Deck 7: Covalent Bonds and Molecular Structure
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/232
Play
Full screen (f)
Deck 7: Covalent Bonds and Molecular Structure
1
When melting S8,________ forces must be overcome and S8 is expected to have a ________ melting point than MgS.
A)covalent bonding,higher
B)covalent bonding,lower
C)intermolecular,higher
D)intermolecular,lower
A)covalent bonding,higher
B)covalent bonding,lower
C)intermolecular,higher
D)intermolecular,lower
intermolecular,lower
2
Covalent bonding is a
A)gain of electrons.
B)loss of electrons.
C)transfer of electrons.
D)sharing of electrons.
A)gain of electrons.
B)loss of electrons.
C)transfer of electrons.
D)sharing of electrons.
sharing of electrons.
3
The Cl-Cl bond energy is 243 kJ/mol.Therefore the formation of a single bond between chlorine atoms
A)should require the absorption of 243 kJ per mole of Cl2 formed.
B)should require the absorption of 486 kJ per mole of Cl2 formed.
C)should result in the release of 243 kJ per mole of Cl2 formed.
D)should result in the release of 486 kJ per mole of Cl2 formed.
A)should require the absorption of 243 kJ per mole of Cl2 formed.
B)should require the absorption of 486 kJ per mole of Cl2 formed.
C)should result in the release of 243 kJ per mole of Cl2 formed.
D)should result in the release of 486 kJ per mole of Cl2 formed.
should result in the release of 243 kJ per mole of Cl2 formed.
4
The electronegativity for both sulfur and carbon is 2.5.Therefore the compound CS2 would be expected to
A)be ionic with C as the anion.
B)be ionic with C as the cation.
C)have nonpolar covalent bonds between C and S.
D)have polar covalent bonds between C and S.
A)be ionic with C as the anion.
B)be ionic with C as the cation.
C)have nonpolar covalent bonds between C and S.
D)have polar covalent bonds between C and S.

Unlock Deck
Unlock for access to all 232 flashcards in this deck.
Unlock Deck
k this deck
5
Compound A is a solid with a melting point of 125°C,and compound B is a gas at 25°C and one atmosphere pressure.Based on these data,one would expect
A)both compounds to be covalent.
B)compound A to be ionic and compound B to be covalent.
C)compound A to be covalent and compound B to be ionic.
D)both compounds to be ionic.
A)both compounds to be covalent.
B)compound A to be ionic and compound B to be covalent.
C)compound A to be covalent and compound B to be ionic.
D)both compounds to be ionic.

Unlock Deck
Unlock for access to all 232 flashcards in this deck.
Unlock Deck
k this deck
6
The compound ICl contains
A)ionic bonds.
B)nonpolar covalent bonds.
C)polar covalent bonds,with partial negative charges on the Cl atoms.
D)polar covalent bonds,with partial negative charges on the I atoms.
A)ionic bonds.
B)nonpolar covalent bonds.
C)polar covalent bonds,with partial negative charges on the Cl atoms.
D)polar covalent bonds,with partial negative charges on the I atoms.

Unlock Deck
Unlock for access to all 232 flashcards in this deck.
Unlock Deck
k this deck
7
The greater the electronegativity difference between two bonded atoms,the
A)greater the bond order.
B)greater the covalent character of the bond.
C)greater the ionic character of the bond.
D)more unstable the bond.
A)greater the bond order.
B)greater the covalent character of the bond.
C)greater the ionic character of the bond.
D)more unstable the bond.

Unlock Deck
Unlock for access to all 232 flashcards in this deck.
Unlock Deck
k this deck
8
At the equilibrium bond length
A)the attractive forces holding the atoms together are less than the repulsive forces.
B)the potential energy is a maximum.
C)the potential energy is a minimum.
D)the repulsive forces are greater than the attractive forces holding the atoms together.
A)the attractive forces holding the atoms together are less than the repulsive forces.
B)the potential energy is a maximum.
C)the potential energy is a minimum.
D)the repulsive forces are greater than the attractive forces holding the atoms together.

Unlock Deck
Unlock for access to all 232 flashcards in this deck.
Unlock Deck
k this deck
9
The electronegativity is 2.1 for H and 1.8 for Si.Based on these electronegativities,SiH4 would be expected to
A)be ionic and contain H- ions.
B)be ionic and contain H+ ions.
C)have polar covalent bonds with a partial negative charges on the H atoms.
D)have polar covalent bonds with a partial positive charges on the H atoms.
A)be ionic and contain H- ions.
B)be ionic and contain H+ ions.
C)have polar covalent bonds with a partial negative charges on the H atoms.
D)have polar covalent bonds with a partial positive charges on the H atoms.

Unlock Deck
Unlock for access to all 232 flashcards in this deck.
Unlock Deck
k this deck
10
In general,at room temperature
A)ionic compounds are all solids and covalent compounds are all gases.
B)ionic compounds are all solids,but covalent compounds may be solids,liquids,or gases.
C)ionic compounds are all solids,and covalent compounds are liquids or gases.
D)covalent compounds are all gases,but ionic compounds may be solids,liquids,or gases.
A)ionic compounds are all solids and covalent compounds are all gases.
B)ionic compounds are all solids,but covalent compounds may be solids,liquids,or gases.
C)ionic compounds are all solids,and covalent compounds are liquids or gases.
D)covalent compounds are all gases,but ionic compounds may be solids,liquids,or gases.

Unlock Deck
Unlock for access to all 232 flashcards in this deck.
Unlock Deck
k this deck
11
Which molecule contains the most polar bonds?
A)CF4
B)CO2
C)CN-
D)CH4
A)CF4
B)CO2
C)CN-
D)CH4

Unlock Deck
Unlock for access to all 232 flashcards in this deck.
Unlock Deck
k this deck
12
Of the following elements,which has the highest electronegativity?
A)P
B)S
C)Sc
D)As
A)P
B)S
C)Sc
D)As

Unlock Deck
Unlock for access to all 232 flashcards in this deck.
Unlock Deck
k this deck
13
Which molecule contains the most easily broken carbon-carbon bond?
A)H3C-CH3
B)H2C=CH2
C)F2C=CF2
D)HC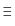 CH
CH
A)H3C-CH3
B)H2C=CH2
C)F2C=CF2
D)HC
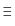 CH
CH
Unlock Deck
Unlock for access to all 232 flashcards in this deck.
Unlock Deck
k this deck
14
Which bond should have the highest bond dissociation energy?
A)N-N
B)N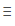 N
N
C)N=N
D)All three bonds should have about the same dissociation energy.
A)N-N
B)N
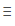 N
NC)N=N
D)All three bonds should have about the same dissociation energy.

Unlock Deck
Unlock for access to all 232 flashcards in this deck.
Unlock Deck
k this deck
15
Which is the longest bond?
A)N-N
B)N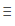 N
N
C)N=N
D)All three bond lengths should be about the same.
A)N-N
B)N
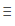 N
NC)N=N
D)All three bond lengths should be about the same.

Unlock Deck
Unlock for access to all 232 flashcards in this deck.
Unlock Deck
k this deck
16
Which molecule has the weakest bonds?
A)CF4
B)CCl4
C)CBr4
D)CI4
A)CF4
B)CCl4
C)CBr4
D)CI4

Unlock Deck
Unlock for access to all 232 flashcards in this deck.
Unlock Deck
k this deck
17
A reactive element with a relatively high electronegativity would be expected to have a relatively
A)small negative electron affinity and a relatively low ionization energy.
B)small negative electron affinity and a relatively high ionization energy.
C)large negative electron affinity and a relatively low ionization energy.
D)large negative electron affinity and a relatively high ionization energy.
A)small negative electron affinity and a relatively low ionization energy.
B)small negative electron affinity and a relatively high ionization energy.
C)large negative electron affinity and a relatively low ionization energy.
D)large negative electron affinity and a relatively high ionization energy.

Unlock Deck
Unlock for access to all 232 flashcards in this deck.
Unlock Deck
k this deck
18
Which electrostatic forces hold atoms together in a molecule?
A)electron-electron forces
B)electron-nucleus forces
C)nucleus-nucleus forces
D)all three forces
A)electron-electron forces
B)electron-nucleus forces
C)nucleus-nucleus forces
D)all three forces

Unlock Deck
Unlock for access to all 232 flashcards in this deck.
Unlock Deck
k this deck
19
Of the following elements,which has the lowest electronegativity?
A)Mg
B)Cl
C)Ca
D)Br
A)Mg
B)Cl
C)Ca
D)Br

Unlock Deck
Unlock for access to all 232 flashcards in this deck.
Unlock Deck
k this deck
20
Which compound is most likely to exist as a gas at room temperature?
A)Al4C3
B)CF4
C)CaF2
D)WC
A)Al4C3
B)CF4
C)CaF2
D)WC

Unlock Deck
Unlock for access to all 232 flashcards in this deck.
Unlock Deck
k this deck
21
Consider a molecule with the following connections: 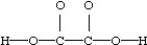
When a valid electron dot structure is written,how many double bonds will the molecule contain?
A)0
B)1
C)2
D)4

When a valid electron dot structure is written,how many double bonds will the molecule contain?
A)0
B)1
C)2
D)4

Unlock Deck
Unlock for access to all 232 flashcards in this deck.
Unlock Deck
k this deck
22
Which molecule contains a triple bond?
A)F2
B)O3
C)HCN
D)H2CO
A)F2
B)O3
C)HCN
D)H2CO

Unlock Deck
Unlock for access to all 232 flashcards in this deck.
Unlock Deck
k this deck
23
How many electrons are in the valence shell of I in IF4-?
A)8
B)10
C)12
D)14
A)8
B)10
C)12
D)14

Unlock Deck
Unlock for access to all 232 flashcards in this deck.
Unlock Deck
k this deck
24
Which is the most acceptable electron dot structure for N2H2?
A)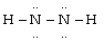
B)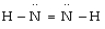
C) H - N =N - H
D)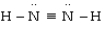
A)
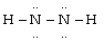
B)
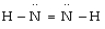
C) H - N =N - H
D)
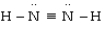

Unlock Deck
Unlock for access to all 232 flashcards in this deck.
Unlock Deck
k this deck
25
Identify the fourth-row element X that forms the ion. 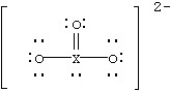
A)Ge
B)As
C)Se
D)Kr

A)Ge
B)As
C)Se
D)Kr

Unlock Deck
Unlock for access to all 232 flashcards in this deck.
Unlock Deck
k this deck
26
How many lone pairs of electrons are on the P atom in PF3?
A)0
B)1
C)2
D)3
A)0
B)1
C)2
D)3

Unlock Deck
Unlock for access to all 232 flashcards in this deck.
Unlock Deck
k this deck
27
The phosphorus atom in PCl3 would be expected to have a
A)partial positive (δ+)charge.
B)partial negative (δ-)charge.
C)3+ charge.
D)3- charge.
A)partial positive (δ+)charge.
B)partial negative (δ-)charge.
C)3+ charge.
D)3- charge.

Unlock Deck
Unlock for access to all 232 flashcards in this deck.
Unlock Deck
k this deck
28
A::A represents
A)a double bond.
B)a quadruple bond.
C)one lone pair of electrons.
D)two lone pairs of electrons.
A)a double bond.
B)a quadruple bond.
C)one lone pair of electrons.
D)two lone pairs of electrons.

Unlock Deck
Unlock for access to all 232 flashcards in this deck.
Unlock Deck
k this deck
29
Arrange the following in order of increasing ionic character: Al2S3,MgS,Na2S,P4S3,S8.
A)MgS,Na2S,Al2S3,P4S3,S8
B)Na2S,MgS,Al2S3,P4S3,S8
C)S8,P4S3,Al2S3,MgS,Na2S
D)S8,P4S3,Al2S3,Na2S,MgS
A)MgS,Na2S,Al2S3,P4S3,S8
B)Na2S,MgS,Al2S3,P4S3,S8
C)S8,P4S3,Al2S3,MgS,Na2S
D)S8,P4S3,Al2S3,Na2S,MgS

Unlock Deck
Unlock for access to all 232 flashcards in this deck.
Unlock Deck
k this deck
30
Elements that can accommodate more than eight electrons in their valence shell occur only in periodic table period
A)2 or lower.
B)3 or lower.
C)4 or lower.
D)5 or lower.
A)2 or lower.
B)3 or lower.
C)4 or lower.
D)5 or lower.

Unlock Deck
Unlock for access to all 232 flashcards in this deck.
Unlock Deck
k this deck
31
How many of the σ bonds in H2SO4 are coordinate covalent bonds?
A)0
B)2
C)4
D)6
A)0
B)2
C)4
D)6

Unlock Deck
Unlock for access to all 232 flashcards in this deck.
Unlock Deck
k this deck
32
Which of the following contains an atom that does not obey the octet rule?
A)KBr
B)CO2
C)ClF3
D)ICl
A)KBr
B)CO2
C)ClF3
D)ICl

Unlock Deck
Unlock for access to all 232 flashcards in this deck.
Unlock Deck
k this deck
33
The nitrogen-nitrogen bond in :N=N: has a bond order of
A)3
B)1
C)2
D)6
A)3
B)1
C)2
D)6

Unlock Deck
Unlock for access to all 232 flashcards in this deck.
Unlock Deck
k this deck
34
The electronegativities for the elements vary from 0.7 for cesium to 4.0 for fluorine.The electronegativity for iodine is 2.5.Based entirely on the general guidelines for electronegativities and bond character,
A)binary compounds with iodine should all be polar covalent with a δ- on I.
B)binary compounds with iodine should all be polar covalent with a δ+ on I.
C)compounds with iodine may be ionic,polar covalent,or nonpolar covalent.
D)no binary compounds with iodine should be substantially ionic.
A)binary compounds with iodine should all be polar covalent with a δ- on I.
B)binary compounds with iodine should all be polar covalent with a δ+ on I.
C)compounds with iodine may be ionic,polar covalent,or nonpolar covalent.
D)no binary compounds with iodine should be substantially ionic.

Unlock Deck
Unlock for access to all 232 flashcards in this deck.
Unlock Deck
k this deck
35
Element A has an electronegativity of 0.8 and element B has an electronegativity of 3.0.Which statement best describes the bonding in A3B?
A)The AB bond is largely covalent with a δ- on A.
B)The AB bond is largely covalent with a δ+ on A.
C)The compound is largely ionic with A as the cation.
D)The compound is largely ionic with A as the anion.
A)The AB bond is largely covalent with a δ- on A.
B)The AB bond is largely covalent with a δ+ on A.
C)The compound is largely ionic with A as the cation.
D)The compound is largely ionic with A as the anion.

Unlock Deck
Unlock for access to all 232 flashcards in this deck.
Unlock Deck
k this deck
36
A chlorine atom in Cl2 should have a
A)charge of 1-.
B)partial charge δ-.
C)partial charge δ+.
D)charge of 0.
A)charge of 1-.
B)partial charge δ-.
C)partial charge δ+.
D)charge of 0.

Unlock Deck
Unlock for access to all 232 flashcards in this deck.
Unlock Deck
k this deck
37
Which element can accommodate more than eight electrons in its valence shell?
A)C
B)O
C)P
D)He
A)C
B)O
C)P
D)He

Unlock Deck
Unlock for access to all 232 flashcards in this deck.
Unlock Deck
k this deck
38
How many lone pairs of electrons are on the Xe atom in XeF6?
A)0
B)1
C)2
D)3
A)0
B)1
C)2
D)3

Unlock Deck
Unlock for access to all 232 flashcards in this deck.
Unlock Deck
k this deck
39
Based on the indicated electronegativities,arrange the following in order of increasing ionic character: CsBr,LaBr3,PBr3,MgBr2. 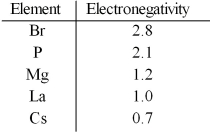
A)CsBr,LaBr3,MgBr2,PBr3
B)CsBr,MgBr2,PBr3,LaBr3
C)PBr3,LaBr3,MgBr2,CsBr
D)PBr3,MgBr2,LaBr3,CsBr

A)CsBr,LaBr3,MgBr2,PBr3
B)CsBr,MgBr2,PBr3,LaBr3
C)PBr3,LaBr3,MgBr2,CsBr
D)PBr3,MgBr2,LaBr3,CsBr

Unlock Deck
Unlock for access to all 232 flashcards in this deck.
Unlock Deck
k this deck
40
In the most acceptable electron-dot structure for carbonyl fluoride,COF2 the central atom is
A)C,which is singly-bonded to O.
B)C,which is doubly-bonded to O.
C)O,which is singly-bonded to C
D)O,which is doubly-bonded to C.
A)C,which is singly-bonded to O.
B)C,which is doubly-bonded to O.
C)O,which is singly-bonded to C
D)O,which is doubly-bonded to C.

Unlock Deck
Unlock for access to all 232 flashcards in this deck.
Unlock Deck
k this deck
41
A molecular compound that obeys the octet rule in which all atoms have a zero formal charge is
A)BaCl2
B)BrF3
C)NCl3
D)XeF4
A)BaCl2
B)BrF3
C)NCl3
D)XeF4

Unlock Deck
Unlock for access to all 232 flashcards in this deck.
Unlock Deck
k this deck
42
NO2- is be expected to have
A)two single bonds.
B)one single and one double bond.
C)two double bonds.
D)two identical bonds intermediate between a single and a double bond.
A)two single bonds.
B)one single and one double bond.
C)two double bonds.
D)two identical bonds intermediate between a single and a double bond.

Unlock Deck
Unlock for access to all 232 flashcards in this deck.
Unlock Deck
k this deck
43
Two resonance forms for SOCl2 are given below. 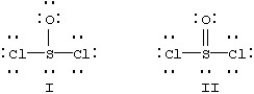
Which is favored by the octet rule and which by formal charge considerations?
A)I is favored by the octet rule and by formal charge considerations.
B)I is favored by the octet rule and II by formal charge considerations.
C)II is favored by the octet rule and I by formal charge considerations.
D)II is favored by the octet rule and by formal charge considerations.

Which is favored by the octet rule and which by formal charge considerations?
A)I is favored by the octet rule and by formal charge considerations.
B)I is favored by the octet rule and II by formal charge considerations.
C)II is favored by the octet rule and I by formal charge considerations.
D)II is favored by the octet rule and by formal charge considerations.

Unlock Deck
Unlock for access to all 232 flashcards in this deck.
Unlock Deck
k this deck
44
Assign formal charges to each atom in the resonance form for SOCl2 given below. 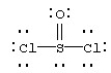
A)0 for Cl,0 for S,and 0 for O
B)0 for Cl,+1 for S,and -1 for O
C)-1 for Cl,+4 for S,and -2 for O
D)-1 for Cl,-2 for S,and -2 for O

A)0 for Cl,0 for S,and 0 for O
B)0 for Cl,+1 for S,and -1 for O
C)-1 for Cl,+4 for S,and -2 for O
D)-1 for Cl,-2 for S,and -2 for O

Unlock Deck
Unlock for access to all 232 flashcards in this deck.
Unlock Deck
k this deck
45
How many double and single bonds are in the resonance form for SO2 in which the formal charges on each atom are zero?
A)two single bonds and no double bonds
B)one single bond and one double bond
C)no single bonds and two double bonds
D)Each of these is possible.
A)two single bonds and no double bonds
B)one single bond and one double bond
C)no single bonds and two double bonds
D)Each of these is possible.

Unlock Deck
Unlock for access to all 232 flashcards in this deck.
Unlock Deck
k this deck
46
Assign formal charges to each atom in the resonance form for SOCl2 given below. 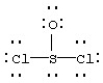
A)0 for Cl,0 for S,and 0 for O
B)0 for Cl,+1 for S,and -1 for O
C)-1 for Cl,+4 for S,and -2 for O
D)-1 for Cl,-2 for S,and -2 for O

A)0 for Cl,0 for S,and 0 for O
B)0 for Cl,+1 for S,and -1 for O
C)-1 for Cl,+4 for S,and -2 for O
D)-1 for Cl,-2 for S,and -2 for O

Unlock Deck
Unlock for access to all 232 flashcards in this deck.
Unlock Deck
k this deck
47
Assign formal charges to all atoms in the following resonance form for HNO3. 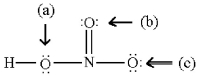
A)0 for all atoms
B)+1 for N,-1 for oxygen (c),0 for all other atoms
C)+1 for N and H,-1 for oxygen (a)and oxygen (c),0 for oxygen (b)
D)+1 for H,-2 for each oxygen,+5 for N

A)0 for all atoms
B)+1 for N,-1 for oxygen (c),0 for all other atoms
C)+1 for N and H,-1 for oxygen (a)and oxygen (c),0 for oxygen (b)
D)+1 for H,-2 for each oxygen,+5 for N

Unlock Deck
Unlock for access to all 232 flashcards in this deck.
Unlock Deck
k this deck
48
What geometric arrangement of charge clouds is expected for an atom that has five charge clouds?
A)tetrahedral
B)square planar
C)trigonal bipyramidal
D)octahedral
A)tetrahedral
B)square planar
C)trigonal bipyramidal
D)octahedral

Unlock Deck
Unlock for access to all 232 flashcards in this deck.
Unlock Deck
k this deck
49
Which of the following should be nonplanar? 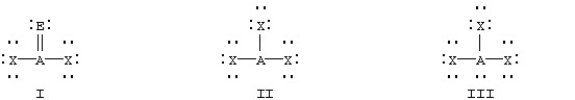
A)only I
B)only II
C)only III
D)I and III

A)only I
B)only II
C)only III
D)I and III

Unlock Deck
Unlock for access to all 232 flashcards in this deck.
Unlock Deck
k this deck
50
In the best Lewis structure for NO+,what is the formal charge on the N atom?
A)-1
B)0
C)+1
D)+2
A)-1
B)0
C)+1
D)+2

Unlock Deck
Unlock for access to all 232 flashcards in this deck.
Unlock Deck
k this deck
51
Which of the following are allowed resonance forms of NCS-?
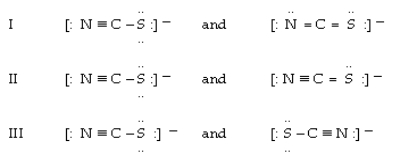
A)only I
B)only II
C)only III
D)I and III
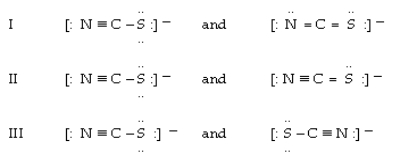
A)only I
B)only II
C)only III
D)I and III

Unlock Deck
Unlock for access to all 232 flashcards in this deck.
Unlock Deck
k this deck
52
Which is expected to have the strongest C-O bond?
A)CH3OH
B)COCl2
C)CH3CO2-
D)CO32-
A)CH3OH
B)COCl2
C)CH3CO2-
D)CO32-

Unlock Deck
Unlock for access to all 232 flashcards in this deck.
Unlock Deck
k this deck
53
How many lone pairs are on the Br atom in BrF2-?
A)0
B)1
C)2
D)3
A)0
B)1
C)2
D)3

Unlock Deck
Unlock for access to all 232 flashcards in this deck.
Unlock Deck
k this deck
54
Which electron dot structure for OCN- has a formal charge of -1 on the most electronegative atom?
A)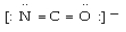
B)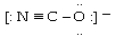
C)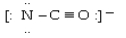
D)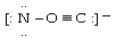
A)
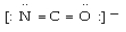
B)
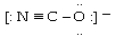
C)
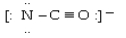
D)
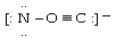

Unlock Deck
Unlock for access to all 232 flashcards in this deck.
Unlock Deck
k this deck
55
What is the approximate carbon-oxygen bond order in CO32-?
A)1
B)4/3
C)5/3
D)2
A)1
B)4/3
C)5/3
D)2

Unlock Deck
Unlock for access to all 232 flashcards in this deck.
Unlock Deck
k this deck
56
Based on formal charge considerations,the electron-dot structure of CO32- ion has
A)two resonance structures involving two single bonds and one double bond.
B)two resonance structures involving one single bond and two double bonds.
C)three resonance structures involving two single bonds and one double bond.
D)three resonance structures involving one single bond and two double bonds.
A)two resonance structures involving two single bonds and one double bond.
B)two resonance structures involving one single bond and two double bonds.
C)three resonance structures involving two single bonds and one double bond.
D)three resonance structures involving one single bond and two double bonds.

Unlock Deck
Unlock for access to all 232 flashcards in this deck.
Unlock Deck
k this deck
57
Based on VSEPR theory,which should have the smallest XAX bond angle?
A)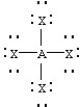
B)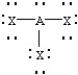
C)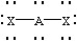
D)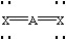
A)

B)

C)

D)
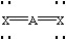

Unlock Deck
Unlock for access to all 232 flashcards in this deck.
Unlock Deck
k this deck
58
Based on formal charges,what is the S-O bond order in SO42-?
A)1
B)1)3
C)1)5
D)2
A)1
B)1)3
C)1)5
D)2

Unlock Deck
Unlock for access to all 232 flashcards in this deck.
Unlock Deck
k this deck
59
Which one of the following is expected to exhibit resonance?
A)NH4+
B)HCN
C)CO2
D)NO2-
A)NH4+
B)HCN
C)CO2
D)NO2-

Unlock Deck
Unlock for access to all 232 flashcards in this deck.
Unlock Deck
k this deck
60
How many resonance structures are required in the electron-dot structure of CO32-?
A)two
B)three
C)four
D)five
A)two
B)three
C)four
D)five

Unlock Deck
Unlock for access to all 232 flashcards in this deck.
Unlock Deck
k this deck
61
The VSEPR model predicts the O-O-O bond angle in O3 to be
A)90°.
B)109.5°.
C)less than 120° but greater than 109.5°.
D)120°.
A)90°.
B)109.5°.
C)less than 120° but greater than 109.5°.
D)120°.

Unlock Deck
Unlock for access to all 232 flashcards in this deck.
Unlock Deck
k this deck
62
A single sp3 hybrid orbital has
A)one lobe.
B)two lobes of equal size.
C)two lobes of unequal size.
D)four lobes of equal size.
A)one lobe.
B)two lobes of equal size.
C)two lobes of unequal size.
D)four lobes of equal size.

Unlock Deck
Unlock for access to all 232 flashcards in this deck.
Unlock Deck
k this deck
63
What orbital hybridization is expected for the central atom in a molecule with a trigonal planar geometry?
A)sp
B)sp2
C)sp3
D)None of these
A)sp
B)sp2
C)sp3
D)None of these

Unlock Deck
Unlock for access to all 232 flashcards in this deck.
Unlock Deck
k this deck
64
Arrange in order from the smallest to the largest bond angle: CH3+,NF3,NH4+,XeF4.
A)CH3+,NF3,NH4+,XeF4
B)NF3,NH4+,XeF4,CH3+
C)XeF4,NH4+,NF3,CH3+
D)XeF4,NF3,NH4+,CH3+
A)CH3+,NF3,NH4+,XeF4
B)NF3,NH4+,XeF4,CH3+
C)XeF4,NH4+,NF3,CH3+
D)XeF4,NF3,NH4+,CH3+

Unlock Deck
Unlock for access to all 232 flashcards in this deck.
Unlock Deck
k this deck
65
Which of the following best describes ICl2-? It has a molecular geometry that is
A)linear molecular shape with no lone pairs on the I atom.
B)linear molecular shape with lone pairs on the I atom.
C)non-linear molecular shape with no lone pairs on the I atom.
D)non-linear molecular shape with lone pairs on the I atom.
A)linear molecular shape with no lone pairs on the I atom.
B)linear molecular shape with lone pairs on the I atom.
C)non-linear molecular shape with no lone pairs on the I atom.
D)non-linear molecular shape with lone pairs on the I atom.

Unlock Deck
Unlock for access to all 232 flashcards in this deck.
Unlock Deck
k this deck
66
What is the smallest bond angle in SF6?
A)60°
B)90°
C)109.5°
D)120°
A)60°
B)90°
C)109.5°
D)120°

Unlock Deck
Unlock for access to all 232 flashcards in this deck.
Unlock Deck
k this deck
67
The orbital hybridization on the carbon atom in HCN is
A)sp.
B)sp2.
C)sp3.
D)None of these
A)sp.
B)sp2.
C)sp3.
D)None of these

Unlock Deck
Unlock for access to all 232 flashcards in this deck.
Unlock Deck
k this deck
68
Which of the following would be expected to have sp2 hybridization on atom A? 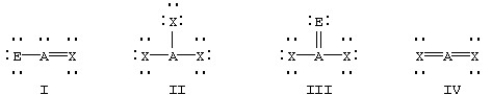
A)II
B)I and III
C)I,II,and III
D)I and IV
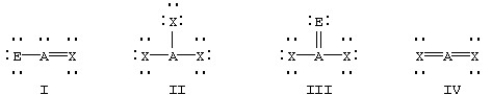
A)II
B)I and III
C)I,II,and III
D)I and IV

Unlock Deck
Unlock for access to all 232 flashcards in this deck.
Unlock Deck
k this deck
69
Which of the following is not true?
A)The sp3 hybrid orbitals are degenerate.
B)An sp3 hybrid orbital may hold a lone pair of electrons.
C)An sp3 hybrid orbital may form a sigma bond by overlap with an orbital on another atom.
D)An sp3 hybrid orbital may form a pi bond by overlap with an orbital on another atom.
A)The sp3 hybrid orbitals are degenerate.
B)An sp3 hybrid orbital may hold a lone pair of electrons.
C)An sp3 hybrid orbital may form a sigma bond by overlap with an orbital on another atom.
D)An sp3 hybrid orbital may form a pi bond by overlap with an orbital on another atom.

Unlock Deck
Unlock for access to all 232 flashcards in this deck.
Unlock Deck
k this deck
70
What is the molecular geometry of BrF4-?
A)seesaw
B)square planar
C)square pyramidal
D)tetrahedral
A)seesaw
B)square planar
C)square pyramidal
D)tetrahedral

Unlock Deck
Unlock for access to all 232 flashcards in this deck.
Unlock Deck
k this deck
71
The number of sp2 hybrid orbitals on the carbon atom in CO32- is
A)one.
B)two.
C)three.
D)four.
A)one.
B)two.
C)three.
D)four.

Unlock Deck
Unlock for access to all 232 flashcards in this deck.
Unlock Deck
k this deck
72
What is the molecular geometry of AsCl3?
A)T-shaped
B)tetrahedral
C)trigonal planar
D)trigonal pyramidal
A)T-shaped
B)tetrahedral
C)trigonal planar
D)trigonal pyramidal

Unlock Deck
Unlock for access to all 232 flashcards in this deck.
Unlock Deck
k this deck
73
Which of the following is not a valence bond concept?
A)The greater the overlap between the orbitals on two atoms,the stronger the bond.
B)Lone pair electrons are in atomic orbitals or in hybrid atomic orbitals.
C)Atomic orbitals on two atoms may overlap to form antibonding orbitals.
D)A pair of electrons in a bond is shared by both atoms.
A)The greater the overlap between the orbitals on two atoms,the stronger the bond.
B)Lone pair electrons are in atomic orbitals or in hybrid atomic orbitals.
C)Atomic orbitals on two atoms may overlap to form antibonding orbitals.
D)A pair of electrons in a bond is shared by both atoms.

Unlock Deck
Unlock for access to all 232 flashcards in this deck.
Unlock Deck
k this deck
74
Which orbital hybridization is associated with a tetrahedral charge cloud arrangement?
A)sp
B)sp2
C)sp3
D)None of these
A)sp
B)sp2
C)sp3
D)None of these

Unlock Deck
Unlock for access to all 232 flashcards in this deck.
Unlock Deck
k this deck
75
What is the O-N-O bond angle in NO3-?
A)less than 109.5°
B)109.5°
C)120°
D)greater than 120°
A)less than 109.5°
B)109.5°
C)120°
D)greater than 120°

Unlock Deck
Unlock for access to all 232 flashcards in this deck.
Unlock Deck
k this deck
76
What is the molecular geometry of IF5?
A)octahedral
B)seesaw
C)square pyramidal
D)trigonal bipyramidal
A)octahedral
B)seesaw
C)square pyramidal
D)trigonal bipyramidal

Unlock Deck
Unlock for access to all 232 flashcards in this deck.
Unlock Deck
k this deck
77
Which of the following should be nonlinear? 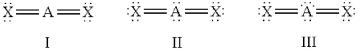
A)only I
B)only II
C)only III
D)II and III
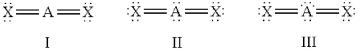
A)only I
B)only II
C)only III
D)II and III

Unlock Deck
Unlock for access to all 232 flashcards in this deck.
Unlock Deck
k this deck
78
What is the angle between adjacent sp3 hybrid orbitals?
A)90°
B)109.5°
C)120°
D)180°
A)90°
B)109.5°
C)120°
D)180°

Unlock Deck
Unlock for access to all 232 flashcards in this deck.
Unlock Deck
k this deck
79
A triple bond is generally composed of
A)three π bonds.
B)two π bonds and one σ bond.
C)one π bond and two σ bonds.
D)three σ bonds.
A)three π bonds.
B)two π bonds and one σ bond.
C)one π bond and two σ bonds.
D)three σ bonds.

Unlock Deck
Unlock for access to all 232 flashcards in this deck.
Unlock Deck
k this deck
80
What is the molecular geometry of TeCl4?
A)seesaw
B)square planar
C)square pyramidal
D)tetrahedral
A)seesaw
B)square planar
C)square pyramidal
D)tetrahedral

Unlock Deck
Unlock for access to all 232 flashcards in this deck.
Unlock Deck
k this deck


