Deck 6: Ionic Bonds and Some Main-Group Chemistry
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/173
Play
Full screen (f)
Deck 6: Ionic Bonds and Some Main-Group Chemistry
1
How many electrons are in the outermost shell of the In3+ ion in its ground state?
A)2
B)3
C)6
D)18
A)2
B)3
C)6
D)18
18
2
What is the ground-state electron configuration of the ion Hg2+?
A)[Xe]4f145d10
B)[Xe]4f145d86s2
C)[Xe]4f145d106s2
D)[Xe]4f145d106s26p2
A)[Xe]4f145d10
B)[Xe]4f145d86s2
C)[Xe]4f145d106s2
D)[Xe]4f145d106s26p2
[Xe]4f145d10
3
Which ion has the smallest ionic radius?
A)Li+
B)Na+
C)K+
D)Rb+
A)Li+
B)Na+
C)K+
D)Rb+
Li+
4
Which contains both covalent bonds and ionic bonds?
A)HCl
B)NaCl
C)NH3
D)NH4Cl
A)HCl
B)NaCl
C)NH3
D)NH4Cl

Unlock Deck
Unlock for access to all 173 flashcards in this deck.
Unlock Deck
k this deck
5
Which ion has the same electron configuration as Kr?
A)Rb+
B)Br-
C)Se2-
D)All of these
A)Rb+
B)Br-
C)Se2-
D)All of these

Unlock Deck
Unlock for access to all 173 flashcards in this deck.
Unlock Deck
k this deck
6
Of the following,which element has the highest first ionization energy?
A)beryllium
B)boron
C)hydrogen
D)lithium
A)beryllium
B)boron
C)hydrogen
D)lithium

Unlock Deck
Unlock for access to all 173 flashcards in this deck.
Unlock Deck
k this deck
7
Which contains covalent bonds?
A)NaH and HCl
B)only HCl
C)only NaCl
D)only NaH
A)NaH and HCl
B)only HCl
C)only NaCl
D)only NaH

Unlock Deck
Unlock for access to all 173 flashcards in this deck.
Unlock Deck
k this deck
8
Of the following,which element has the highest first ionization energy?
A)aluminum
B)magnesium
C)silicon
D)sodium
A)aluminum
B)magnesium
C)silicon
D)sodium

Unlock Deck
Unlock for access to all 173 flashcards in this deck.
Unlock Deck
k this deck
9
Which two ions have the same electron configuration in the ground state?
A)Rb+ and Cs+
B)Ba2+ and I-
C)Se2+ and I-
D)Fe2+ and Fe3+
A)Rb+ and Cs+
B)Ba2+ and I-
C)Se2+ and I-
D)Fe2+ and Fe3+

Unlock Deck
Unlock for access to all 173 flashcards in this deck.
Unlock Deck
k this deck
10
Which contains ionic bonds?
A)CCl4
B)CaCl2
C)Cl2
D)HCl
A)CCl4
B)CaCl2
C)Cl2
D)HCl

Unlock Deck
Unlock for access to all 173 flashcards in this deck.
Unlock Deck
k this deck
11
Most of the compounds of the 2+ ions of the first row of the transition metals from Mn to Zn are colored due to absorption of visible light promoting an electron from one 3d orbital to another.Which of these ions should tend to form colorless compounds?
A)Mn2+
B)Co2+
C)Cu2+
D)Zn2+
A)Mn2+
B)Co2+
C)Cu2+
D)Zn2+

Unlock Deck
Unlock for access to all 173 flashcards in this deck.
Unlock Deck
k this deck
12
What is the ground-state electron configuration of the ion Cu2+?
A)[Ar] 3d9
B)[Ar] 4s1 3d8
C)[Ar] 4s2 3d7
D)[Ar] 4s2 3d10 4p1
A)[Ar] 3d9
B)[Ar] 4s1 3d8
C)[Ar] 4s2 3d7
D)[Ar] 4s2 3d10 4p1

Unlock Deck
Unlock for access to all 173 flashcards in this deck.
Unlock Deck
k this deck
13
What is the ground-state electron configuration of Se2-?
A)[Ar]3d104s24p2
B)[Ar]3d104s24p4
C)[Ar]3d124s24p4
D)[Ar]3d104s24p6
A)[Ar]3d104s24p2
B)[Ar]3d104s24p4
C)[Ar]3d124s24p4
D)[Ar]3d104s24p6

Unlock Deck
Unlock for access to all 173 flashcards in this deck.
Unlock Deck
k this deck
14
Arrange the ions N3-,O2-,Mg2+,Na+,and F- in order of increasing ionic radius,starting with the smallest first.
A)Mg2+,Na+,F-,O2-,N3-
B)N3-,Mg2+,O2-,Na+,F-
C)N3-,O2-,Mg2+,F-,Na+
D)N3-,O2-,F-,Na+,Mg2+
A)Mg2+,Na+,F-,O2-,N3-
B)N3-,Mg2+,O2-,Na+,F-
C)N3-,O2-,Mg2+,F-,Na+
D)N3-,O2-,F-,Na+,Mg2+

Unlock Deck
Unlock for access to all 173 flashcards in this deck.
Unlock Deck
k this deck
15
Which ion does not have a noble gas configuration in its ground state?
A)Sc3+
B)Al3+
C)Ga3+
D)As3-
A)Sc3+
B)Al3+
C)Ga3+
D)As3-

Unlock Deck
Unlock for access to all 173 flashcards in this deck.
Unlock Deck
k this deck
16
Indicate which is larger in each of the following two sets.
(I)Cr3+ or Cr (II)Se2- or Se
A)Cr3+ is larger than Cr and Se2- is larger than Se.
B)Cr3+ is larger than Cr and Se is larger than Se2-.
C)Cr is larger than Cr3+ and Se2- is larger than Se.
D)Cr is larger than Cr3+ and Se is larger than Se2-.
(I)Cr3+ or Cr (II)Se2- or Se
A)Cr3+ is larger than Cr and Se2- is larger than Se.
B)Cr3+ is larger than Cr and Se is larger than Se2-.
C)Cr is larger than Cr3+ and Se2- is larger than Se.
D)Cr is larger than Cr3+ and Se is larger than Se2-.

Unlock Deck
Unlock for access to all 173 flashcards in this deck.
Unlock Deck
k this deck
17
Which of the following three sets consist of atoms or ions with the same electron configuration in the ground state?
(I)O2-,Ne,and Mg2+
(II)Ni,Cu+,and Zn2+
(III)Hg,Tl+,and Pb2+
A)all three sets
B)all but (I)
C)all but (II)
D)only (I)
(I)O2-,Ne,and Mg2+
(II)Ni,Cu+,and Zn2+
(III)Hg,Tl+,and Pb2+
A)all three sets
B)all but (I)
C)all but (II)
D)only (I)

Unlock Deck
Unlock for access to all 173 flashcards in this deck.
Unlock Deck
k this deck
18
Which ion has the smallest ionic radius?
A)F-
B)Cl-
C)Br-
D)I-
A)F-
B)Cl-
C)Br-
D)I-

Unlock Deck
Unlock for access to all 173 flashcards in this deck.
Unlock Deck
k this deck
19
Consider Li+,F-,and O2-.Which ratio should be the largest?
A)(radius Li+)/(radius F-)
B)(radius Li+)/(radius O2-)
C)(radius F-)/(radius Li+)
D)(radius O2-)/(radius Li+)
A)(radius Li+)/(radius F-)
B)(radius Li+)/(radius O2-)
C)(radius F-)/(radius Li+)
D)(radius O2-)/(radius Li+)

Unlock Deck
Unlock for access to all 173 flashcards in this deck.
Unlock Deck
k this deck
20
Which of the following most likely represent the atomic radius of a Cr atom,the ionic radius of a Cr2+ ion,and the ionic radius of a Cr3+ ion?
A)128 pm for Cr,167 pm for Cr2+,and 193 pm for Cr3+
B)128 pm for Cr,147 pm for Cr2+,and 193 pm for Cr3+
C)128 pm for Cr,109 pm for Cr2+,and 63 pm for Cr3+
D)128 pm for Cr,89 pm for Cr2+,and 63 pm for Cr3+
A)128 pm for Cr,167 pm for Cr2+,and 193 pm for Cr3+
B)128 pm for Cr,147 pm for Cr2+,and 193 pm for Cr3+
C)128 pm for Cr,109 pm for Cr2+,and 63 pm for Cr3+
D)128 pm for Cr,89 pm for Cr2+,and 63 pm for Cr3+

Unlock Deck
Unlock for access to all 173 flashcards in this deck.
Unlock Deck
k this deck
21
Which element has the least favorable (least negative)electron affinity?
A)B
B)C
C)N
D)O
A)B
B)C
C)N
D)O

Unlock Deck
Unlock for access to all 173 flashcards in this deck.
Unlock Deck
k this deck
22
Which period 3 element has successive first through seventh ionization energies (kJ/mol)of
Ei1 = 578;Ei2 = 1,817;Ei3 = 2,745;Ei4 = 11,575;Ei5 = 14,830;Ei6 = 18,376;and Ei7 = 23,293?
A)Mg
B)Al
C)S
D)Cl
Ei1 = 578;Ei2 = 1,817;Ei3 = 2,745;Ei4 = 11,575;Ei5 = 14,830;Ei6 = 18,376;and Ei7 = 23,293?
A)Mg
B)Al
C)S
D)Cl

Unlock Deck
Unlock for access to all 173 flashcards in this deck.
Unlock Deck
k this deck
23
Which ionization process requires the most energy?
A)S(g)→ S+(g)+ e-
B)S+(g)→ S2+(g)+ e-
C)Cl(g)→ Cl+(g)+ e-
D)Cl+(g)→ Cl2+(g)+ e-
A)S(g)→ S+(g)+ e-
B)S+(g)→ S2+(g)+ e-
C)Cl(g)→ Cl+(g)+ e-
D)Cl+(g)→ Cl2+(g)+ e-

Unlock Deck
Unlock for access to all 173 flashcards in this deck.
Unlock Deck
k this deck
24
Which liberates the most energy?
A)Li(g)+ e- → Li-(g)
B)Na(g)+ e- → Na-(g)
C)K(g)+ e- → K-(g)
D)Rb(g)+ e- → Rb-(g)
A)Li(g)+ e- → Li-(g)
B)Na(g)+ e- → Na-(g)
C)K(g)+ e- → K-(g)
D)Rb(g)+ e- → Rb-(g)

Unlock Deck
Unlock for access to all 173 flashcards in this deck.
Unlock Deck
k this deck
25
Which of the following represents the change in electronic configuration that is associated with the first ionization energy of magnesium?
A)[Ne]3s13p1 → [Ne]3s1 + e-
B)[Ne]3s2 → [Ne]3s13p1
C)[Ne]3s2 → [Ne]3s1 + e-
D)[Ne]3s2 + e- → [Ne]3s23p1
A)[Ne]3s13p1 → [Ne]3s1 + e-
B)[Ne]3s2 → [Ne]3s13p1
C)[Ne]3s2 → [Ne]3s1 + e-
D)[Ne]3s2 + e- → [Ne]3s23p1

Unlock Deck
Unlock for access to all 173 flashcards in this deck.
Unlock Deck
k this deck
26
Which element has the most favorable (most negative)electron affinity?
A)B
B)C
C)Li
D)N
A)B
B)C
C)Li
D)N

Unlock Deck
Unlock for access to all 173 flashcards in this deck.
Unlock Deck
k this deck
27
Of the following,which element has the highest first ionization energy?
A)Al
B)Cl
C)Na
D)P
A)Al
B)Cl
C)Na
D)P

Unlock Deck
Unlock for access to all 173 flashcards in this deck.
Unlock Deck
k this deck
28
List the elements Cs,Ca,Ne,Na,Ar in order of decreasing first ionization energy.
A)Ar > Ca > Cs > Na > Ne
B)Ne > Ar > Ca > Na > Cs
C)Ne > Ar > Na > Cs > Ca
D)Ne > Na > Cs > Ca > Ar
A)Ar > Ca > Cs > Na > Ne
B)Ne > Ar > Ca > Na > Cs
C)Ne > Ar > Na > Cs > Ca
D)Ne > Na > Cs > Ca > Ar

Unlock Deck
Unlock for access to all 173 flashcards in this deck.
Unlock Deck
k this deck
29
Of the following,which element has the highest first ionization energy?
A)Cl
B)F
C)O
D)S
A)Cl
B)F
C)O
D)S

Unlock Deck
Unlock for access to all 173 flashcards in this deck.
Unlock Deck
k this deck
30
Which period 2 element has successive first through seventh ionization energies (kJ/mol)of
Ei1 = 1,402;Ei2 =2,856;Ei3 =4,578;Ei4 =7,475;Ei5 =9,445;Ei6 =53,267;and Ei7 = 64,360?
A)B
B)C
C)N
D)O
Ei1 = 1,402;Ei2 =2,856;Ei3 =4,578;Ei4 =7,475;Ei5 =9,445;Ei6 =53,267;and Ei7 = 64,360?
A)B
B)C
C)N
D)O

Unlock Deck
Unlock for access to all 173 flashcards in this deck.
Unlock Deck
k this deck
31
Which element has the most favorable (most negative)electron affinity?
A)Na
B)Mg
C)O
D)Ne
A)Na
B)Mg
C)O
D)Ne

Unlock Deck
Unlock for access to all 173 flashcards in this deck.
Unlock Deck
k this deck
32
Of the following,which element has the highest first ionization energy?
A)Ca
B)K
C)Li
D)Mg
A)Ca
B)K
C)Li
D)Mg

Unlock Deck
Unlock for access to all 173 flashcards in this deck.
Unlock Deck
k this deck
33
Which of the following atoms with the specified electronic configurations would have the lowest first ionization energy?
A)[He]2s22p3
B)[Ne]3s23p4
C)[Xe]6s1
D)[Xe]6s24f145d106p1
A)[He]2s22p3
B)[Ne]3s23p4
C)[Xe]6s1
D)[Xe]6s24f145d106p1

Unlock Deck
Unlock for access to all 173 flashcards in this deck.
Unlock Deck
k this deck
34
Which electron affinity process would liberate the most energy?
A)[He] 2s2 + e- → [He] 2s2 2p1
B)[He] 2s2 2p2 + e- → [He] 2s2 2p3
C)[He] 2s2 2p3 + e- → [He] 2s2 2p4
D)[He] 2s2 2p6 + e- → [He] 2s2 2p6 3s1
A)[He] 2s2 + e- → [He] 2s2 2p1
B)[He] 2s2 2p2 + e- → [He] 2s2 2p3
C)[He] 2s2 2p3 + e- → [He] 2s2 2p4
D)[He] 2s2 2p6 + e- → [He] 2s2 2p6 3s1

Unlock Deck
Unlock for access to all 173 flashcards in this deck.
Unlock Deck
k this deck
35
Which liberates the most energy?
A)Br(g)+ e⁻ → Br⁻(g)
B)Cl(g)+ e⁻ → Cl⁻(g)
C)F(g)+ e⁻ → F⁻(g)
D)I(g)→ I⁻(g)
A)Br(g)+ e⁻ → Br⁻(g)
B)Cl(g)+ e⁻ → Cl⁻(g)
C)F(g)+ e⁻ → F⁻(g)
D)I(g)→ I⁻(g)

Unlock Deck
Unlock for access to all 173 flashcards in this deck.
Unlock Deck
k this deck
36
Of the following,which element has the highest first ionization energy?
A)Ca
B)Cl
C)Na
D)Se
A)Ca
B)Cl
C)Na
D)Se

Unlock Deck
Unlock for access to all 173 flashcards in this deck.
Unlock Deck
k this deck
37
List the elements Na,Ca,Rb,Cl,He in order of decreasing first ionization energy.
A)He > Cl > Ca > Na > Rb
B)He > Na > Ca > Cl > Rb
C)He > Na > Cl > Ca > Rb
D)Rb > Ca > Cl > Na > He
A)He > Cl > Ca > Na > Rb
B)He > Na > Ca > Cl > Rb
C)He > Na > Cl > Ca > Rb
D)Rb > Ca > Cl > Na > He

Unlock Deck
Unlock for access to all 173 flashcards in this deck.
Unlock Deck
k this deck
38
Which of the following species will have the highest ionization energy?
A)Na+
B)Ne
C)F-
D)O2-
A)Na+
B)Ne
C)F-
D)O2-

Unlock Deck
Unlock for access to all 173 flashcards in this deck.
Unlock Deck
k this deck
39
Consider the following electron configurations for neutral atoms:
Atom I = 1s22s22p63s2
Atom II = 1s22s22p63s23p4
Atom III = 1s22s22p63s23p6
Which atom would be expected to have the largest third ionization energy?
A)atom I
B)atom II
C)atom III
D)All of these atoms would be expected to have the same third ionization energy.
Atom I = 1s22s22p63s2
Atom II = 1s22s22p63s23p4
Atom III = 1s22s22p63s23p6
Which atom would be expected to have the largest third ionization energy?
A)atom I
B)atom II
C)atom III
D)All of these atoms would be expected to have the same third ionization energy.

Unlock Deck
Unlock for access to all 173 flashcards in this deck.
Unlock Deck
k this deck
40
Which ionization process requires the most energy?
A)P(g)→ P+(g)+ e-
B)P+(g)→ P2+(g)+ e-
C)P2+(g)→ P3+(g)+ e-
D)P3+(g)→ P4+(g)+ e-
A)P(g)→ P+(g)+ e-
B)P+(g)→ P2+(g)+ e-
C)P2+(g)→ P3+(g)+ e-
D)P3+(g)→ P4+(g)+ e-

Unlock Deck
Unlock for access to all 173 flashcards in this deck.
Unlock Deck
k this deck
41
An element M reacts with chlorine to form MCl2,with oxygen to form MO,and with nitrogen to form M3N2.The most likely candidate for the element is
A)Li
B)Mg
C)Al
D)Si
A)Li
B)Mg
C)Al
D)Si

Unlock Deck
Unlock for access to all 173 flashcards in this deck.
Unlock Deck
k this deck
42
Calculate the lattice energy for NaCl(s)using a Born-Haber cycle and the following information: 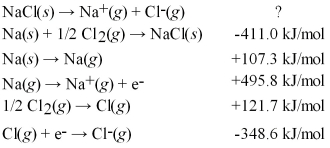
A)+34.8 kJ/mol
B)+690.3 kJ/mol
C)+787.2 kJ/mol
D)+1512 kJ/mol
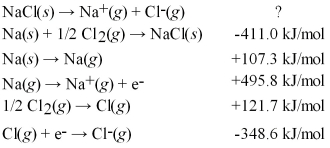
A)+34.8 kJ/mol
B)+690.3 kJ/mol
C)+787.2 kJ/mol
D)+1512 kJ/mol

Unlock Deck
Unlock for access to all 173 flashcards in this deck.
Unlock Deck
k this deck
43
To reach a noble gas electron configuration how many electrons would sulfur have to adopt?
A)1
B)2
C)6
D)8
A)1
B)2
C)6
D)8

Unlock Deck
Unlock for access to all 173 flashcards in this deck.
Unlock Deck
k this deck
44
Calculate the energy change for the formation of MgBr2(s)from its elements in their standard states: 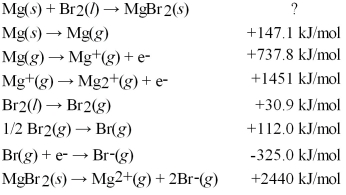
A)-150.8 kJ/mol
B)-286.0 kJ/mol
C)-499.2 kJ/mol
D)-5682 kJ/mol
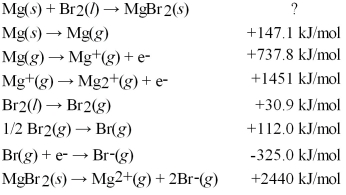
A)-150.8 kJ/mol
B)-286.0 kJ/mol
C)-499.2 kJ/mol
D)-5682 kJ/mol

Unlock Deck
Unlock for access to all 173 flashcards in this deck.
Unlock Deck
k this deck
45
Calculate the energy change for the formation of LiCl(s)from its elements in their standard states and the following tabulated information: 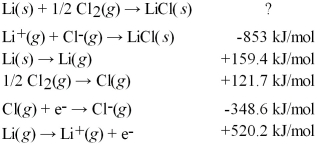
A)+1305.7 kJ/mol
B)+296.9 kJ/mol
C)-400.3 kJ/mol
D)-627.2 kJ/mol
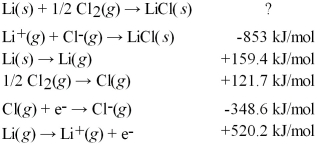
A)+1305.7 kJ/mol
B)+296.9 kJ/mol
C)-400.3 kJ/mol
D)-627.2 kJ/mol

Unlock Deck
Unlock for access to all 173 flashcards in this deck.
Unlock Deck
k this deck
46
Calculate the energy change for the formation of CaF2(s)from its elements in their standard states and the following information: 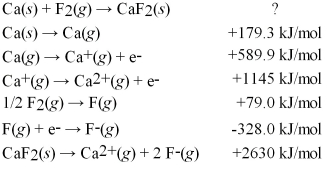
A)+4046 kJ/mol
B)-965 kJ/mol
C)-1214 kJ/mol
D)-3286 kJ/mol
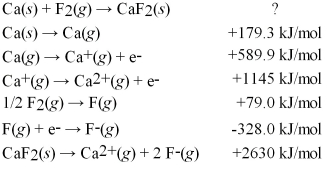
A)+4046 kJ/mol
B)-965 kJ/mol
C)-1214 kJ/mol
D)-3286 kJ/mol

Unlock Deck
Unlock for access to all 173 flashcards in this deck.
Unlock Deck
k this deck
47
Which of these elements has the most favorable (most negative)electron affinity?
A)Ca
B)N
C)Ne
D)S
A)Ca
B)N
C)Ne
D)S

Unlock Deck
Unlock for access to all 173 flashcards in this deck.
Unlock Deck
k this deck
48
What is the general trend in ionization energy and electron affinity values?
A)Both decrease as one traverses a period from left to right and both decrease as one descends a group.
B)Both decrease as one traverses a period from left to right and both increase as one descends a group.
C)Both increase as one traverses a period from left to right and both decrease as one descends a group.
D)Both increase as one traverses a period from left to right and both increase as one descends a group.
A)Both decrease as one traverses a period from left to right and both decrease as one descends a group.
B)Both decrease as one traverses a period from left to right and both increase as one descends a group.
C)Both increase as one traverses a period from left to right and both decrease as one descends a group.
D)Both increase as one traverses a period from left to right and both increase as one descends a group.

Unlock Deck
Unlock for access to all 173 flashcards in this deck.
Unlock Deck
k this deck
49
Calculate the energy change in kJ/mol for the reaction Li+(g)+ F-(g)→ Li(g)+ F(g)using the following information:
Li(g)→ Li+(g)+ e- +520 kJ/mol
F(g)+ e- → F-(g)-328 kJ/mol
A)-848 kJ/mol
B)-192 kJ/mol
C)+192 kJ/mol
D)+848 kJ/mol
Li(g)→ Li+(g)+ e- +520 kJ/mol
F(g)+ e- → F-(g)-328 kJ/mol
A)-848 kJ/mol
B)-192 kJ/mol
C)+192 kJ/mol
D)+848 kJ/mol

Unlock Deck
Unlock for access to all 173 flashcards in this deck.
Unlock Deck
k this deck
50
An element that has the valence electron configuration 3s23p3 belongs to which period and group?
A)period 3;group 3A
B)period 3;group 5A
C)period 4;group 3A
D)period 4;group 5A
A)period 3;group 3A
B)period 3;group 5A
C)period 4;group 3A
D)period 4;group 5A

Unlock Deck
Unlock for access to all 173 flashcards in this deck.
Unlock Deck
k this deck
51
How many electrons does magnesium lose and nitrogen need to form Mg3N2?
A)magnesium loses 2 and nitrogen gains 2
B)magnesium loses 2 and nitrogen gains 3
C)magnesium loses 3 and nitrogen gains 2
D)magnesium loses 3 and nitrogen gains 3
A)magnesium loses 2 and nitrogen gains 2
B)magnesium loses 2 and nitrogen gains 3
C)magnesium loses 3 and nitrogen gains 2
D)magnesium loses 3 and nitrogen gains 3

Unlock Deck
Unlock for access to all 173 flashcards in this deck.
Unlock Deck
k this deck
52
Calculate the electron affinity for the formation of the hydride ion from the following information: 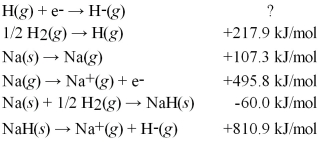
A)-50.1 kJ/mol
B)-70.1 kJ/mol
C)-816 kJ/mol
D)-1632 kJ/mol
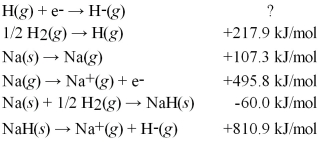
A)-50.1 kJ/mol
B)-70.1 kJ/mol
C)-816 kJ/mol
D)-1632 kJ/mol

Unlock Deck
Unlock for access to all 173 flashcards in this deck.
Unlock Deck
k this deck
53
The octet rule is most likely to fail occasionally for which of the following elements?
A)C
B)N
C)Na
D)S
A)C
B)N
C)Na
D)S

Unlock Deck
Unlock for access to all 173 flashcards in this deck.
Unlock Deck
k this deck
54
Which species does not have an octet of electrons for its outer core?
A)C4-
B)P3-
C)O2-
D)Mg+
A)C4-
B)P3-
C)O2-
D)Mg+

Unlock Deck
Unlock for access to all 173 flashcards in this deck.
Unlock Deck
k this deck
55
Which of the following ionic compounds would be expected to have the highest lattice energy?
A)NaF
B)NaCl
C)NaBr
D)NaI
A)NaF
B)NaCl
C)NaBr
D)NaI

Unlock Deck
Unlock for access to all 173 flashcards in this deck.
Unlock Deck
k this deck
56
Which chemical process is associated with the lattice energy for sodium chloride?
A)NaCl(s)→ Na+(g)+ Cl-(g)
B)NaCl(g)→ Na+(g)+ Cl-(g)
C)Na(s)+ 1/2 Cl2(g)→ NaCl(s)
D)NaCl(s)+ H2O(l)→ Na+(aq)+ Cl-(aq)
A)NaCl(s)→ Na+(g)+ Cl-(g)
B)NaCl(g)→ Na+(g)+ Cl-(g)
C)Na(s)+ 1/2 Cl2(g)→ NaCl(s)
D)NaCl(s)+ H2O(l)→ Na+(aq)+ Cl-(aq)

Unlock Deck
Unlock for access to all 173 flashcards in this deck.
Unlock Deck
k this deck
57
In the reaction of sodium metal with chlorine gas which of the following processes releases energy?
A)Cl2(g)→ 2 Cl(g)
B)Cl(g)+ e- → Cl-(g)
C)Na(s)→ Na(g)
D)Na(g)→ Na+(g)+ e-
A)Cl2(g)→ 2 Cl(g)
B)Cl(g)+ e- → Cl-(g)
C)Na(s)→ Na(g)
D)Na(g)→ Na+(g)+ e-

Unlock Deck
Unlock for access to all 173 flashcards in this deck.
Unlock Deck
k this deck
58
How many valence shell electrons does an atom of aluminum have?
A)1
B)2
C)3
D)13
A)1
B)2
C)3
D)13

Unlock Deck
Unlock for access to all 173 flashcards in this deck.
Unlock Deck
k this deck
59
Calculate the lattice energy for MgO(s)using a Born-Haber cycle and the following information: 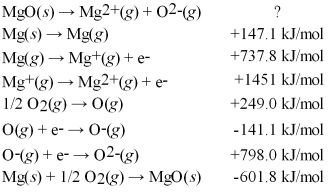
A)+1842 kJ/mol
B)+2444 kJ/mol
C)+3844 kJ/mol
D)+4108 kJ/mol
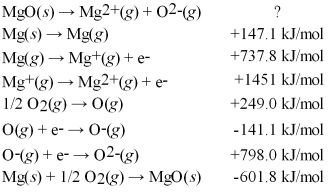
A)+1842 kJ/mol
B)+2444 kJ/mol
C)+3844 kJ/mol
D)+4108 kJ/mol

Unlock Deck
Unlock for access to all 173 flashcards in this deck.
Unlock Deck
k this deck
60
Calculate the lattice energy for MgCl2(s)using a Born-Haber cycle and the following information: 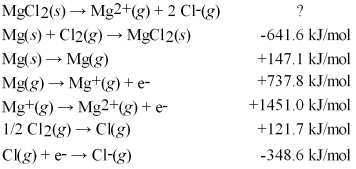
A)+641.6 kJ/mol
B)+1240.5 kJ/mol
C)+1882.1 kJ/mol
D)+2523.7 kJ/mol
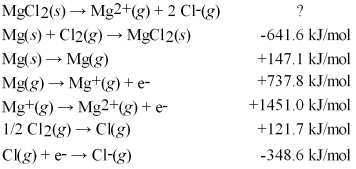
A)+641.6 kJ/mol
B)+1240.5 kJ/mol
C)+1882.1 kJ/mol
D)+2523.7 kJ/mol

Unlock Deck
Unlock for access to all 173 flashcards in this deck.
Unlock Deck
k this deck
61
Which alkaline earth metal reacts the most vigorously with water at room temperature?
A)Be
B)Ca
C)Ba
D)Sr
A)Be
B)Ca
C)Ba
D)Sr

Unlock Deck
Unlock for access to all 173 flashcards in this deck.
Unlock Deck
k this deck
62
Which is not a chemical reaction of the alkali metal potassium?
A)K(s)+ O2(g)→ KO2(s)
B)2 K(s)+ 2 H2O(l)→ 2 KOH(aq)+ H2(g)
C)2 K(s)+ H2(g)→ 2 KH(s)
D)6 K(s)+ N2(g)→ 2 K3N(s)
A)K(s)+ O2(g)→ KO2(s)
B)2 K(s)+ 2 H2O(l)→ 2 KOH(aq)+ H2(g)
C)2 K(s)+ H2(g)→ 2 KH(s)
D)6 K(s)+ N2(g)→ 2 K3N(s)

Unlock Deck
Unlock for access to all 173 flashcards in this deck.
Unlock Deck
k this deck
63
Which is not generally considered to be a chemical reaction of the alkaline earth metal calcium?
A)Ca(s)+ Cl2(g)→ CaCl2(s)
B)2 Ca(s)+ 2 H2O(l)→ 2 Ca(OH)2(aq)+ H2(g)
C)6 Ca(s)+ 2 N2(g)→ 2 Ca3N2(s)
D)Ca(s)+ O2(g)→ CaO2(s)
A)Ca(s)+ Cl2(g)→ CaCl2(s)
B)2 Ca(s)+ 2 H2O(l)→ 2 Ca(OH)2(aq)+ H2(g)
C)6 Ca(s)+ 2 N2(g)→ 2 Ca3N2(s)
D)Ca(s)+ O2(g)→ CaO2(s)

Unlock Deck
Unlock for access to all 173 flashcards in this deck.
Unlock Deck
k this deck
64
The alkali metals K,Rb,and Cs are commercially produced by
A)chemical reduction of their molten salts.
B)electrolysis of their molten salts.
C)thermal decomposition of their molten metal halides.
D)thermal decomposition of their molten metal oxides.
A)chemical reduction of their molten salts.
B)electrolysis of their molten salts.
C)thermal decomposition of their molten metal halides.
D)thermal decomposition of their molten metal oxides.

Unlock Deck
Unlock for access to all 173 flashcards in this deck.
Unlock Deck
k this deck
65
Which alkali metal reacts with nitrogen to form a nitride?
A)Li
B)Na
C)K
D)All of these
A)Li
B)Na
C)K
D)All of these

Unlock Deck
Unlock for access to all 173 flashcards in this deck.
Unlock Deck
k this deck
66
Which alkali metal forms preferentially an oxide rather than a peroxide or superoxide?
A)Li
B)Na
C)K
D)Rb
A)Li
B)Na
C)K
D)Rb

Unlock Deck
Unlock for access to all 173 flashcards in this deck.
Unlock Deck
k this deck
67
Predict the product(s)when the reactants Be(s)+ Br2(l)are mixed.
A)BeBr(s)
B)BeBr2(s)
C)Be2Br(s)
D)BeBr3(s)
A)BeBr(s)
B)BeBr2(s)
C)Be2Br(s)
D)BeBr3(s)

Unlock Deck
Unlock for access to all 173 flashcards in this deck.
Unlock Deck
k this deck
68
Which of the following ionic compounds would be expected to have the highest lattice energy?
A)LiCl
B)NaCl
C)KCl
D)RbCl
A)LiCl
B)NaCl
C)KCl
D)RbCl

Unlock Deck
Unlock for access to all 173 flashcards in this deck.
Unlock Deck
k this deck
69
Which ionic compound would be expected to have the highest lattice energy?
A)NaCl
B)MgO
C)AlF3
D)Al2O3
A)NaCl
B)MgO
C)AlF3
D)Al2O3

Unlock Deck
Unlock for access to all 173 flashcards in this deck.
Unlock Deck
k this deck
70
Which of the following elements is a solid at room temperature?
A)fluorine
B)chlorine
C)bromine
D)iodine
A)fluorine
B)chlorine
C)bromine
D)iodine

Unlock Deck
Unlock for access to all 173 flashcards in this deck.
Unlock Deck
k this deck
71
Which ionic compound would be expected to have the highest lattice energy?
A)Li2O
B)Na2O2
C)KO2
D)RbO2
A)Li2O
B)Na2O2
C)KO2
D)RbO2

Unlock Deck
Unlock for access to all 173 flashcards in this deck.
Unlock Deck
k this deck
72
Which ionic compound would be expected to have the highest lattice energy?
A)Na2O
B)MgO
C)Al2O3
D)CO2
A)Na2O
B)MgO
C)Al2O3
D)CO2

Unlock Deck
Unlock for access to all 173 flashcards in this deck.
Unlock Deck
k this deck
73
Which alkali metal forms preferentially a peroxide and superoxide?
A)Li
B)Na
C)K
D)Mg
A)Li
B)Na
C)K
D)Mg

Unlock Deck
Unlock for access to all 173 flashcards in this deck.
Unlock Deck
k this deck
74
Group 2A metals tend to be somewhat less reactive than alkali metals,and the order of their reactivity is
A)Ba > Sr > Ca > Mg > Be.
B)Be > Mg > Ca > Sr > Ba.
C)Ca > Mg > Be > Ba > Sr.
D)Sr > Ca > Mg > Be > Ba.
A)Ba > Sr > Ca > Mg > Be.
B)Be > Mg > Ca > Sr > Ba.
C)Ca > Mg > Be > Ba > Sr.
D)Sr > Ca > Mg > Be > Ba.

Unlock Deck
Unlock for access to all 173 flashcards in this deck.
Unlock Deck
k this deck
75
The alkali metals Li and Na are commercially produced by
A)chemical reduction of their molten salts.
B)electrolysis of their molten salts.
C)thermal decomposition of their molten metal halides.
D)thermal decomposition of their molten metal oxides.
A)chemical reduction of their molten salts.
B)electrolysis of their molten salts.
C)thermal decomposition of their molten metal halides.
D)thermal decomposition of their molten metal oxides.

Unlock Deck
Unlock for access to all 173 flashcards in this deck.
Unlock Deck
k this deck
76
An incorrect statement about the alkaline earth metals is:
A)Melting points generally decrease as one descends the group.
B)Densities are less than those of the corresponding alkali elements of the same period.
C)Ionic radii of the M2+ ion increases as one descends the group.
D)The first ionization energy is less than that of the second ionization energy.
A)Melting points generally decrease as one descends the group.
B)Densities are less than those of the corresponding alkali elements of the same period.
C)Ionic radii of the M2+ ion increases as one descends the group.
D)The first ionization energy is less than that of the second ionization energy.

Unlock Deck
Unlock for access to all 173 flashcards in this deck.
Unlock Deck
k this deck
77
Which of the following elements is a liquid at room temperature?
A)fluorine
B)chlorine
C)bromine
D)iodine
A)fluorine
B)chlorine
C)bromine
D)iodine

Unlock Deck
Unlock for access to all 173 flashcards in this deck.
Unlock Deck
k this deck
78
Which is not a chemical reaction of the alkali metal sodium?
A)4 Na(s)+ 2 O2(g)→ 2 Na2O(s)+ Na2O2(s)
B)2 Na(s)+ 2 NH3(l)→ 2 NaNH2(sol)+ H2(g)
C)2 Na(s)+ Cl2(g)→ 2 NaCl(s)
D)2 Na(s)+ 2 H2O(l)→ 2 NaOH(aq)+ H2(g)
A)4 Na(s)+ 2 O2(g)→ 2 Na2O(s)+ Na2O2(s)
B)2 Na(s)+ 2 NH3(l)→ 2 NaNH2(sol)+ H2(g)
C)2 Na(s)+ Cl2(g)→ 2 NaCl(s)
D)2 Na(s)+ 2 H2O(l)→ 2 NaOH(aq)+ H2(g)

Unlock Deck
Unlock for access to all 173 flashcards in this deck.
Unlock Deck
k this deck
79
Calcium,strontium,and barium are all prepared commercially by the same method which is
A)electrolysis of the molten metal oxides.
B)electrolysis of the molten metal halides.
C)chemical reduction of the metal oxides with aluminium.
D)chemical reduction of the metal halides with oxygen.
A)electrolysis of the molten metal oxides.
B)electrolysis of the molten metal halides.
C)chemical reduction of the metal oxides with aluminium.
D)chemical reduction of the metal halides with oxygen.

Unlock Deck
Unlock for access to all 173 flashcards in this deck.
Unlock Deck
k this deck
80
Which is not generally considered to be a chemical reaction of the alkaline earth metal magnesium?
A)2 Mg(g)+ O2(g)→ 2 MgO(s)at room temperature
B)3 Mg(s)+ N2(g)→ Mg3N2(s)at elevated temperatures
C)Mg(s)+ 2 H2O(l)→ Mg(OH)2(s)+ H2(g)at room temperature
D)Mg(s)+ F2(g)→ MgF2(s)at room temperature
A)2 Mg(g)+ O2(g)→ 2 MgO(s)at room temperature
B)3 Mg(s)+ N2(g)→ Mg3N2(s)at elevated temperatures
C)Mg(s)+ 2 H2O(l)→ Mg(OH)2(s)+ H2(g)at room temperature
D)Mg(s)+ F2(g)→ MgF2(s)at room temperature

Unlock Deck
Unlock for access to all 173 flashcards in this deck.
Unlock Deck
k this deck


