Deck 1: Chemistry: Matter and Measurement
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/219
Play
Full screen (f)
Deck 1: Chemistry: Matter and Measurement
1
What is the chemical symbol for tin?
A)Sb
B)Sn
C)Ta
D)Ti
A)Sb
B)Sn
C)Ta
D)Ti
Sn
2
Which horizontal row of the periodic table contains the most elements?
A)row 4
B)row 5
C)row 6
D)They all contain the same number of elements.
A)row 4
B)row 5
C)row 6
D)They all contain the same number of elements.
row 6
3
Calcium belongs to the ________ group of the periodic table.
A)alkali metal
B)alkaline earth
C)halogen
D)noble gas
A)alkali metal
B)alkaline earth
C)halogen
D)noble gas
alkaline earth
4
The horizontal rows of the periodic table are called
A)groups.
B)periods.
C)triads.
D)elements.
A)groups.
B)periods.
C)triads.
D)elements.

Unlock Deck
Unlock for access to all 219 flashcards in this deck.
Unlock Deck
k this deck
5
Which element has the chemical symbol,Au?
A)aluminum
B)argon
C)gold
D)silver
A)aluminum
B)argon
C)gold
D)silver

Unlock Deck
Unlock for access to all 219 flashcards in this deck.
Unlock Deck
k this deck
6
What is the chemical symbol for platinum?
A)P
B)Pa
C)Pt
D)Pu
A)P
B)Pa
C)Pt
D)Pu

Unlock Deck
Unlock for access to all 219 flashcards in this deck.
Unlock Deck
k this deck
7
Which is not true?
A)Mendeleev ended each row in his periodic table with an inert gas.
B)Mendeleev left gaps in his periodic table for undiscovered elements.
C)Mendeleev ordered the elements in his periodic table by atomic weight.
D)Mendeleev's periodic table predated the concept of electron configuration.
A)Mendeleev ended each row in his periodic table with an inert gas.
B)Mendeleev left gaps in his periodic table for undiscovered elements.
C)Mendeleev ordered the elements in his periodic table by atomic weight.
D)Mendeleev's periodic table predated the concept of electron configuration.

Unlock Deck
Unlock for access to all 219 flashcards in this deck.
Unlock Deck
k this deck
8
Elements in a periodic group have similar
A)chemical properties.
B)densities.
C)masses.
D)physical properties.
A)chemical properties.
B)densities.
C)masses.
D)physical properties.

Unlock Deck
Unlock for access to all 219 flashcards in this deck.
Unlock Deck
k this deck
9
A consistent explanation of known observations is called
A)an experiment.
B)a hypothesis.
C)a prediction.
D)a theory.
A)an experiment.
B)a hypothesis.
C)a prediction.
D)a theory.

Unlock Deck
Unlock for access to all 219 flashcards in this deck.
Unlock Deck
k this deck
10
An interpretation of the results of many tests is called
A)an experiment.
B)a hypothesis.
C)a prediction.
D)a theory.
A)an experiment.
B)a hypothesis.
C)a prediction.
D)a theory.

Unlock Deck
Unlock for access to all 219 flashcards in this deck.
Unlock Deck
k this deck
11
The vertical columns of the periodic table are called
A)groups.
B)periods.
C)triads.
D)elements.
A)groups.
B)periods.
C)triads.
D)elements.

Unlock Deck
Unlock for access to all 219 flashcards in this deck.
Unlock Deck
k this deck
12
Which of the following elements has chemical properties similar to oxygen?
A)fluorine
B)hydrogen
C)nitrogen
D)sulfur
A)fluorine
B)hydrogen
C)nitrogen
D)sulfur

Unlock Deck
Unlock for access to all 219 flashcards in this deck.
Unlock Deck
k this deck
13
Bromine belongs to the ________ group of the periodic table.
A)alkali metal
B)alkaline earth metal
C)halogen
D)noble gas
A)alkali metal
B)alkaline earth metal
C)halogen
D)noble gas

Unlock Deck
Unlock for access to all 219 flashcards in this deck.
Unlock Deck
k this deck
14
What is the chemical symbol for manganese?
A)Hg
B)Mg
C)Mn
D)Na
A)Hg
B)Mg
C)Mn
D)Na

Unlock Deck
Unlock for access to all 219 flashcards in this deck.
Unlock Deck
k this deck
15
Lithium belongs to the ________ group of the periodic table.
A)alkali metal
B)alkaline earth metal
C)halogen
D)noble gas
A)alkali metal
B)alkaline earth metal
C)halogen
D)noble gas

Unlock Deck
Unlock for access to all 219 flashcards in this deck.
Unlock Deck
k this deck
16
Mendeleev arranged the elements according to
A)atomic number and atomic weight.
B)atomic weight and chemical reactivity.
C)electron configuration and atomic weight.
D)physical state and relative abundance.
A)atomic number and atomic weight.
B)atomic weight and chemical reactivity.
C)electron configuration and atomic weight.
D)physical state and relative abundance.

Unlock Deck
Unlock for access to all 219 flashcards in this deck.
Unlock Deck
k this deck
17
Which element has the chemical symbol,P?
A)lead
B)phosphorus
C)platinum
D)potassium
A)lead
B)phosphorus
C)platinum
D)potassium

Unlock Deck
Unlock for access to all 219 flashcards in this deck.
Unlock Deck
k this deck
18
Which of the following statements does not describe a physical property of chlorine?
A)Chlorine combines with sodium to form table salt.
B)The color of chorine gas is green.
C)The density of chlorine gas at standard temperature and pressure is 3.17 g/L.
D)The freezing point of chlorine is -101°C.
A)Chlorine combines with sodium to form table salt.
B)The color of chorine gas is green.
C)The density of chlorine gas at standard temperature and pressure is 3.17 g/L.
D)The freezing point of chlorine is -101°C.

Unlock Deck
Unlock for access to all 219 flashcards in this deck.
Unlock Deck
k this deck
19
Argon belongs to the ________ group of the periodic table.
A)alkali metal
B)alkaline earth
C)halogen
D)noble gas
A)alkali metal
B)alkaline earth
C)halogen
D)noble gas

Unlock Deck
Unlock for access to all 219 flashcards in this deck.
Unlock Deck
k this deck
20
Most elements in the periodic table are
A)metals.
B)non-metals.
C)noble gases.
D)semi-metals.
A)metals.
B)non-metals.
C)noble gases.
D)semi-metals.

Unlock Deck
Unlock for access to all 219 flashcards in this deck.
Unlock Deck
k this deck
21
Which of the following underlined items is not an extensive property?
A)the color of a cobalt compound
B)the diameter of a soap bubble
C)the mass of a diamond
D)the volume of a glucose solution
A)the color of a cobalt compound
B)the diameter of a soap bubble
C)the mass of a diamond
D)the volume of a glucose solution

Unlock Deck
Unlock for access to all 219 flashcards in this deck.
Unlock Deck
k this deck
22
Which of the following elements is classified as a semimetal?
A)calcium
B)germanium
C)fluorine
D)uranium
A)calcium
B)germanium
C)fluorine
D)uranium

Unlock Deck
Unlock for access to all 219 flashcards in this deck.
Unlock Deck
k this deck
23
The fundamental SI unit of mass is the
A)centigram.
B)gram.
C)kilogram.
D)milligram.
A)centigram.
B)gram.
C)kilogram.
D)milligram.

Unlock Deck
Unlock for access to all 219 flashcards in this deck.
Unlock Deck
k this deck
24
Which group 1A element is not a metal?
A)H
B)Li
C)Fr
D)None of these
A)H
B)Li
C)Fr
D)None of these

Unlock Deck
Unlock for access to all 219 flashcards in this deck.
Unlock Deck
k this deck
25
What symbol is used to represent the factor 10-3?
A)M
B)m
C)μ
D)n
A)M
B)m
C)μ
D)n

Unlock Deck
Unlock for access to all 219 flashcards in this deck.
Unlock Deck
k this deck
26
The factor 0.01 corresponds to which prefix?
A)deka
B)deci
C)centi
D)milli
A)deka
B)deci
C)centi
D)milli

Unlock Deck
Unlock for access to all 219 flashcards in this deck.
Unlock Deck
k this deck
27
Which of the following underlined items is not an intensive property?
A)A chemical reaction requires 3.00 g of oxygen.
B)Solid copper hydroxide is blue colored.
C)The density of helium at 25°C is 1.64 × 10-4 g/cm3.
D)The melting point of aluminum metal is 933 K.
A)A chemical reaction requires 3.00 g of oxygen.
B)Solid copper hydroxide is blue colored.
C)The density of helium at 25°C is 1.64 × 10-4 g/cm3.
D)The melting point of aluminum metal is 933 K.

Unlock Deck
Unlock for access to all 219 flashcards in this deck.
Unlock Deck
k this deck
28
What symbol is used to express the factor,10-6?
A)M
B)m
C)μ
D)n
A)M
B)m
C)μ
D)n

Unlock Deck
Unlock for access to all 219 flashcards in this deck.
Unlock Deck
k this deck
29
All of the following are fundamental SI units except the
A)gram.
B)Kelvin.
C)meter.
D)second.
A)gram.
B)Kelvin.
C)meter.
D)second.

Unlock Deck
Unlock for access to all 219 flashcards in this deck.
Unlock Deck
k this deck
30
Which of the following statements does not describe a chemical property of oxygen?
A)Iron will rust in the presence of oxygen.
B)Oxygen combines with carbon to form carbon dioxide gas.
C)The pressure is caused by collision of oxygen molecules with the sides of a container.
D)When coal is burned in oxygen,the process is called combustion.
A)Iron will rust in the presence of oxygen.
B)Oxygen combines with carbon to form carbon dioxide gas.
C)The pressure is caused by collision of oxygen molecules with the sides of a container.
D)When coal is burned in oxygen,the process is called combustion.

Unlock Deck
Unlock for access to all 219 flashcards in this deck.
Unlock Deck
k this deck
31
All of the following elements are nonmetals except
A)antimony.
B)carbon.
C)hydrogen.
D)oxygen.
A)antimony.
B)carbon.
C)hydrogen.
D)oxygen.

Unlock Deck
Unlock for access to all 219 flashcards in this deck.
Unlock Deck
k this deck
32
Which of the following elements is not a solid at room temperature?
A)Ag
B)Al
C)Br
D)Fe
A)Ag
B)Al
C)Br
D)Fe

Unlock Deck
Unlock for access to all 219 flashcards in this deck.
Unlock Deck
k this deck
33
Which group 5A element is most metallic?
A)N
B)P
C)Sb
D)Bi
A)N
B)P
C)Sb
D)Bi

Unlock Deck
Unlock for access to all 219 flashcards in this deck.
Unlock Deck
k this deck
34
Which one of the following elements is a poor conductor of heat and electricity?
A)copper
B)fluorine
C)iron
D)lead
A)copper
B)fluorine
C)iron
D)lead

Unlock Deck
Unlock for access to all 219 flashcards in this deck.
Unlock Deck
k this deck
35
Which of the following elements is a liquid at room temperature?
A)chlorine
B)helium
C)mercury
D)sodium
A)chlorine
B)helium
C)mercury
D)sodium

Unlock Deck
Unlock for access to all 219 flashcards in this deck.
Unlock Deck
k this deck
36
Which group of elements reacts violently with water?
A)halogens
B)noble gases
C)alkali metals
D)alkaline earth metals
A)halogens
B)noble gases
C)alkali metals
D)alkaline earth metals

Unlock Deck
Unlock for access to all 219 flashcards in this deck.
Unlock Deck
k this deck
37
Gaseous elements characterized by low reactivity are found in group ________ of the periodic table.
A)5A
B)6A
C)7A
D)8A
A)5A
B)6A
C)7A
D)8A

Unlock Deck
Unlock for access to all 219 flashcards in this deck.
Unlock Deck
k this deck
38
Which of the following is a fundamental SI Unit?
A)centimeter
B)kilogram
C)microsecond
D)milliliter
A)centimeter
B)kilogram
C)microsecond
D)milliliter

Unlock Deck
Unlock for access to all 219 flashcards in this deck.
Unlock Deck
k this deck
39
Which of the following elements is a good conductor of heat and electricity?
A)carbon
B)chlorine
C)neon
D)zinc
A)carbon
B)chlorine
C)neon
D)zinc

Unlock Deck
Unlock for access to all 219 flashcards in this deck.
Unlock Deck
k this deck
40
What symbol is used to represent the factor 10-9?
A)M
B)m
C)μ
D)n
A)M
B)m
C)μ
D)n

Unlock Deck
Unlock for access to all 219 flashcards in this deck.
Unlock Deck
k this deck
41
The number of cm in 1.0 in is closest to ________.
A)1)0
B)1)5
C)2)0
D)2)5
A)1)0
B)1)5
C)2)0
D)2)5

Unlock Deck
Unlock for access to all 219 flashcards in this deck.
Unlock Deck
k this deck
42
An astronaut uses a laboratory balance and weighs an object on earth and again on the moon.Which statement below about the weight and mass of the object is true?
A)The mass and weight will be identical on the earth and the moon.
B)The mass will be the same on earth and moon but the weight will be less on the moon.
C)The weight will be the same on earth and moon but the mass will be less on the moon.
D)Both the mass and weight will be different on earth and moon.
A)The mass and weight will be identical on the earth and the moon.
B)The mass will be the same on earth and moon but the weight will be less on the moon.
C)The weight will be the same on earth and moon but the mass will be less on the moon.
D)Both the mass and weight will be different on earth and moon.

Unlock Deck
Unlock for access to all 219 flashcards in this deck.
Unlock Deck
k this deck
43
Convert 5.100 × 10-3 to ordinary notation.
A)0)0005100
B)0)005100
C)510.0
D)5100
A)0)0005100
B)0)005100
C)510.0
D)5100

Unlock Deck
Unlock for access to all 219 flashcards in this deck.
Unlock Deck
k this deck
44
Convert 0.003002 to standard scientific notation.
A)3)002 × 10-3
B)3002 × 10-6
C)3)002 × 103
D)3002 × 106
A)3)002 × 10-3
B)3002 × 10-6
C)3)002 × 103
D)3002 × 106

Unlock Deck
Unlock for access to all 219 flashcards in this deck.
Unlock Deck
k this deck
45
The diameter of the nucleus of an atom is approximately 1 × 10-15 meters.If 1 nm is equal to 10 Ångstroms,what is the diameter of the nucleus in Ångstroms?
A)1 × 10-23 Å
B)1 × 10-8 Å
C)1 × 10-7 Å
D)1 × 10-5 Å
A)1 × 10-23 Å
B)1 × 10-8 Å
C)1 × 10-7 Å
D)1 × 10-5 Å

Unlock Deck
Unlock for access to all 219 flashcards in this deck.
Unlock Deck
k this deck
46
A sailor circumnavigated the earth and covered 4,264,000 meters.Express this number in standard scientific notation.
A)4)264 × 10-7 m
B)4)264 × 10-6 m
C)4)264 × 106 m
D)4)264 × 107 m
A)4)264 × 10-7 m
B)4)264 × 10-6 m
C)4)264 × 106 m
D)4)264 × 107 m

Unlock Deck
Unlock for access to all 219 flashcards in this deck.
Unlock Deck
k this deck
47
Which of the following is the greatest mass?
A)1000 μg
B)1)000 × 10-4 kg
C)1)000 × 10-2 cg
D)1)000 × 10-8 Mg
A)1000 μg
B)1)000 × 10-4 kg
C)1)000 × 10-2 cg
D)1)000 × 10-8 Mg

Unlock Deck
Unlock for access to all 219 flashcards in this deck.
Unlock Deck
k this deck
48
One gram is approximately the same as half the mass of a new U.S.
A)penny.
B)dime.
C)quarter.
D)dollar.
A)penny.
B)dime.
C)quarter.
D)dollar.

Unlock Deck
Unlock for access to all 219 flashcards in this deck.
Unlock Deck
k this deck
49
The factor 10-2 corresponds to which prefix?
A)deka
B)deci
C)centi
D)milli
A)deka
B)deci
C)centi
D)milli

Unlock Deck
Unlock for access to all 219 flashcards in this deck.
Unlock Deck
k this deck
50
The average distance between nitrogen and oxygen atoms is 115 pm in a compound called nitric oxide.What is this distance in centimeters?
A)1)15 × 10-9 cm
B)1)15 × 10-8 cm
C)1)15 × 1012 cm
D)1)15 × 1016 cm
A)1)15 × 10-9 cm
B)1)15 × 10-8 cm
C)1)15 × 1012 cm
D)1)15 × 1016 cm

Unlock Deck
Unlock for access to all 219 flashcards in this deck.
Unlock Deck
k this deck
51
One kilogram is slightly more than ________ U.S.pounds?
A)0)5
B)1
C)2
D)5
A)0)5
B)1
C)2
D)5

Unlock Deck
Unlock for access to all 219 flashcards in this deck.
Unlock Deck
k this deck
52
Convert 1 μm to meters.
A)1 × 10-9 m
B)1 × 10-6 m
C)1 × 10-3 m
D)1 × 106 m
A)1 × 10-9 m
B)1 × 10-6 m
C)1 × 10-3 m
D)1 × 106 m

Unlock Deck
Unlock for access to all 219 flashcards in this deck.
Unlock Deck
k this deck
53
Without using a calculator,solve the following problem: 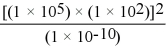
A)1 × 10-6
B)1 × 104
C)1 × 1024
D)1 × 1034
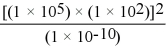
A)1 × 10-6
B)1 × 104
C)1 × 1024
D)1 × 1034

Unlock Deck
Unlock for access to all 219 flashcards in this deck.
Unlock Deck
k this deck
54
The mass of a proton is 1.67 × 10-27 kg.What is the mass of a proton in Gigagrams?
A)1)67 × 10-39 Gg
B)1)67 × 10-36 Gg
C)1)67 × 10-33 Gg
D)1)67 × 10-30 Gg
A)1)67 × 10-39 Gg
B)1)67 × 10-36 Gg
C)1)67 × 10-33 Gg
D)1)67 × 10-30 Gg

Unlock Deck
Unlock for access to all 219 flashcards in this deck.
Unlock Deck
k this deck
55
When measuring a solid metal block at constant temperature,which measurement will change in numerical value depending on the location where it is taken?
A)length
B)mass
C)volume
D)weight
A)length
B)mass
C)volume
D)weight

Unlock Deck
Unlock for access to all 219 flashcards in this deck.
Unlock Deck
k this deck
56
Without using a calculator,solve the following problem: 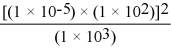
A)1 × 100
B)1 × 10-3
C)1 × 10-9
D)1 × 10-12
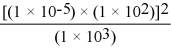
A)1 × 100
B)1 × 10-3
C)1 × 10-9
D)1 × 10-12

Unlock Deck
Unlock for access to all 219 flashcards in this deck.
Unlock Deck
k this deck
57
The diameter of an atom is approximately 1 × 10-10 m.What is the diameter in millimeters?
A)1 × 10-16 mm
B)1 × 10-13 mm
C)1 × 10-7 mm
D)1 × 10-4 mm
A)1 × 10-16 mm
B)1 × 10-13 mm
C)1 × 10-7 mm
D)1 × 10-4 mm

Unlock Deck
Unlock for access to all 219 flashcards in this deck.
Unlock Deck
k this deck
58
The mass of a single copper atom is 1.055 × 10-22 g.This is the same mass as
A)1)055 × 10-16 mg.
B)1)055 × 10-25 kg.
C)1)055 × 10-28 μg.
D)1)055 × 10-31 ng.
A)1)055 × 10-16 mg.
B)1)055 × 10-25 kg.
C)1)055 × 10-28 μg.
D)1)055 × 10-31 ng.

Unlock Deck
Unlock for access to all 219 flashcards in this deck.
Unlock Deck
k this deck
59
A student weighed 3000.μg of sulfur in the lab.This is the same mass as
A)3)000 × 10-6 g.
B)3)000 × 10-3 kg.
C)3)000 × 103 mg.
D)3)000 × 106 ng.
A)3)000 × 10-6 g.
B)3)000 × 10-3 kg.
C)3)000 × 103 mg.
D)3)000 × 106 ng.

Unlock Deck
Unlock for access to all 219 flashcards in this deck.
Unlock Deck
k this deck
60
The thickness of a U.S.dime is approximately one
A)m)
B)cm.
C)mm.
D)μm.
A)m)
B)cm.
C)mm.
D)μm.

Unlock Deck
Unlock for access to all 219 flashcards in this deck.
Unlock Deck
k this deck
61
The estimated mass of the planet Jupiter is 1.90 × 1027 kg and the density is believed to be 1.34 g/cm3.If Jupiter were a perfect sphere,what would be its diameter?
A)6)96 × 106 m
B)6)96 × 107 m
C)1)39 × 107 m
D)1)39 × 108 m
A)6)96 × 106 m
B)6)96 × 107 m
C)1)39 × 107 m
D)1)39 × 108 m

Unlock Deck
Unlock for access to all 219 flashcards in this deck.
Unlock Deck
k this deck
62
If the melting point of titanium metal is 1672°C,what is its melting point in Kelvins?
A)897 K
B)1399 K
C)1945 K
D)3042 K
A)897 K
B)1399 K
C)1945 K
D)3042 K

Unlock Deck
Unlock for access to all 219 flashcards in this deck.
Unlock Deck
k this deck
63
What is the coldest temperature possible?
A)0°C
B)0°F
C)0 K
D)None of these
A)0°C
B)0°F
C)0 K
D)None of these

Unlock Deck
Unlock for access to all 219 flashcards in this deck.
Unlock Deck
k this deck
64
The density of mercury is 13.5 g/mL.What is the mass in kg of mercury that fills a 0.250-L flask?
A)0)0540 kg
B)3)38 kg
C)54.0 kg
D)3380 kg
A)0)0540 kg
B)3)38 kg
C)54.0 kg
D)3380 kg

Unlock Deck
Unlock for access to all 219 flashcards in this deck.
Unlock Deck
k this deck
65
The freezing point of methane is -295°F and the boiling point is -263°F.The temperature of the surface of Titan,a moon of Saturn,is 93 K.If methane exists on Titan,it is
A)a gas.
B)a liquid.
C)a solid.
D)a plasma.
A)a gas.
B)a liquid.
C)a solid.
D)a plasma.

Unlock Deck
Unlock for access to all 219 flashcards in this deck.
Unlock Deck
k this deck
66
Which one of the following statements about temperature scales is false?
A)The boiling point of water on the Fahrenheit scale is 212 degrees.
B)The Celsius degree is smaller than the Fahrenheit degree.
C)The freezing point of water on the Celsius scale is 0 degrees.
D)All temperatures on the Kelvin scale are positive numbers.
A)The boiling point of water on the Fahrenheit scale is 212 degrees.
B)The Celsius degree is smaller than the Fahrenheit degree.
C)The freezing point of water on the Celsius scale is 0 degrees.
D)All temperatures on the Kelvin scale are positive numbers.

Unlock Deck
Unlock for access to all 219 flashcards in this deck.
Unlock Deck
k this deck
67
Convert 100 cm3 to m3.
A)1 × 10-4 m3
B)1 × 100 m3
C)1 × 104 m3
D)1 × 108 m3
A)1 × 10-4 m3
B)1 × 100 m3
C)1 × 104 m3
D)1 × 108 m3

Unlock Deck
Unlock for access to all 219 flashcards in this deck.
Unlock Deck
k this deck
68
Convert 15 m3 to liters.
A)1)5 × 10-2 L
B)1)5 L
C)1)5 × 102 L
D)1)5 × 104 L
A)1)5 × 10-2 L
B)1)5 L
C)1)5 × 102 L
D)1)5 × 104 L

Unlock Deck
Unlock for access to all 219 flashcards in this deck.
Unlock Deck
k this deck
69
Which of the following is the lowest temperature?
A)37°C
B)54°F
C)313 K
D)All of these temperatures are all equal.
A)37°C
B)54°F
C)313 K
D)All of these temperatures are all equal.

Unlock Deck
Unlock for access to all 219 flashcards in this deck.
Unlock Deck
k this deck
70
The density of copper is 8.96 g/cm3.What is the mass in mg of a cube of copper that measures 2.31 mm on each side?
A)0)0207 g
B)0)110 g
C)2)07 g
D)110 g
A)0)0207 g
B)0)110 g
C)2)07 g
D)110 g

Unlock Deck
Unlock for access to all 219 flashcards in this deck.
Unlock Deck
k this deck
71
The density of aluminum is 2.702 g/cm3.What is the final liquid level of water if 1.130 ounces of aluminum is dropped into a graduated cylinder containing 15.90 mL of water?
A)17.08 mL
B)21.66 mL
C)27.76 mL
D)47.95 mL
A)17.08 mL
B)21.66 mL
C)27.76 mL
D)47.95 mL

Unlock Deck
Unlock for access to all 219 flashcards in this deck.
Unlock Deck
k this deck
72
Because of the high heat and low humidity in the summer in Death Valley,California,a visitor requires about one quart of water for every two miles traveled on foot.If the density of water is 0.990 g/mL at 45°C,how many kilograms of water are required for a person to walk 30 kilometers in Death Valley?
A)8)7 kg
B)70 kg
C)3)5 × 102 kg
D)7)0 × 102 kg
A)8)7 kg
B)70 kg
C)3)5 × 102 kg
D)7)0 × 102 kg

Unlock Deck
Unlock for access to all 219 flashcards in this deck.
Unlock Deck
k this deck
73
A mass of mercury occupies 0.750 L.What volume would an equal mass of ethanol occupy? The density of mercury is 13.546 g/mL and the density of ethanol is 0.789 g/mL.
A)0)0437 L
B)0)0777 L
C)12.9 L
D)22.9 L
A)0)0437 L
B)0)0777 L
C)12.9 L
D)22.9 L

Unlock Deck
Unlock for access to all 219 flashcards in this deck.
Unlock Deck
k this deck
74
Which of the following is the smallest volume?
A)44 cm3
B)1)0 dL
C)5)5 × 103 mL
D)1 × 108 nL
A)44 cm3
B)1)0 dL
C)5)5 × 103 mL
D)1 × 108 nL

Unlock Deck
Unlock for access to all 219 flashcards in this deck.
Unlock Deck
k this deck
75
Which of the following volumes is equal to 10 mL?
A)10 cm3
B)10 dm3
C)0)10 L
D)0)00010 kL
A)10 cm3
B)10 dm3
C)0)10 L
D)0)00010 kL

Unlock Deck
Unlock for access to all 219 flashcards in this deck.
Unlock Deck
k this deck
76
A gold ingot weighs 5.50 lbs.If the density of gold is 19.31 g/cm3,and the length and width of the ingot are 12.0 cm and 3.00 cm respectively,what is the height of the ingot?
A)6)50 × 10-3 cm
B)3)59 cm
C)10.2 cm
D)1)34 × 103 cm
A)6)50 × 10-3 cm
B)3)59 cm
C)10.2 cm
D)1)34 × 103 cm

Unlock Deck
Unlock for access to all 219 flashcards in this deck.
Unlock Deck
k this deck
77
The volume of a well is 40.0 ft3.How many kilograms of concrete will it take to fill the well if the density of concrete is 2.85 g/cm3?
A)3)47 kg
B)3)23 × 103 kg
C)3)47 × 103 kg
D)3)23 × 106 kg
A)3)47 kg
B)3)23 × 103 kg
C)3)47 × 103 kg
D)3)23 × 106 kg

Unlock Deck
Unlock for access to all 219 flashcards in this deck.
Unlock Deck
k this deck
78
A piece of metal ore weighs 8.25 g.When a student places it into a graduated cylinder containing water,the liquid level rises from 21.25 mL to 26.47 mL.What is the density of the ore?
A)0)312 g/mL
B)0)633 g/mL
C)1)58 g/mL
D)3)21 g/mL
A)0)312 g/mL
B)0)633 g/mL
C)1)58 g/mL
D)3)21 g/mL

Unlock Deck
Unlock for access to all 219 flashcards in this deck.
Unlock Deck
k this deck
79
The nighttime and daytime temperatures on Mercury are 13 K and 683 K respectively.The melting point and boiling point of sulfur is 246°F and 832°F.Which of the following statements is true? On Mercury sulfur exists
A)only in the liquid state.
B)only in the solid state.
C)as both a liquid and a gas.
D)as both a liquid and a solid.
A)only in the liquid state.
B)only in the solid state.
C)as both a liquid and a gas.
D)as both a liquid and a solid.

Unlock Deck
Unlock for access to all 219 flashcards in this deck.
Unlock Deck
k this deck
80
One liter is approximately the same as one U.S.
A)ounce.
B)pint.
C)quart.
D)gallon.
A)ounce.
B)pint.
C)quart.
D)gallon.

Unlock Deck
Unlock for access to all 219 flashcards in this deck.
Unlock Deck
k this deck


