Deck 18: Hydrogen, oxygen, and Water
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/175
Play
Full screen (f)
Deck 18: Hydrogen, oxygen, and Water
1
Which is not an isotope of hydrogen?
A)hydrogen-1
B)hydrogen-2
C)hydrogen-3
D)hydrogen-4
A)hydrogen-1
B)hydrogen-2
C)hydrogen-3
D)hydrogen-4
hydrogen-4
2
What is the total volume of the mixture of hydrogen gas and oxygen gas can be obtained from the electrolysis of 90.0 grams of water at 25.0°C and 1.00 atm pressure according to the chemical equation shown below?
2 H2O(l)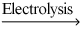 2 H2(g)+ O2(g)
2 H2(g)+ O2(g)
A)61.1 L
B)122 L
C)183 L
D)366 L
2 H2O(l)
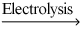 2 H2(g)+ O2(g)
2 H2(g)+ O2(g)A)61.1 L
B)122 L
C)183 L
D)366 L
183 L
3
If the molar mass of monoatomic deuterium (D)is 2.0141 grams then what is the density of diatomic deuterium (D2)gas at 25.0°C and 1.00 atmosphere pressure?
A)0)082 g/L
B)0)165 g/L
C)0)329 g/L
D)12.2 g/L
A)0)082 g/L
B)0)165 g/L
C)0)329 g/L
D)12.2 g/L
0)165 g/L
4
Hydrogen,H2,has a very low boiling point.What is the force that must be overcome in order to boil hydrogen?
A)dipole-dipole
B)H-H covalent bonding
C)hydrogen bonding
D)London dispersion
A)dipole-dipole
B)H-H covalent bonding
C)hydrogen bonding
D)London dispersion

Unlock Deck
Unlock for access to all 175 flashcards in this deck.
Unlock Deck
k this deck
5
The strongest homonuclear single bond is
A)H-H
B)C-C
C)N-N
D)O-O
A)H-H
B)C-C
C)N-N
D)O-O

Unlock Deck
Unlock for access to all 175 flashcards in this deck.
Unlock Deck
k this deck
6
What is not a commercial method for the preparation of hydrogen gas?
A)H2O(g)+ CH4(g)→ CO(g)+ 3 H2(g)
B)2 H2O(l)→ 2 H2(g)+ O2(g)
C)H2O(g)+ C(s)→ CO(g)+ H2(g)
D)4 H2O(g)+ 3 Fe → Fe3O4(s)+ 4 H2(g)
A)H2O(g)+ CH4(g)→ CO(g)+ 3 H2(g)
B)2 H2O(l)→ 2 H2(g)+ O2(g)
C)H2O(g)+ C(s)→ CO(g)+ H2(g)
D)4 H2O(g)+ 3 Fe → Fe3O4(s)+ 4 H2(g)

Unlock Deck
Unlock for access to all 175 flashcards in this deck.
Unlock Deck
k this deck
7
How many grams of zinc metal are required to produce 1.00 liter of hydrogen gas at STP according to the chemical equation shown below? 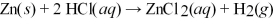
A)0)0343 g
B)2)92 g
C)5)84 g
D)65.4 g
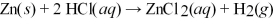
A)0)0343 g
B)2)92 g
C)5)84 g
D)65.4 g

Unlock Deck
Unlock for access to all 175 flashcards in this deck.
Unlock Deck
k this deck
8
Which is an ionic binary hydride?
A)HCl
B)KH
C)both HCl and KH
D)neither HCl nor KH
A)HCl
B)KH
C)both HCl and KH
D)neither HCl nor KH

Unlock Deck
Unlock for access to all 175 flashcards in this deck.
Unlock Deck
k this deck
9
How many liters of hydrogen gas are needed to produce 90.0 grams of water at 25.0°C and 1.00 atm pressure according to the chemical equation shown below?
2 H2(g)+ O2(g)→ 2 H2O(l)
A)61.1 L
B)122 L
C)183 L
D)366 L
2 H2(g)+ O2(g)→ 2 H2O(l)
A)61.1 L
B)122 L
C)183 L
D)366 L

Unlock Deck
Unlock for access to all 175 flashcards in this deck.
Unlock Deck
k this deck
10
How many kilojoules of energy are released when 90.0 grams of water are produced in the reaction shown below?
2 H2(g)+ O2(g)→ 2 H2O(l)ΔH° = -572 kJ
A)5720 kJ
B)2860 kJ
C)1430 kJ
D)715 kJ
2 H2(g)+ O2(g)→ 2 H2O(l)ΔH° = -572 kJ
A)5720 kJ
B)2860 kJ
C)1430 kJ
D)715 kJ

Unlock Deck
Unlock for access to all 175 flashcards in this deck.
Unlock Deck
k this deck
11
The dissociation of D2O is shown below.
D2O ⇌ D+(aq)+ OD-(aq)K = 1.95 × 10-15 at 25°C
In order to be acidic a D2O solution must have a pH (= pD)that is
A)greater than 7.00
B)greater than 7.35
C)less than 7.00
D)less than 7.35
D2O ⇌ D+(aq)+ OD-(aq)K = 1.95 × 10-15 at 25°C
In order to be acidic a D2O solution must have a pH (= pD)that is
A)greater than 7.00
B)greater than 7.35
C)less than 7.00
D)less than 7.35

Unlock Deck
Unlock for access to all 175 flashcards in this deck.
Unlock Deck
k this deck
12
Which isotope of diatomic hydrogen should have the highest heat of vaporization?
A)H2
B)D2
C)T2
D)The values are equal.
A)H2
B)D2
C)T2
D)The values are equal.

Unlock Deck
Unlock for access to all 175 flashcards in this deck.
Unlock Deck
k this deck
13
The largest single industrial use of hydrogen is
A)in the synthesis of methanol.
B)in the synthesis of ammonia.
C)in the hydrogenation of fats and oils.
D)as an automobile fuel.
A)in the synthesis of methanol.
B)in the synthesis of ammonia.
C)in the hydrogenation of fats and oils.
D)as an automobile fuel.

Unlock Deck
Unlock for access to all 175 flashcards in this deck.
Unlock Deck
k this deck
14
Differences in the physical properties of H2O and D2O are due to isotope effects.Which comparison is not correct? Compared to H2O,D2O has a higher
A)boiling point.
B)density.
C)dissociation constant.
D)melting point.
A)boiling point.
B)density.
C)dissociation constant.
D)melting point.

Unlock Deck
Unlock for access to all 175 flashcards in this deck.
Unlock Deck
k this deck
15
Write a chemical equation representing the water gas shift reaction.
A)H2O(g)+ CH4(g)→ CO(g)+ 3 H2(g)
B)2 H2O(l)→ 2 H2(g)+ O2(g)
C)CO(g)+ H2O(g)→ CO2(g)+ H2(g)
D)4 H2O(g)+ 3 Fe → Fe3O4(s)+ 4 H2(g)
A)H2O(g)+ CH4(g)→ CO(g)+ 3 H2(g)
B)2 H2O(l)→ 2 H2(g)+ O2(g)
C)CO(g)+ H2O(g)→ CO2(g)+ H2(g)
D)4 H2O(g)+ 3 Fe → Fe3O4(s)+ 4 H2(g)

Unlock Deck
Unlock for access to all 175 flashcards in this deck.
Unlock Deck
k this deck
16
What is the Lewis electron dot structure for the hydride ion?
A)H+
B)H: -
C)H ∙
D)H3O+
A)H+
B)H: -
C)H ∙
D)H3O+

Unlock Deck
Unlock for access to all 175 flashcards in this deck.
Unlock Deck
k this deck
17
The first step in the steam-hydrocarbon re-forming process is shown below.
H2O(g)+ CH4(g)→ CO(g)+ 3 H2(g
What is the change in oxidation number of the atom undergoing oxidation in this reaction?
A)- 2
B)+ 1
C)+ 2
D)+ 6
H2O(g)+ CH4(g)→ CO(g)+ 3 H2(g
What is the change in oxidation number of the atom undergoing oxidation in this reaction?
A)- 2
B)+ 1
C)+ 2
D)+ 6

Unlock Deck
Unlock for access to all 175 flashcards in this deck.
Unlock Deck
k this deck
18
How many grams of H2 gas gas can be produced by the reaction of 27.0 grams of Al(s)with an excess of dilute hydrochloric acid in the reaction shown below?
2 Al(s)+ 6 HCl(aq)→ 2 AlCl3(aq)+ 3 H2(g)
A)1)34 g
B)2)02 g
C)3)03 g
D)6)06 g
2 Al(s)+ 6 HCl(aq)→ 2 AlCl3(aq)+ 3 H2(g)
A)1)34 g
B)2)02 g
C)3)03 g
D)6)06 g

Unlock Deck
Unlock for access to all 175 flashcards in this deck.
Unlock Deck
k this deck
19
Consider the hypothetical isotope of hydrogen,quadrium,Q = 4H.Q2O would have a melting point that is (higher,lower)and a dissociation constant Kw that is (larger smaller)than H2O.
A)higher,larger
B)higher,smaller
C)lower,larger
D)lower,smaller
A)higher,larger
B)higher,smaller
C)lower,larger
D)lower,smaller

Unlock Deck
Unlock for access to all 175 flashcards in this deck.
Unlock Deck
k this deck
20
Which individual is generally given credit for the discovery of hydrogen gas?
A)Cavendish
B)Davy
C)Lavoisier
D)Priestley
A)Cavendish
B)Davy
C)Lavoisier
D)Priestley

Unlock Deck
Unlock for access to all 175 flashcards in this deck.
Unlock Deck
k this deck
21
How many liters of hydrogen gas can be generated by reacting 6.25 grams of barium hydride with water at 20°C and 755 mm Hg pressure according to the chemical equation shown below?
BaH2(s)+ 2 H2O(l)→ Ba(OH)2(aq)+ 2 H2(g)
A)0)148 L
B)0)540 L
C)1)08 L
D)2)17 L
BaH2(s)+ 2 H2O(l)→ Ba(OH)2(aq)+ 2 H2(g)
A)0)148 L
B)0)540 L
C)1)08 L
D)2)17 L

Unlock Deck
Unlock for access to all 175 flashcards in this deck.
Unlock Deck
k this deck
22
How many liters of O2 gas at 25°C and 1.00 atm pressure are needed to react with 120.5 grams of potassium hydride according to the chemical equation shown below?
2 KH(s)+ O2(g)→ H2O(g)+ K2O(s)
A)18.4 L
B)36.7 L
C)73.5 L
D)147 L
2 KH(s)+ O2(g)→ H2O(g)+ K2O(s)
A)18.4 L
B)36.7 L
C)73.5 L
D)147 L

Unlock Deck
Unlock for access to all 175 flashcards in this deck.
Unlock Deck
k this deck
23
Which transition metal hydride is commonly used in the purification of hydrogen gas?
A)Cu
B)Fe
C)Pt
D)Pd
A)Cu
B)Fe
C)Pt
D)Pd

Unlock Deck
Unlock for access to all 175 flashcards in this deck.
Unlock Deck
k this deck
24
Covalent hydrides of the type MH3 are likely to form with elements of what group?
A)3A
B)4A
C)5A
D)6A
A)3A
B)4A
C)5A
D)6A

Unlock Deck
Unlock for access to all 175 flashcards in this deck.
Unlock Deck
k this deck
25
How many mL of O2 gas at 25°C and 755 mm Hg pressure can be produced from the thermal decomposition of 0.300 grams of KClO3(s)according to the chemical equation shown below?
2 KClO3(s)→ 2 KCl(s)+ 3 O2(g)
A)30.1 mL
B)40.2 mL
C)90.4 mL
D)181 mL
2 KClO3(s)→ 2 KCl(s)+ 3 O2(g)
A)30.1 mL
B)40.2 mL
C)90.4 mL
D)181 mL

Unlock Deck
Unlock for access to all 175 flashcards in this deck.
Unlock Deck
k this deck
26
How many grams of calcium hydride are required to produce 2.28 L of hydrogen gas at 25.0°C and 0.975 atm pressure according to the chemical equation shown below?
CaH2(s)+ 2 H2O(l)→ Ca(OH)2(aq)+ 2 H2(g)
A)1)91 g
B)3)82 g
C)7)64 g
D)22.8 g
CaH2(s)+ 2 H2O(l)→ Ca(OH)2(aq)+ 2 H2(g)
A)1)91 g
B)3)82 g
C)7)64 g
D)22.8 g

Unlock Deck
Unlock for access to all 175 flashcards in this deck.
Unlock Deck
k this deck
27
In which set are all compounds considered to be covalent binary hydrides?
A)NaH,KH,CaH2,BaH2
B)MgH2,SrH2,AlH3,SiH4
C)BeH2,B2H6,CH4,NH3
D)MgH2,AlH3,SiH4,H2S
A)NaH,KH,CaH2,BaH2
B)MgH2,SrH2,AlH3,SiH4
C)BeH2,B2H6,CH4,NH3
D)MgH2,AlH3,SiH4,H2S

Unlock Deck
Unlock for access to all 175 flashcards in this deck.
Unlock Deck
k this deck
28
There are three isotopes of oxygen 16O,17O and 18O.Indicate the number of different types of isotopically substituted diatomic oxygen.
A)3
B)5
C)6
D)9
A)3
B)5
C)6
D)9

Unlock Deck
Unlock for access to all 175 flashcards in this deck.
Unlock Deck
k this deck
29
Dissolving 0.010 mole of which of the following in enough water to make 0.250 L of solution would result in a solution with the highest pH?
A)NaH
B)CaH2
C)NH3
D)PH3
A)NaH
B)CaH2
C)NH3
D)PH3

Unlock Deck
Unlock for access to all 175 flashcards in this deck.
Unlock Deck
k this deck
30
Which is not true about elemental oxygen?
A)Oxygen exists in two allotropic forms.
B)Oxygen has a double bond.
C)Oxygen is a diamagnetic element.
D)Solid oxygen is pale blue in color
A)Oxygen exists in two allotropic forms.
B)Oxygen has a double bond.
C)Oxygen is a diamagnetic element.
D)Solid oxygen is pale blue in color

Unlock Deck
Unlock for access to all 175 flashcards in this deck.
Unlock Deck
k this deck
31
In the preparation of oxygen by the thermal decomposition of potassium chlorate shown below,what is the oxidation number change of the atom undergoing reduction?
2 KClO3(s)→ 2 KCl(s)+ 3 O2(g)
A)- 8
B)- 6
C)+ 1
D)+ 2
2 KClO3(s)→ 2 KCl(s)+ 3 O2(g)
A)- 8
B)- 6
C)+ 1
D)+ 2

Unlock Deck
Unlock for access to all 175 flashcards in this deck.
Unlock Deck
k this deck
32
Using the three different isotopes of oxygen 16O,17O,18O and the major isotope of hydrogen,1H.Determine the number of different types of isotopically substituted hydrogen peroxide,H2O2,that could be formed.
A)3
B)5
C)6
D)9
A)3
B)5
C)6
D)9

Unlock Deck
Unlock for access to all 175 flashcards in this deck.
Unlock Deck
k this deck
33
There are three different isotopes of hydrogen 1H,2H,3H and three different isotopes of oxygen 16O,17O,18O.Indicate the number of different types of isotopically substituted water,H2O,that could be formed.
A)9
B)12
C)15
D)18
A)9
B)12
C)15
D)18

Unlock Deck
Unlock for access to all 175 flashcards in this deck.
Unlock Deck
k this deck
34
The net ionic reaction of solid CaH2 with water is
A)CaH2(s)+ 2 H2O(l)→ Ca(OH)2(aq)+ 2 H2(g)
B)CaH2(s)+ 2 H2O(l)→ Ca2+(aq)+ 2 OH-(aq)+ 2 H2(g)
C)H-(aq)+ H2O(l)→ OH-(aq)+ H2(g)
D)CaH2(s)+ H2O(l)→ CaO(s)+ 2 H2(g)
A)CaH2(s)+ 2 H2O(l)→ Ca(OH)2(aq)+ 2 H2(g)
B)CaH2(s)+ 2 H2O(l)→ Ca2+(aq)+ 2 OH-(aq)+ 2 H2(g)
C)H-(aq)+ H2O(l)→ OH-(aq)+ H2(g)
D)CaH2(s)+ H2O(l)→ CaO(s)+ 2 H2(g)

Unlock Deck
Unlock for access to all 175 flashcards in this deck.
Unlock Deck
k this deck
35
How many grams of KCl(s)are produced from the thermal decomposition of KClO3(s)which produces 25.0 mL of O2(g)at 25°C and 1.00 atm pressure according to the chemical equation shown below?
2 KClO3(s)→ 2 KCl(s)+ 3 O2(g)
A)0)0508 g
B)0)0762 g
C)0)0835 g
D)0)152 g
2 KClO3(s)→ 2 KCl(s)+ 3 O2(g)
A)0)0508 g
B)0)0762 g
C)0)0835 g
D)0)152 g

Unlock Deck
Unlock for access to all 175 flashcards in this deck.
Unlock Deck
k this deck
36
Commercially oxygen is usually obtained by
A)decomposition of mercury(II)oxide.
B)decomposition of potassium chlorate.
C)electrolytic decomposition of water.
D)fractional distillation of air.
A)decomposition of mercury(II)oxide.
B)decomposition of potassium chlorate.
C)electrolytic decomposition of water.
D)fractional distillation of air.

Unlock Deck
Unlock for access to all 175 flashcards in this deck.
Unlock Deck
k this deck
37
What is the oxidation number of N in NH3?
A)-3
B)-1
C)+1
D)+3
A)-3
B)-1
C)+1
D)+3

Unlock Deck
Unlock for access to all 175 flashcards in this deck.
Unlock Deck
k this deck
38
How many grams of water are required to produce 6.50 L of hydrogen gas at 25.0°C and 755 mm Hg pressure according to the chemical equation shown below?
BaH2(s)+ 2 H2O(l)→ Ba(OH)2(aq)+ 2 H2(g)
A)2)38 g
B)4)75 g
C)4)81 g
D)9)50 g
BaH2(s)+ 2 H2O(l)→ Ba(OH)2(aq)+ 2 H2(g)
A)2)38 g
B)4)75 g
C)4)81 g
D)9)50 g

Unlock Deck
Unlock for access to all 175 flashcards in this deck.
Unlock Deck
k this deck
39
Which of the following binary hydrides has the highest melting point?
A)AlH3
B)CaH2
C)NH3
D)SiH4
A)AlH3
B)CaH2
C)NH3
D)SiH4

Unlock Deck
Unlock for access to all 175 flashcards in this deck.
Unlock Deck
k this deck
40
In which set are all compounds considered to be ionic binary hydrides?
A)NaH,KH,CaH2,BaH2
B)MgH2,SrH2,AlH3,SiH4
C)BeH2,B2H6,CH4,NH3
D)MgH2,AlH3,SiH4,H2S
A)NaH,KH,CaH2,BaH2
B)MgH2,SrH2,AlH3,SiH4
C)BeH2,B2H6,CH4,NH3
D)MgH2,AlH3,SiH4,H2S

Unlock Deck
Unlock for access to all 175 flashcards in this deck.
Unlock Deck
k this deck
41
Which is classified as an ionic binary oxide?
A)MgO
B)CO2
C)P2O5
D)SO3
A)MgO
B)CO2
C)P2O5
D)SO3

Unlock Deck
Unlock for access to all 175 flashcards in this deck.
Unlock Deck
k this deck
42
What is not a good laboratory method for the preparation of oxygen?
A)photosynthesis of sugars: 6 CO2(g)+ 6 H2O(l)→ 6 O2(g)+ 6H12O6(s)
B)electrolysis of water: 2 H2O(l)→ 2 H2(g)+ O2(g)
C)catalytic decomposition of hydrogen peroxide: 2 H2O2(aq)→ 2 H2O(l)+ O2(g)
D)thermal decomposition of potassium chlorate: 2 KClO3(s)→ 2 KCl(s)+ 3 O2(g)
A)photosynthesis of sugars: 6 CO2(g)+ 6 H2O(l)→ 6 O2(g)+ 6H12O6(s)
B)electrolysis of water: 2 H2O(l)→ 2 H2(g)+ O2(g)
C)catalytic decomposition of hydrogen peroxide: 2 H2O2(aq)→ 2 H2O(l)+ O2(g)
D)thermal decomposition of potassium chlorate: 2 KClO3(s)→ 2 KCl(s)+ 3 O2(g)

Unlock Deck
Unlock for access to all 175 flashcards in this deck.
Unlock Deck
k this deck
43
Which one of the following binary oxides is the most basic?
A)B2O3
B)Al2O3
C)Ga2O3
D)In2O3
A)B2O3
B)Al2O3
C)Ga2O3
D)In2O3

Unlock Deck
Unlock for access to all 175 flashcards in this deck.
Unlock Deck
k this deck
44
When sodium metal is heated in excess oxygen it tends to form ________.
A)an oxide
B)a peroxide
C)a superoxide
D)an oxide and a superoxide
A)an oxide
B)a peroxide
C)a superoxide
D)an oxide and a superoxide

Unlock Deck
Unlock for access to all 175 flashcards in this deck.
Unlock Deck
k this deck
45
Which reaction is not consistent with the chemistry of binary oxides?
A)Na2O(s)+ H2O(l)→ 2 Na+(aq)+ 2 OH-(aq)
B)SiO2(s)+ 2 OH-(aq)→ SiO32-(aq)+ H2O(l)
C)Al2O3(s)+ 6 H+(aq)→ 2 Al3+(aq)+ 3 H2O(l)
D)N2O5(aq)+ 2 H+(aq)→ 2 NO2+(aq)+ H2O(l)
A)Na2O(s)+ H2O(l)→ 2 Na+(aq)+ 2 OH-(aq)
B)SiO2(s)+ 2 OH-(aq)→ SiO32-(aq)+ H2O(l)
C)Al2O3(s)+ 6 H+(aq)→ 2 Al3+(aq)+ 3 H2O(l)
D)N2O5(aq)+ 2 H+(aq)→ 2 NO2+(aq)+ H2O(l)

Unlock Deck
Unlock for access to all 175 flashcards in this deck.
Unlock Deck
k this deck
46
What is the bond length trend for molecular oxygen,the peroxide ion,and superoxide ion?
A)O2 > O2- > O22-
B)O2 > O22- > O2-
C)O22- > O2- > O2
D)O22- > O2 > O2-
A)O2 > O2- > O22-
B)O2 > O22- > O2-
C)O22- > O2- > O2
D)O22- > O2 > O2-

Unlock Deck
Unlock for access to all 175 flashcards in this deck.
Unlock Deck
k this deck
47
What is the chemical formula for the superoxide ion?
A)O-
B)O2-
C)O2-
D)O22-
A)O-
B)O2-
C)O2-
D)O22-

Unlock Deck
Unlock for access to all 175 flashcards in this deck.
Unlock Deck
k this deck
48
What is the total volume of hydrogen gas and oxygen gas that can be produced from the thermal decomposition of 0.340 grams of H2O2 at 700°C and 755 mm Hg according to the chemical equation shown below?
H2O2(l)→ H2(g)+ O2(g)
A)402 mL
B)804 mL
C)1210 mL
D)1610 mL
H2O2(l)→ H2(g)+ O2(g)
A)402 mL
B)804 mL
C)1210 mL
D)1610 mL

Unlock Deck
Unlock for access to all 175 flashcards in this deck.
Unlock Deck
k this deck
49
What is the oxidation number of oxygen in BaO2?
A)0
B)-1/2
C)-1
D)-2
A)0
B)-1/2
C)-1
D)-2

Unlock Deck
Unlock for access to all 175 flashcards in this deck.
Unlock Deck
k this deck
50
What is the oxidation number of oxygen in KO2?
A)0
B)-1/2
C)-1
D)-2
A)0
B)-1/2
C)-1
D)-2

Unlock Deck
Unlock for access to all 175 flashcards in this deck.
Unlock Deck
k this deck
51
Which one of the following binary oxides is the most acidic?
A)P2O5
B)As2O5
C)Bi2O5
D)Cl2O7
A)P2O5
B)As2O5
C)Bi2O5
D)Cl2O7

Unlock Deck
Unlock for access to all 175 flashcards in this deck.
Unlock Deck
k this deck
52
What is the reaction of potassium peroxide in water?
A)2 KO2(s)+ H2O(l)→ O2(g)+ 2 K+(aq)+ HO2-(aq)+ OH-(aq)
B)K2O2(s)+ H2O(l)→ 2 K+(aq)+ HO2-(aq)+ OH-(aq)
C)2 K2O2(s)+ 2 H2O(l)→ 4 K+(aq)+ 4 OH-(aq)+ O2(g)
D)K2O(s)+ H2O(l)→ 2 K+(aq)+ 2 OH-(aq)
A)2 KO2(s)+ H2O(l)→ O2(g)+ 2 K+(aq)+ HO2-(aq)+ OH-(aq)
B)K2O2(s)+ H2O(l)→ 2 K+(aq)+ HO2-(aq)+ OH-(aq)
C)2 K2O2(s)+ 2 H2O(l)→ 4 K+(aq)+ 4 OH-(aq)+ O2(g)
D)K2O(s)+ H2O(l)→ 2 K+(aq)+ 2 OH-(aq)

Unlock Deck
Unlock for access to all 175 flashcards in this deck.
Unlock Deck
k this deck
53
When KO2 is dissolved in water the evolution of oxygen takes place according to the reaction
2 KO2(s)+ H2O(l)→ O2(g)+ 2 K+(aq)+ HO2-(aq)+ OH-(aq).In this reaction the O2- ion undergoes
A)disproportionation.
B)isomerization.
C)only oxidation.
D)only reduction.
2 KO2(s)+ H2O(l)→ O2(g)+ 2 K+(aq)+ HO2-(aq)+ OH-(aq).In this reaction the O2- ion undergoes
A)disproportionation.
B)isomerization.
C)only oxidation.
D)only reduction.

Unlock Deck
Unlock for access to all 175 flashcards in this deck.
Unlock Deck
k this deck
54
Which has the greatest number of unpaired electrons?
A)O2-
B)O2
C)O2-
D)O22-
A)O2-
B)O2
C)O2-
D)O22-

Unlock Deck
Unlock for access to all 175 flashcards in this deck.
Unlock Deck
k this deck
55
Which is classified as an amphoteric binary oxide?
A)B2O3
B)CO2
C)Al2O3
D)SiO2
A)B2O3
B)CO2
C)Al2O3
D)SiO2

Unlock Deck
Unlock for access to all 175 flashcards in this deck.
Unlock Deck
k this deck
56
Which of the following is a peroxide?
A)Li2O
B)K2O2
C)CaO
D)CsO2
A)Li2O
B)K2O2
C)CaO
D)CsO2

Unlock Deck
Unlock for access to all 175 flashcards in this deck.
Unlock Deck
k this deck
57
Which one of the following binary oxides is the most acidic?
A)Na2O
B)Al2O3
C)P2O5
D)Cl2O7
A)Na2O
B)Al2O3
C)P2O5
D)Cl2O7

Unlock Deck
Unlock for access to all 175 flashcards in this deck.
Unlock Deck
k this deck
58
At 25°C CO2 is a gas and SiO2 is a high-melting solid.In these compounds of oxygen
A)both carbon in CO2 and silicon in SiO2 are π-bonded to two oxygen atoms.
B)both carbon in CO2 and silicon in SiO2 are σ-bonded to four oxygen atoms.
C)carbon in CO2 is π-bonded to two oxygen atoms and silicon in SiO2 is σ-bonded to four oxygen atoms.
D)silicon in SiO2 is π-bonded to two oxygen atoms and carbon in CO2 is σ-bonded to four oxygen atoms.
A)both carbon in CO2 and silicon in SiO2 are π-bonded to two oxygen atoms.
B)both carbon in CO2 and silicon in SiO2 are σ-bonded to four oxygen atoms.
C)carbon in CO2 is π-bonded to two oxygen atoms and silicon in SiO2 is σ-bonded to four oxygen atoms.
D)silicon in SiO2 is π-bonded to two oxygen atoms and carbon in CO2 is σ-bonded to four oxygen atoms.

Unlock Deck
Unlock for access to all 175 flashcards in this deck.
Unlock Deck
k this deck
59
Which one of the following binary oxides is the most basic?
A)Na2O
B)Al2O3
C)P2O5
D)SO3
A)Na2O
B)Al2O3
C)P2O5
D)SO3

Unlock Deck
Unlock for access to all 175 flashcards in this deck.
Unlock Deck
k this deck
60
How many liters of oxygen gas can be produced at STP from the decomposition of 0.500 L of 3.00 M H2O2 according to the chemical equation shown below?
2 H2O2(l)→ 2 H2O(l)+ O2 (g)
A)16.8 L
B)22.4 L
C)33.6 L
D)67.2 L
2 H2O2(l)→ 2 H2O(l)+ O2 (g)
A)16.8 L
B)22.4 L
C)33.6 L
D)67.2 L

Unlock Deck
Unlock for access to all 175 flashcards in this deck.
Unlock Deck
k this deck
61
All of the following are reactions commonly associated with water except:
A)2 Na(s)+ 2 H2O(l)→ H2(g)+ 2 Na+(aq)+ 2 OH-(aq)
B)Ca(s)+ 2 H2O(l)→ H2(g)+ Ca2+(aq)+ 2 OH-(aq)
C)F2(g)+ H2O(l)→ HOF(aq)+ HF(aq)
D)Cl2(g)+ H2O(l)→ HOCl(aq)+ H+(aq)+ Cl-(aq)
A)2 Na(s)+ 2 H2O(l)→ H2(g)+ 2 Na+(aq)+ 2 OH-(aq)
B)Ca(s)+ 2 H2O(l)→ H2(g)+ Ca2+(aq)+ 2 OH-(aq)
C)F2(g)+ H2O(l)→ HOF(aq)+ HF(aq)
D)Cl2(g)+ H2O(l)→ HOCl(aq)+ H+(aq)+ Cl-(aq)

Unlock Deck
Unlock for access to all 175 flashcards in this deck.
Unlock Deck
k this deck
62
What are the allotropes of oxygen?
A)O and O2
B)O2 and O3
C)O,O2 and O3
D)O2,O3 and O4
A)O and O2
B)O2 and O3
C)O,O2 and O3
D)O2,O3 and O4

Unlock Deck
Unlock for access to all 175 flashcards in this deck.
Unlock Deck
k this deck
63
Indicate the coefficient in front of H2O2 when the following redox equation is balanced in an acidic medium.
H2O2(aq)+ Cl-(aq)→ H2O(l)+ Cl2(aq)
A)1
B)2
C)3
D)4
H2O2(aq)+ Cl-(aq)→ H2O(l)+ Cl2(aq)
A)1
B)2
C)3
D)4

Unlock Deck
Unlock for access to all 175 flashcards in this deck.
Unlock Deck
k this deck
64
A binary compound that forms when potassium reacts with oxygen has the composition 54.99% K and 45.01% O.This compound contains
A)one oxygen molecule and one potassium atom.
B)one peroxide ion and one potassium ion.
C)one superoxide ion and one potassium ion.
D)two oxide ions and one potassium ion.
A)one oxygen molecule and one potassium atom.
B)one peroxide ion and one potassium ion.
C)one superoxide ion and one potassium ion.
D)two oxide ions and one potassium ion.

Unlock Deck
Unlock for access to all 175 flashcards in this deck.
Unlock Deck
k this deck
65
What are the three major chemicals that are commercially obtained from sea water?
A)sodium chloride,magnesium,and bromine
B)sodium chloride,calcium carbonate,sodium sulfate
C)sodium chloride,sodium bromide,sodium carbonate
D)sodium chloride,calcium chloride,calcium carbonate
A)sodium chloride,magnesium,and bromine
B)sodium chloride,calcium carbonate,sodium sulfate
C)sodium chloride,sodium bromide,sodium carbonate
D)sodium chloride,calcium chloride,calcium carbonate

Unlock Deck
Unlock for access to all 175 flashcards in this deck.
Unlock Deck
k this deck
66
Write a balanced net ionic equation for the reaction of potassium with water.
A)2 K(s)+ H2O(l)→ 2 K+(aq)+ O2-(aq)+ H2(g)
B)2 K(s)+ 2 H2O(l)→ 2 K+(aq)+ 2 OH-(aq)+ H2(g)
C)2 K(s)+ 2 H2O(l)→ 2 K+(aq)+ O22-(aq)+ 2 H2(g)
D)K(s)+ H2O(l)→ no reaction
A)2 K(s)+ H2O(l)→ 2 K+(aq)+ O2-(aq)+ H2(g)
B)2 K(s)+ 2 H2O(l)→ 2 K+(aq)+ 2 OH-(aq)+ H2(g)
C)2 K(s)+ 2 H2O(l)→ 2 K+(aq)+ O22-(aq)+ 2 H2(g)
D)K(s)+ H2O(l)→ no reaction

Unlock Deck
Unlock for access to all 175 flashcards in this deck.
Unlock Deck
k this deck
67
What is not a characteristic of ozone,O3?
A)It is a nonlinear triatomic system.
B)It has one single bond and one double bond.
C)It is an extremely powerful oxidizing agent.
D)It is made by passing an electrical discharge through oxygen.
A)It is a nonlinear triatomic system.
B)It has one single bond and one double bond.
C)It is an extremely powerful oxidizing agent.
D)It is made by passing an electrical discharge through oxygen.

Unlock Deck
Unlock for access to all 175 flashcards in this deck.
Unlock Deck
k this deck
68
Indicate the order of reactivity of the halogens (F2,Cl2,Br2,I2)with water (fastest to slowest).
A)F2 > Cl2 > Br2 > I2
B)Cl2 > Br2 > I2 > F2
C)Br2 > I2 > F2 > Cl2
D)I2 > Br2 > Cl2 > F2
A)F2 > Cl2 > Br2 > I2
B)Cl2 > Br2 > I2 > F2
C)Br2 > I2 > F2 > Cl2
D)I2 > Br2 > Cl2 > F2

Unlock Deck
Unlock for access to all 175 flashcards in this deck.
Unlock Deck
k this deck
69
In which of the following reactions does hydrogen peroxide act as a reducing agent?
A)PbS(s)+ 4 H2O2(aq)→ PbSO4(s)+ 4 H2O(l)
B)H2O2(aq)+ 2 H+(aq)+ 2I-(aq)→ 2 H2O(l)+ I2(aq)
C)5 H2O2(aq)+ 2 MnO4-(aq)+ 6 H+(aq)→ 8 H2O(l)+ 5 O2(g)+ 2 Mn2+(aq)
D)2 Fe2+(aq)+ H2O2(aq)+ 2 H+(aq)→ 2 Fe3+(aq)+ 2 H2O(l)
A)PbS(s)+ 4 H2O2(aq)→ PbSO4(s)+ 4 H2O(l)
B)H2O2(aq)+ 2 H+(aq)+ 2I-(aq)→ 2 H2O(l)+ I2(aq)
C)5 H2O2(aq)+ 2 MnO4-(aq)+ 6 H+(aq)→ 8 H2O(l)+ 5 O2(g)+ 2 Mn2+(aq)
D)2 Fe2+(aq)+ H2O2(aq)+ 2 H+(aq)→ 2 Fe3+(aq)+ 2 H2O(l)

Unlock Deck
Unlock for access to all 175 flashcards in this deck.
Unlock Deck
k this deck
70
How many milliliters of ozone gas at at 25.0°C and 1.00 atm pressure are needed to react with 45.00 mL of a 0.100 M aqueous solution of KI according to the chemical equation shown below?
O3(g)+ 2 I-(aq)+ H2O(l)→ O2(g)+ I2(s)+ 2 OH-(aq)
A)55.0 mL
B)110.mL
C)165 mL
D)220.mL
O3(g)+ 2 I-(aq)+ H2O(l)→ O2(g)+ I2(s)+ 2 OH-(aq)
A)55.0 mL
B)110.mL
C)165 mL
D)220.mL

Unlock Deck
Unlock for access to all 175 flashcards in this deck.
Unlock Deck
k this deck
71
What are the four major ionic constituents of seawater?
A)Cl-,Na+,Mg2+,HCO3-
B)Cl-,Na+,SO42-,Mg2+
C)Cl-,Na+,SO42-,HCO3-
D)Na+,SO42-,Mg2+,Ca2+
A)Cl-,Na+,Mg2+,HCO3-
B)Cl-,Na+,SO42-,Mg2+
C)Cl-,Na+,SO42-,HCO3-
D)Na+,SO42-,Mg2+,Ca2+

Unlock Deck
Unlock for access to all 175 flashcards in this deck.
Unlock Deck
k this deck
72
In the reaction of ozone shown below,which of the following unbalanced half-reactions shows the species undergoing reduction and the reduced product?
O3(g)+ 2 I-(aq)+ H2O(l)→ O2(g)+ I2(s)+ 2 OH-(aq)
A)O3(g)→ O2(g)
B)O3(g)→ OH-(aq)
C)H2O(l)→ O2(g)
D)H2O(l)→ OH-(aq)
O3(g)+ 2 I-(aq)+ H2O(l)→ O2(g)+ I2(s)+ 2 OH-(aq)
A)O3(g)→ O2(g)
B)O3(g)→ OH-(aq)
C)H2O(l)→ O2(g)
D)H2O(l)→ OH-(aq)

Unlock Deck
Unlock for access to all 175 flashcards in this deck.
Unlock Deck
k this deck
73
What is not a characteristic of the aqueous chemical reactions of hydrogen peroxide?
A)It is a strong oxidizing agent.
B)It is a reducing agent.
C)It behaves as a weak base.
D)It undergoes disproportionation.
A)It is a strong oxidizing agent.
B)It is a reducing agent.
C)It behaves as a weak base.
D)It undergoes disproportionation.

Unlock Deck
Unlock for access to all 175 flashcards in this deck.
Unlock Deck
k this deck
74
Indicate the coefficient in front of H2O2 when the following redox equation is balanced in an acidic medium.
H2O2(aq)+ MnO4-(aq)→ O2(g)+ Mn2+(aq)
A)2
B)3
C)5
D)7
H2O2(aq)+ MnO4-(aq)→ O2(g)+ Mn2+(aq)
A)2
B)3
C)5
D)7

Unlock Deck
Unlock for access to all 175 flashcards in this deck.
Unlock Deck
k this deck
75
Water accounts for approximately two-thirds of the mass of the adult human body.If an adult human has a mass of 145 pounds,how many water molecules are there in the adult human's body?
A)96.7 molecules
B)2)43 × 103 molecules
C)1)47 × 1027 molecules
D)2)20 × 1027 molecules
A)96.7 molecules
B)2)43 × 103 molecules
C)1)47 × 1027 molecules
D)2)20 × 1027 molecules

Unlock Deck
Unlock for access to all 175 flashcards in this deck.
Unlock Deck
k this deck
76
Write a balanced net ionic equation for the reaction of bromine with water.
A)2 Br2(l)+ 2 H2O(l)→ O2(g)+ 4 HBr(aq)
B)Br2(l)+ H2O(l)→ HOBr(aq)+ H+(aq)+ Br-(aq)
C)Br2(l)+ 2 H2O(l)→ 2 HOBr(aq)+ H2(g)
D)2 Br2(g)+ 2 H2O(l)→ O2(g)+ 4 H+(aq)+ 4 Br-(aq)
A)2 Br2(l)+ 2 H2O(l)→ O2(g)+ 4 HBr(aq)
B)Br2(l)+ H2O(l)→ HOBr(aq)+ H+(aq)+ Br-(aq)
C)Br2(l)+ 2 H2O(l)→ 2 HOBr(aq)+ H2(g)
D)2 Br2(g)+ 2 H2O(l)→ O2(g)+ 4 H+(aq)+ 4 Br-(aq)

Unlock Deck
Unlock for access to all 175 flashcards in this deck.
Unlock Deck
k this deck
77
Which of the following chemical reagents is not used in the purification of water?
A)NaNO3
B)Cl2
C)CaO
D)Al2(SO4)3
A)NaNO3
B)Cl2
C)CaO
D)Al2(SO4)3

Unlock Deck
Unlock for access to all 175 flashcards in this deck.
Unlock Deck
k this deck
78
In which compound does oxygen have a -1 oxidation state?
A)H2O
B)H2O2
C)O2
D)MgO
A)H2O
B)H2O2
C)O2
D)MgO

Unlock Deck
Unlock for access to all 175 flashcards in this deck.
Unlock Deck
k this deck
79
A binary compound that forms when potassium reacts with oxygen has the composition 58.96% Na and 41.04% O.What is the formula of one formula unit of this compound?
A)Na2O
B)NaO
C)NaO2
D)Na2O2
A)Na2O
B)NaO
C)NaO2
D)Na2O2

Unlock Deck
Unlock for access to all 175 flashcards in this deck.
Unlock Deck
k this deck
80
An estimated 1.35 × 1018 m3 of water is present in the earth's oceans.Assuming water has a density of 1 g/mL then how many kilograms of water does this volume contain?
A)1)35 × 109 kg
B)1)35 × 1012 kg
C)1)35 × 1021 kg
D)1)35 × 1034 kg
A)1)35 × 109 kg
B)1)35 × 1012 kg
C)1)35 × 1021 kg
D)1)35 × 1034 kg

Unlock Deck
Unlock for access to all 175 flashcards in this deck.
Unlock Deck
k this deck


