(s)
B)HF(g)< Al(s)< NaF(s)< SiF4(g)< SiH4(g)
C)Al(s)< NaF(s)< HF(g)< SiH4(g)< SiF4(g)
D)Al(s)< HF(g)< NaF(s)< SiF4(g)< SiH4(g)
E)NaF(s)< Al(s)< HF(g)< SiF4(g)< SiH4(g)

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
7
Which of these species has the highest entropy (S°)at 25°C?
A)CH3OH(l)
B)CO(g)
C)MgCO3(s)
D)H2O(l)
E)Ni(s)
A)CH3OH(l)
B)CO(g)
C)MgCO3(s)
D)H2O(l)
E)Ni(s)

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
8
MgCO3(s) MgO(s)+ CO2(g)
A)1 < 2 < 3
B)2 < 3 < 1
C)3 < 2 < 1
D)2 < 1 < 3
E)1 < 3 < 2
A)1 < 2 < 3
B)2 < 3 < 1
C)3 < 2 < 1
D)2 < 1 < 3
E)1 < 3 < 2

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
9
Which of these species would you expect to have the lowest standard entropy (S°)?
A)CH4(g)
B)HF(g)
C)NH3(g)
D)H2O(g)
A)CH4(g)
B)HF(g)
C)NH3(g)
D)H2O(g)

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
10
Which one of the following reactions would you expect to have the lowest S°?
A)CH4(g)+ 2O2(g) CO2(g)+ 2H2O(g)
B)C2H2(g)+ 5/2O2(g) 2CO2(g)+ H2O(g)
C)C2H4(g)+ O2(g) 2CO2(g)+ 2H2O(g)
D)C2H6(g)+ 7/2O2(g) 2CO2(g)+ 3H2O(g)
A)CH4(g)+ 2O2(g) CO2(g)+ 2H2O(g)
B)C2H2(g)+ 5/2O2(g) 2CO2(g)+ H2O(g)
C)C2H4(g)+ O2(g) 2CO2(g)+ 2H2O(g)
D)C2H6(g)+ 7/2O2(g) 2CO2(g)+ 3H2O(g)

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
11
Calculate S° for the reaction SO2(s)+ NO2(g) SO3(g)+ NO(g). 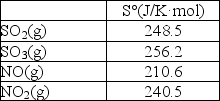
A)53.6 J/K·mol
B)-53.6 J/K·mol
C)-22.2 J/K·mol
D)474.8 J/K·mol
E)-474.8 J/K·mol

A)53.6 J/K·mol
B)-53.6 J/K·mol
C)-22.2 J/K·mol
D)474.8 J/K·mol
E)-474.8 J/K·mol

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
12
Determine S° for the reaction SO3(g)+ H2O(l) H2SO4(l). 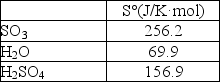
A)169.2 J/K·mol
B)1343.2 J/K·mol
C)-169.2 J/K·mol
D)-29.4 J/K·mol
E)29.4 J/K·mol

A)169.2 J/K·mol
B)1343.2 J/K·mol
C)-169.2 J/K·mol
D)-29.4 J/K·mol
E)29.4 J/K·mol

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
13
H2O2(l) H2O(l)+ 1/2O2(g)
A)1 < 3 < 2
B)2 < 3 < 1
C)2 < 1 < 3
D)3 < 2 < 1
E)3 < 1 < 2
A)1 < 3 < 2
B)2 < 3 < 1
C)2 < 1 < 3
D)3 < 2 < 1
E)3 < 1 < 2

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
14
Which of these species has the highest entropy (S°)at 25°C?
A)CO(g)
B)CH4(g)
C)NaCl(s)
D)H2O(l)
E)Fe(s)
A)CO(g)
B)CH4(g)
C)NaCl(s)
D)H2O(l)
E)Fe(s)

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
15
Which of these species would you expect to have the lowest standard entropy (S°)?
A)Br2(l)
B)Cl2(g)
C)F2(g)
D)H2(g)
E)I2(s)
A)Br2(l)
B)Cl2(g)
C)F2(g)
D)H2(g)
E)I2(s)

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
16
Sulfur can be separated from lead in the mineral galena, PbS(s), by "roasting" the ore in the presence of oxygen as shown in the following reaction: 2PbS(s)+ 3O2(g) 2PbO(s)+ 2SO2(g)
Calculate S° for this reaction using the thermodynamic data provided below.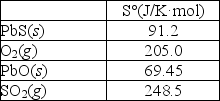
A)-410 J/K·mol
B)-161.5 J/K·mol
C)-47.7 J/K·mol
D)21.8 J/K·mol
E)43.5 J/K·mol
Calculate S° for this reaction using the thermodynamic data provided below.

A)-410 J/K·mol
B)-161.5 J/K·mol
C)-47.7 J/K·mol
D)21.8 J/K·mol
E)43.5 J/K·mol

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
17
The entropy change on vaporization ( Svap)of a compound or element is
A)always negative.
B)always positive.
C)sometimes is positive and sometimes is negative.
A)always negative.
B)always positive.
C)sometimes is positive and sometimes is negative.

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
18
Mg2+(aq)+ 2OH-(aq) Mg(OH)2(s)
A)1, 2
B)1, 3
C)3, 4
D)3
E)2, 4
A)1, 2
B)1, 3
C)3, 4
D)3
E)2, 4

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
19
H2O2(l) H2O(l)+ 1/2O2(g)
A)1, 2, 3, 4
B)1, 2
C)2, 3, 4
D)3, 4
E)1, 4
A)1, 2, 3, 4
B)1, 2
C)2, 3, 4
D)3, 4
E)1, 4

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
20
HI has a normal boiling point of -35.4°C, and its Hvap is 21.16 kJ/mol. Calculate the molar entropy of vaporization ( Svap).
A)598 J/K·mol
B)68.6 J/K·mol
C)75.2 J/K·mol
D)0.068 J/K·mol
E)89.0 J/K·mol
A)598 J/K·mol
B)68.6 J/K·mol
C)75.2 J/K·mol
D)0.068 J/K·mol
E)89.0 J/K·mol

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
21
At 1500°C the equilibrium constant for the reaction CO(g)+ 2H2(g) CH3OH(g)has the value Kp = 1.4 * 10-7. Calculate G° for this reaction at 1500°C.
A)105 kJ/mol
B)1.07 kJ/mol
C)-233 kJ/mol
D)-105 kJ/mol
E)233 kJ/mol
A)105 kJ/mol
B)1.07 kJ/mol
C)-233 kJ/mol
D)-105 kJ/mol
E)233 kJ/mol

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
22
A negative sign for G indicates that, at constant T and P,
A)the reaction is exothermic.
B)the reaction is endothermic.
C)the reaction is fast.
D)the reaction is spontaneous.
E)( S) must be > 0.
A)the reaction is exothermic.
B)the reaction is endothermic.
C)the reaction is fast.
D)the reaction is spontaneous.
E)( S) must be > 0.

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
23
Determine the equilibrium constant Kp at 25°C for the reaction N2(g)+ 3H2(g) 2NH3(g) [ G°f (NH3(g))= -16.6 kJ/mol].
A)1.52 * 10-6
B)6.60 * 105
C)8.28 * 10-2
D)2.60
E)13.4
A)1.52 * 10-6
B)6.60 * 105
C)8.28 * 10-2
D)2.60
E)13.4

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
24
Kw for the auto-ionization of water, H2O(l) H+(aq)+ OH- (aq), is 1.0 * 10-14. What are the signs (+/-)of S° and H° for the reaction at 25°C?
A)( S°) = (+)and H° = (+)
B)( S°) = (+)and H° = (-)
C)( S°) = (-)and H° = (+)
D)( S°) = (-)and H° = (-)
A)( S°) = (+)and H° = (+)
B)( S°) = (+)and H° = (-)
C)( S°) = (-)and H° = (+)
D)( S°) = (-)and H° = (-)

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
25
Sodium carbonate can be made by heating sodium bicarbonate: 2NaHCO3(s) Na2CO3(s)+ CO2(g)+ H2O(g)
Given that H° = 128.9 kJ/mol and G° = 33.1 kJ/mol at 25°C, above what minimum temperature will the reaction become spontaneous under standard state conditions?
A)0.4 K
B)3.9 K
C)321 K
D)401 K
E)525 K
Given that H° = 128.9 kJ/mol and G° = 33.1 kJ/mol at 25°C, above what minimum temperature will the reaction become spontaneous under standard state conditions?
A)0.4 K
B)3.9 K
C)321 K
D)401 K
E)525 K

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
26
Calculate Kp at 298 K for the reaction SO2(g)+ NO2(g) SO3(g)+ NO(g). 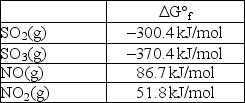
A)6.99 * 10-7
B)5.71 * 10-8
C)14.2
D)475
E)1.42 * 106

A)6.99 * 10-7
B)5.71 * 10-8
C)14.2
D)475
E)1.42 * 106

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
27
The element oxygen was prepared by Joseph Priestley in 1774 by heating mercury(II)oxide: HgO(s) Hg(l)+ 1/2O2(g), H° = 90.84 kJ/mol.
Estimate the temperature at which this reaction will become spontaneous under standard state conditions.
S°(Hg)= 76.02 J/K·mol
S°(O2)= 205.0 J/K·mol
S°(HgO)= 70.29 J/K·mol
A)108 K
B)430 K
C)620 K
D)775 K
E)840 K
Estimate the temperature at which this reaction will become spontaneous under standard state conditions.
S°(Hg)= 76.02 J/K·mol
S°(O2)= 205.0 J/K·mol
S°(HgO)= 70.29 J/K·mol
A)108 K
B)430 K
C)620 K
D)775 K
E)840 K

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
28
Ozone (O3)in the atmosphere can reaction with nitric oxide (NO): O3(g)+ NO(g) NO2(g)+ O2(g).
Calculate the G° for this reaction at 25°C. ( H° = -199 kJ/mol, S° = -4.1 J/K·mol)
A)1020 kJ/mol
B)-1.22 * 103 kJ/mol
C)2.00 * 103 kJ/mol
D)-1.42 * 103 kJ/mol
E)-198 kJ/mol
Calculate the G° for this reaction at 25°C. ( H° = -199 kJ/mol, S° = -4.1 J/K·mol)
A)1020 kJ/mol
B)-1.22 * 103 kJ/mol
C)2.00 * 103 kJ/mol
D)-1.42 * 103 kJ/mol
E)-198 kJ/mol

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
29
Determine the equilibrium constant (Kp)at 25°C for the reaction CO(g)+ H2O(g) CO2(g)+ H2(g) G° = -28.5 kJ/mol.
A)2.9 * 10-60
B)1.0 * 10-4
C)1.2
D)1.0 * 105
E)3.4 * 1059
A)2.9 * 10-60
B)1.0 * 10-4
C)1.2
D)1.0 * 105
E)3.4 * 1059

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
30
Nitrosyl chloride (NOCl)decomposes at elevated temperatures according to the equation 2NOCl(g) 2NO(g)+ Cl2(g). Calculate Kp for this reaction at 227°C. ( H° = 81.2 kJ/mol, S° = 128 J/K·mol)
A)1.59 * 10-2
B)2.10 * 10-7
C)62.8
D)4.90 * 106
E)3.20 * 109
A)1.59 * 10-2
B)2.10 * 10-7
C)62.8
D)4.90 * 106
E)3.20 * 109

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
31
For the reaction 2C(graphite)+ H2(g) C2H2(g), G°= +209.2 kJ/mol at 25°C. If P(H2)= 100. atm, and P(C2H2)= 0.10 atm, calculate G for this reaction.
A)+207.8 kJ/mol
B)+226.3 kJ/mol
C)+192.1 kJ/mol
D)+17.3 kJ/mol
E)-16.9 kJ/mol
A)+207.8 kJ/mol
B)+226.3 kJ/mol
C)+192.1 kJ/mol
D)+17.3 kJ/mol
E)-16.9 kJ/mol

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
32
Aluminum forms a layer of aluminum oxide when exposed to air which protects the bulk metal from further corrosion. 4Al(s)+ 3O2(g) 2Al2O3(s)
Calculate G° for this reaction, given that G°f of aluminum oxide is -1576.4 kJ/mol.
A)-3152.8 kJ/mol
B)-1576.4 kJ/mol
C)-788.2 kJ/mol
D)1576.4 kJ/mol
E)3152.8 kJ/mol
Calculate G° for this reaction, given that G°f of aluminum oxide is -1576.4 kJ/mol.
A)-3152.8 kJ/mol
B)-1576.4 kJ/mol
C)-788.2 kJ/mol
D)1576.4 kJ/mol
E)3152.8 kJ/mol

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
33
The equilibrium constant at 427°C for the reaction N2(g)+ 3H2(g) 2NH3(g)is Kp = 9.4 * 10-5. Calculate the value of G° for the reaction under these conditions.
A)-33 kJ/mol
B)-54 kJ/mol
C)54 kJ/mol
D)33 kJ/mol
E)1.3 J/mol
A)-33 kJ/mol
B)-54 kJ/mol
C)54 kJ/mol
D)33 kJ/mol
E)1.3 J/mol

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
34
Calculate the equilibrium constant for the decomposition of water 2H2O(l) 2H2(g)+ O2(g)
At 25°C, given that G°f (H2O(l))= -237.2 kJ/mol.
A)0.83
B)6.3 * 10-84
C)2.5 * 10-42
D)1.6 * 1083
E)4.7 * 105
At 25°C, given that G°f (H2O(l))= -237.2 kJ/mol.
A)0.83
B)6.3 * 10-84
C)2.5 * 10-42
D)1.6 * 1083
E)4.7 * 105

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
35
A spontaneous endothermic reaction always
A)causes the surroundings to get colder.
B)bursts into flame.
C)requires a spark to initiate it.
D)releases heat to the surroundings.
A)causes the surroundings to get colder.
B)bursts into flame.
C)requires a spark to initiate it.
D)releases heat to the surroundings.

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
36
Calculate G° for the reaction 3NO2(g)+ H2O(l) 2HNO3(l)+ NO(g). 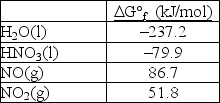
A)8.7 kJ/mol
B)192 kJ/mol
C)-414 kJ/mol
D)-192 kJ/mol
E)-155 kJ/mol

A)8.7 kJ/mol
B)192 kJ/mol
C)-414 kJ/mol
D)-192 kJ/mol
E)-155 kJ/mol

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
37
For the reaction H2(g)+ S(s) H2S(g), H° = -20.2 kJ/mol and S° = +43.1 J/K·mol. Which of these statements is true?
A)The reaction is only spontaneous at low temperatures.
B)The reaction is spontaneous at all temperatures.
C)( G°) becomes less favorable as temperature increases.
D)The reaction is spontaneous only at high temperatures.
E)The reaction is at equilibrium at 25°C under standard conditions.
A)The reaction is only spontaneous at low temperatures.
B)The reaction is spontaneous at all temperatures.
C)( G°) becomes less favorable as temperature increases.
D)The reaction is spontaneous only at high temperatures.
E)The reaction is at equilibrium at 25°C under standard conditions.

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
38
Hydrogen peroxide (H2O2)decomposes according to the equation H2O2(l) H2O(l)+ 1/2O2(g).
Calculate Kp for this reaction at 25°C. ( H° = -98.2 kJ/mol, S° = 70.1 J/K·mol)
A)1.3 * 10-21
B)20.9
C)3.46 * 1017
D)7.5 * 1020
E)8.6 * 104
Calculate Kp for this reaction at 25°C. ( H° = -98.2 kJ/mol, S° = 70.1 J/K·mol)
A)1.3 * 10-21
B)20.9
C)3.46 * 1017
D)7.5 * 1020
E)8.6 * 104

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
39
The equilibrium constant for the reaction AgBr(s) Ag+(aq)+ Br- (aq)is the solubility product constant, Ksp = 7.7* 10-13 at 25°C. Calculate G for the reaction when [Ag+] = 1.0 * 10-2 M and [Br-] = 1.0 * 10-3 M. Is the reaction spontaneous or nonspontaneous at these concentrations?
A)( G) = 69.1 kJ/mol, nonspontaneous
B)( G) = -69.1 kJ/mol, spontaneous
C)( G) = 97.5 kJ/mol, spontaneous
D)( G) = 40.6 kJ/mol, nonspontaneous
E)( G) = -97.5 kJ/mol, nonspontaneous
A)( G) = 69.1 kJ/mol, nonspontaneous
B)( G) = -69.1 kJ/mol, spontaneous
C)( G) = 97.5 kJ/mol, spontaneous
D)( G) = 40.6 kJ/mol, nonspontaneous
E)( G) = -97.5 kJ/mol, nonspontaneous

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
40
The normal freezing point of ammonia is -78°C. Predict the signs of H, S, and G for ammonia when it freezes at -80°C and 1 atm: NH3(l) NH3(s) H S G
A)- - 0
B)- + -
C)+ - +
D)+ + 0
E)- - -
A)- - 0
B)- + -
C)+ - +
D)+ + 0
E)- - -

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
41
For the reaction CuS(s)+ H2(g) H2S(g)+ Cu(s),
G°f (CuS)= -53.6 kJ/mol
G°f (H2S)= -33.6 kJ/mol
H°f (CuS)= -53.1 kJ/mol
H°f (H2S)= -20.6 kJ/mol
Calculate the value of the equilibrium constant (Kp)at 798 K and 1 atm pressure.
G°f (CuS)= -53.6 kJ/mol
G°f (H2S)= -33.6 kJ/mol
H°f (CuS)= -53.1 kJ/mol
H°f (H2S)= -20.6 kJ/mol
Calculate the value of the equilibrium constant (Kp)at 798 K and 1 atm pressure.

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
42
The reaction rates of many spontaneous reactions are actually very slow. Which of these statements is the best explanation for this observation?
A)Kp for the reaction is less than one.
B)The activation energy of the reaction is large.
C)( G°) for the reaction is positive.
D)Such reactions are endothermic.
E)The entropy change is negative.
A)Kp for the reaction is less than one.
B)The activation energy of the reaction is large.
C)( G°) for the reaction is positive.
D)Such reactions are endothermic.
E)The entropy change is negative.

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
43
In the gas phase, methyl isocyanate (CH3NC)isomerizes to acetonitrile (CH3CN), H3C-N C (g) H3C-C N (g)
With H° = -89.5 kJ/mol and G° = -73.8 kJ/mol at 25°C. Find the equilibrium constant for this reaction at 100°C.
A)1.68 * 10-10
B)5.96 * 109
C)2.16 * 1010
D)4.63 * 10-11
E)8.64 * 1012
With H° = -89.5 kJ/mol and G° = -73.8 kJ/mol at 25°C. Find the equilibrium constant for this reaction at 100°C.
A)1.68 * 10-10
B)5.96 * 109
C)2.16 * 1010
D)4.63 * 10-11
E)8.64 * 1012

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
44
For the reaction HCONH2(g) NH3(g)+ CO(g), Kc = 4.84 at 400 K. If H° for this reaction is 29 kJ/mol, find Kc at 500 K.
A)5.8
B)0.17
C)27
D)0.88
E)10.3
A)5.8
B)0.17
C)27
D)0.88
E)10.3

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
45
Find the temperature at which the reaction N2O4(g) 2NO2(g)will be in equilibrium when both gases are present at partial pressures of 1.00 atm. 
A)300°C
B)28°C
C)55°C
D)32°C
E)562°C

A)300°C
B)28°C
C)55°C
D)32°C
E)562°C

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
46
Predict the normal boiling point of triethylborane (C6H15B)using the following data: 
A)92°C
B)-21°C
C)21°C
D)365°C
E)256°C

A)92°C
B)-21°C
C)21°C
D)365°C
E)256°C

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
47
In the gas phase, formic acid forms a dimmer, 2HCOOH(g) (HCOOH)2(g). For this reaction, H° = -60.1 kJ/mol and G° = -13.9 kJ/mol at 25°C. Find the equilibrium constant (Kp)for this reaction at 75 °C.
A)8960
B)273
C)0.120
D)8.33
E)1.12 * 10-4
A)8960
B)273
C)0.120
D)8.33
E)1.12 * 10-4

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
48
Using the thermodynamic data provided below, calculate the standard change in entropy when one mole of sodium sulfate is dissolved in water? 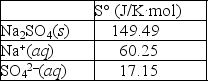 Will the solubility of sodium nitrate increase or decrease if the temperature of the system is increased?
Will the solubility of sodium nitrate increase or decrease if the temperature of the system is increased?
 Will the solubility of sodium nitrate increase or decrease if the temperature of the system is increased?
Will the solubility of sodium nitrate increase or decrease if the temperature of the system is increased?
Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
49
Which species will have the lowest absolute entropy at 25°C?
A)C2H5OH(l)
B)C2H2(g)
C)C3H8(g)
D)C3H7OH(l)
E)C2H6(g)
A)C2H5OH(l)
B)C2H2(g)
C)C3H8(g)
D)C3H7OH(l)
E)C2H6(g)

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
50
For the reaction CuS(s)+ H2(g) H2S(g)+ Cu(s),
G°f (CuS)= -53.6 kJ/mol
G°f (H2S)= -33.6 kJ/mol
H°f (CuS)= -53.1 kJ/mol
H°f (H2S)= -20.6 kJ/mol
Will this reaction proceed spontaneously at 298 K and 1 atm pressure?
G°f (CuS)= -53.6 kJ/mol
G°f (H2S)= -33.6 kJ/mol
H°f (CuS)= -53.1 kJ/mol
H°f (H2S)= -20.6 kJ/mol
Will this reaction proceed spontaneously at 298 K and 1 atm pressure?

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
51
For the reaction CuS(s)+ H2(g) H2S(g)+ Cu(s),
G°f (CuS)= -53.6 kJ/mol
G°f (H2S)= -33.6 kJ/mol
H°f (CuS)= -53.1 kJ/mol
H°f (H2S)= -20.6 kJ/mol
Calculate the value of the equilibrium constant (Kp)for this reaction at 298 K.
G°f (CuS)= -53.6 kJ/mol
G°f (H2S)= -33.6 kJ/mol
H°f (CuS)= -53.1 kJ/mol
H°f (H2S)= -20.6 kJ/mol
Calculate the value of the equilibrium constant (Kp)for this reaction at 298 K.

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
52
Using the thermodynamic data provided below, calculate the standard change in entropy when one mole of sodium nitrate is dissolved in water? 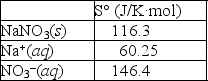 Will the solubility of sodium nitrate increase or decrease if the temperature of the system is increased?
Will the solubility of sodium nitrate increase or decrease if the temperature of the system is increased?
 Will the solubility of sodium nitrate increase or decrease if the temperature of the system is increased?
Will the solubility of sodium nitrate increase or decrease if the temperature of the system is increased?
Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
53
Find the temperature at which Kp = 42.0 for the reaction H2(g)+ I2(g) 2HI(g). [Given: at 25°C, for H2(g), H°f = 0, S° = 131.0 J/mol·K; for I2(g), H°f = 62.26 kJ/mol, S° = 260.6 J/mol·K; for HI(g), H°f = 25.9 kJ/mol, S° = 206.3 J/mol·K; assume that H° and S° are independent of temperature.]
A)1040 K
B)168 K
C)539 K
D)1400 K
E)34,200 K
A)1040 K
B)168 K
C)539 K
D)1400 K
E)34,200 K

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
54
The standard free energy of formation of gaseous hydrogen iodide is 1.30 kJ/mol at 25°C. Find Kp for the reaction H2(g)+ I2(s) 2HI(g)at this temperature.
A)7.0
B)7100
C)1.0
D)2.4
E)2.9
A)7.0
B)7100
C)1.0
D)2.4
E)2.9

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
55
Find the temperature at which Kp = 4.00 for the reaction N2O4(g) 2NO2(g). [Given: at 25°C, for NO2(g), H°f = 33.85 kJ/mol, S° = 240.46 J/mol·K; for N2O4(g), H°f = 9.66 kJ/mol, S° = 304.3 J/mol·K; assume that H° and S° are independent of temperature.]
A)197 °C
B)56 °C
C)36 °C
D)79 °C
E)476°C
A)197 °C
B)56 °C
C)36 °C
D)79 °C
E)476°C

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
56
The solubility product constant at 25°C for AgI(s)in water has the value 8.3 * 10-17. Calculate Grxn at 25°C for the process AgI(s) Ag+(aq)+ I- (aq)where [Ag+] = 9.1 * 10-9 and [I-] = 9.1 * 10-9.
A)+4.4 kJ/mol
B)+91.7 kJ/mol
C)0.0 kJ/mol
D)-91.7 kJ/mol
E)-4.4 kJ/mol
A)+4.4 kJ/mol
B)+91.7 kJ/mol
C)0.0 kJ/mol
D)-91.7 kJ/mol
E)-4.4 kJ/mol

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
57
For the reaction CuS(s)+ H2(g) H2S(g)+ Cu(s),
G°f (CuS)= -53.6 kJ/mol
G°f (H2S)= -33.6 kJ/mol
H°f (CuS)= -53.1 kJ/mol
H°f (H2S)= -20.6 kJ/mol
Calculate G at 798 K and 1 atm pressure (assume S° and H° do not change with temperature).
G°f (CuS)= -53.6 kJ/mol
G°f (H2S)= -33.6 kJ/mol
H°f (CuS)= -53.1 kJ/mol
H°f (H2S)= -20.6 kJ/mol
Calculate G at 798 K and 1 atm pressure (assume S° and H° do not change with temperature).

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
58
A sample of solid naphthalene is introduced into an evacuated flask. Use the data below to calculate the equilibrium vapor pressure of naphthalene (C10H8)in the flask at 35°C. 
A)890. mmHg
B)0.21 mmHg
C)696 mmHg
D)0.086 mmHg
E)833 mmHg

A)890. mmHg
B)0.21 mmHg
C)696 mmHg
D)0.086 mmHg
E)833 mmHg

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
59
Which species will have the greatest absolute entropy at 25°C?
A)Ne(g)
B)C2H2(g)
C)H2O(l)
D)C2H5OH(l)
E)C4H10(g)
A)Ne(g)
B)C2H2(g)
C)H2O(l)
D)C2H5OH(l)
E)C4H10(g)

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
60
Calculate G° for the combustion of ethanol vapor, C2H5OH(g), at 750°C in oxygen to form carbon dioxide and water vapor. The following data is valid at 25°C: 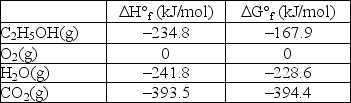
A)-1407 kJ/mol
B)-2151 kJ/mol
C)-1307 kJ/mol
D)-4486 kJ/mol
E)-1377 kJ/mol

A)-1407 kJ/mol
B)-2151 kJ/mol
C)-1307 kJ/mol
D)-4486 kJ/mol
E)-1377 kJ/mol

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
61
For the reaction 3H2(g)+ N2(g) 2NH3(g), Kc = 9.0 at 350°C. What is the value of G at this temperature when 1.0 mol NH3, 5.0 mol N2, and 5.0 mol H2 are mixed in a 2.5 L reactor?

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
62
Is the reaction SiO2(s)+ Pb(s) PbO2(s)+ Si(s)spontaneous?
G°f (PbO2)= -217 kJ/mol
G°f (SiO2)= -856 kJ/mol
G°f (PbO2)= -217 kJ/mol
G°f (SiO2)= -856 kJ/mol

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
63
Predict the sign of S for the process Ni(s, 50°C) Ni(s, 25°C).

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
64
Assuming S° and H° do not vary with temperature, at what temperature will the reaction shown below become spontaneous?
C(s)+ H2O(g) H2(g)+ CO(s)( S° = 133.6 J/K·mol; H° = 131.3 kJ/mol)
C(s)+ H2O(g) H2(g)+ CO(s)( S° = 133.6 J/K·mol; H° = 131.3 kJ/mol)

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
65
Predict the sign of S for the reaction O2(g) 2O(g).

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
66
For the reaction SbCl5(g) SbCl3(g)+ Cl2(g),
G°f (SbCl5)= -334.34 kJ/mol
G°f (SbCl3)= -301.25 kJ/mol
H°f (SbCl5)= -394.34 kJ/mol
H°f (SbCl3)= -313.80 kJ/mol
Calculate G at 800 K and 1 atm pressure (assume S° and H° do not change with temperature).
G°f (SbCl5)= -334.34 kJ/mol
G°f (SbCl3)= -301.25 kJ/mol
H°f (SbCl5)= -394.34 kJ/mol
H°f (SbCl3)= -313.80 kJ/mol
Calculate G at 800 K and 1 atm pressure (assume S° and H° do not change with temperature).

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
67
For the reaction SbCl5(g) SbCl3(g)+ Cl2(g),
G°f (SbCl5)= -334.34 kJ/mol
G°f (SbCl3)= -301.25 kJ/mol
H°f (SbCl5)= -394.34 kJ/mol
H°f (SbCl3)= -313.80 kJ/mol
Calculate the value of the equilibrium constant (Kp)at 800 K and 1 atm pressure.
G°f (SbCl5)= -334.34 kJ/mol
G°f (SbCl3)= -301.25 kJ/mol
H°f (SbCl5)= -394.34 kJ/mol
H°f (SbCl3)= -313.80 kJ/mol
Calculate the value of the equilibrium constant (Kp)at 800 K and 1 atm pressure.

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
68
For the reaction SbCl5(g) SbCl3(g)+ Cl2(g),
G°f (SbCl5)= -334.34 kJ/mol
G°f (SbCl3)= -301.25 kJ/mol
H°f (SbCl5)= -394.34 kJ/mol
H°f (SbCl3)= -313.80 kJ/mol
Calculate the value of the equilibrium constant (Kp)for this reaction at 298 K.
G°f (SbCl5)= -334.34 kJ/mol
G°f (SbCl3)= -301.25 kJ/mol
H°f (SbCl5)= -394.34 kJ/mol
H°f (SbCl3)= -313.80 kJ/mol
Calculate the value of the equilibrium constant (Kp)for this reaction at 298 K.

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
69
For a certain reaction, G° = 87 kJ/mol, H° = 100 kJ/mol at STP. At what temperature, in K, is the reaction in equilibrium, assuming that S° and H° are temperature-independent?

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
70
What is the free energy change for the reaction SiO2(s)+ Pb(s) PbO2(s)+ Si(s)?
G°f (PbO2)= -217 kJ/mol
G°f (SiO2)= -856 kJ/mol
G°f (PbO2)= -217 kJ/mol
G°f (SiO2)= -856 kJ/mol

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
71
Rubidium has a heat of vaporization of 69.0 kJ/mol at its boiling point (686°C). Calculate S for this process, Rb(l) Rb(g), at 1 atm and 686°C.

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
72
The following reaction is nonspontaneous at 25°C:
Cu2O(s) 2Cu(s)+ 1/2O2(g), G° = 141 kJ/mol
If S° = 75.8 J/K·mol, what is the lowest temperature at which the reaction will be spontaneous?
Cu2O(s) 2Cu(s)+ 1/2O2(g), G° = 141 kJ/mol
If S° = 75.8 J/K·mol, what is the lowest temperature at which the reaction will be spontaneous?

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
73
For the reaction 3H2(g)+ N2(g) 2NH3(g), Kc = 9.0 at 350°C. Calculate G° at 350°C.

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
74
The heat of vaporization of water is 2.27 kJ/g. What is Svap per mole at the normal boiling point?

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
75
Predict the sign of S for the process N2(g, 10 atm) N2(g, 1atm).

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
76
For the reaction 3H2(g)+ N2(g) 2NH3(g), Kc = 9.0 at 350°C. In what direction does this reaction proceed at 350°C under standard state conditions?

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
77
For the reaction SbCl5(g) SbCl3(g)+ Cl2(g),
G°f (SbCl5)= -334.34 kJ/mol
G°f (SbCl3)= -301.25 kJ/mol
H°f (SbCl5)= -394.34 kJ/mol
H°f (SbCl3)= -313.80 kJ/mol
Will this reaction proceed spontaneously at 298 K and 1 atm pressure?
G°f (SbCl5)= -334.34 kJ/mol
G°f (SbCl3)= -301.25 kJ/mol
H°f (SbCl5)= -394.34 kJ/mol
H°f (SbCl3)= -313.80 kJ/mol
Will this reaction proceed spontaneously at 298 K and 1 atm pressure?

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
78
Predict the sign of S for the reaction 6CO2(g)+ 6H2O(g) C6H12O6(g)+ 6O2(g).

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
79
Calculate the free energy of formation of NaBr(s)given the following information:
NaBr(s) Na(s)+ 1/2Br2(l), G° = 349 kJ/mol
NaBr(s) Na(s)+ 1/2Br2(l), G° = 349 kJ/mol

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck
80
The free energy of formation of nitric oxide, NO, at 1000 K (roughly the temperature in an automobile engine during ignition)is about 78 kJ/mol. Calculate the equilibrium constant Kp for the reaction N2(g)+ O2(g) 2NO(g)at this temperature.

Unlock Deck
Unlock for access to all 112 flashcards in this deck.
Unlock Deck
k this deck


