Deck 11: Solutions and Their Properties
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/192
Play
Full screen (f)
Deck 11: Solutions and Their Properties
1
Which of the following mixtures have components which can be separated by filtration?
A)colloids
B)solutions
C)suspensions
D)All of these
A)colloids
B)solutions
C)suspensions
D)All of these
suspensions
2
When two similar liquids mix to form a solution,the entropy of solution (ΔSsoln)is expected to be
A)negative.
B)zero.
C)positive.
D)negative at low temperatures but positive at high temperatures.
A)negative.
B)zero.
C)positive.
D)negative at low temperatures but positive at high temperatures.
positive.
3
Commercial cold packs often contain solid NH4NO3 and a pouch of water.The temperature of the pack drops as the NH4NO3 dissolves in water.Therefore,for the dissolving of NH4NO3 in water,
A)ΔHsoln is negative and ΔSsoln may be negative or positive.
B)ΔHsoln is negative and ΔSsoln is positive.
C)ΔHsoln is positive and ΔSsoln may be negative or positive.
D)ΔHsoln is positive and ΔSsoln is positive.
A)ΔHsoln is negative and ΔSsoln may be negative or positive.
B)ΔHsoln is negative and ΔSsoln is positive.
C)ΔHsoln is positive and ΔSsoln may be negative or positive.
D)ΔHsoln is positive and ΔSsoln is positive.
ΔHsoln is positive and ΔSsoln is positive.
4
Which cation in each set is expected to have the larger (more negative)hydration energy?
I.Mg2+ or Ba2+
II.K+ or Al3+
A)Mg2+ in set I and K+ in set II
B)Mg2+ in set I and Al3+ in set II
C)Ba2+ in set I and K+ in set II
D)Ba2+ in set I and Al3+ in set II
I.Mg2+ or Ba2+
II.K+ or Al3+
A)Mg2+ in set I and K+ in set II
B)Mg2+ in set I and Al3+ in set II
C)Ba2+ in set I and K+ in set II
D)Ba2+ in set I and Al3+ in set II

Unlock Deck
Unlock for access to all 192 flashcards in this deck.
Unlock Deck
k this deck
5
Which is not a solution?
A)brass
B)fog
C)hydrochloric acid
D)wine
A)brass
B)fog
C)hydrochloric acid
D)wine

Unlock Deck
Unlock for access to all 192 flashcards in this deck.
Unlock Deck
k this deck
6
When an ionic solute dissolves in water to form an unsaturated solution,the free energy change (ΔGsoln)is
A)negative.
B)zero.
C)positive.
D)either A or C,depending on the ionic compound
A)negative.
B)zero.
C)positive.
D)either A or C,depending on the ionic compound

Unlock Deck
Unlock for access to all 192 flashcards in this deck.
Unlock Deck
k this deck
7
Which cation in each set would be expected to have the larger (more negative)hydration energy?
I.Fe2+ or Fe3+
II.Li+ or NH4+
A)Fe2+ in set I and Li+ in set II
B)Fe2+ in set I and NH4+ in set II
C)Fe3+ in set I and Li+ in set II
D)Fe3+ in set I and NH4+ in set II
I.Fe2+ or Fe3+
II.Li+ or NH4+
A)Fe2+ in set I and Li+ in set II
B)Fe2+ in set I and NH4+ in set II
C)Fe3+ in set I and Li+ in set II
D)Fe3+ in set I and NH4+ in set II

Unlock Deck
Unlock for access to all 192 flashcards in this deck.
Unlock Deck
k this deck
8
KBr does not dissolve well in nonpolar solvents because
A)solute-solute interactions are much larger than solvent-solvent or solute-solvent interactions.
B)solvent-solvent interactions are much larger than solute-solvent or solute-solute interactions.
C)solute-solvent interactions are much larger than solvent-solvent or solute-solute interactions.
D)solute-solvent interactions are similar to solvent-solvent and solute-solute interactions.
A)solute-solute interactions are much larger than solvent-solvent or solute-solvent interactions.
B)solvent-solvent interactions are much larger than solute-solvent or solute-solute interactions.
C)solute-solvent interactions are much larger than solvent-solvent or solute-solute interactions.
D)solute-solvent interactions are similar to solvent-solvent and solute-solute interactions.

Unlock Deck
Unlock for access to all 192 flashcards in this deck.
Unlock Deck
k this deck
9
In the process of dissolving ionic compounds,the cations and anions are separated from the crystal lattice and surrounded by an ordered shell of solvent molecules.If the solvent is water,the dissolved ions are said to be
A)halogenated.
B)homogenized.
C)hybridized.
D)hydrated.
A)halogenated.
B)homogenized.
C)hybridized.
D)hydrated.

Unlock Deck
Unlock for access to all 192 flashcards in this deck.
Unlock Deck
k this deck
10
For a liquid solution made by dissolving a solid or a gas in a liquid,the
A)liquid is the solute.
B)liquid is the solvent.
C)solute is the component present in the greatest amount.
D)solvent is the component present in the greatest amount.
A)liquid is the solute.
B)liquid is the solvent.
C)solute is the component present in the greatest amount.
D)solvent is the component present in the greatest amount.

Unlock Deck
Unlock for access to all 192 flashcards in this deck.
Unlock Deck
k this deck
11
The change in the Gibbs free energy for dissolving more solute in a supersaturated solution is
A)negative.
B)zero.
C)positive.
D)positive at low temperatures and negative at high temperatures.
A)negative.
B)zero.
C)positive.
D)positive at low temperatures and negative at high temperatures.

Unlock Deck
Unlock for access to all 192 flashcards in this deck.
Unlock Deck
k this deck
12
Although there are exceptions,which is most likely to be true for the dissolving of a solid in a liquid?
A)ΔHsoln is positive.
B)ΔHsoln is negative.
C)ΔSsoln is positive.
D)ΔSsoln is negative.
A)ΔHsoln is positive.
B)ΔHsoln is negative.
C)ΔSsoln is positive.
D)ΔSsoln is negative.

Unlock Deck
Unlock for access to all 192 flashcards in this deck.
Unlock Deck
k this deck
13
One reason ionic compounds do not dissolve well in nonpolar solvents is that
A)ion-dipole interactions are too large for effective solvation to occur.
B)ion-solvent interactions are not strong enough to solvate the ions in solution.
C)not all cations and anions have the same magnitude of charge and therefore do not form neutral ion pairs.
D)there are no forces of attraction between ions and nonpolar molecules.
A)ion-dipole interactions are too large for effective solvation to occur.
B)ion-solvent interactions are not strong enough to solvate the ions in solution.
C)not all cations and anions have the same magnitude of charge and therefore do not form neutral ion pairs.
D)there are no forces of attraction between ions and nonpolar molecules.

Unlock Deck
Unlock for access to all 192 flashcards in this deck.
Unlock Deck
k this deck
14
When a particular solid begins to dissolve in water,the temperature rises dramatically.For the dissolving of this solid in pure water
A)ΔHsoln is always negative and ΔSsoln may be negative or positive.
B)ΔHsoln is always negative and ΔSsoln is always positive.
C)ΔHsoln is always positive and ΔSsoln may be negative or positive.
D)ΔHsoln is always positive and ΔSsoln is always positive.
A)ΔHsoln is always negative and ΔSsoln may be negative or positive.
B)ΔHsoln is always negative and ΔSsoln is always positive.
C)ΔHsoln is always positive and ΔSsoln may be negative or positive.
D)ΔHsoln is always positive and ΔSsoln is always positive.

Unlock Deck
Unlock for access to all 192 flashcards in this deck.
Unlock Deck
k this deck
15
In most liquid solutions,the component present in the larger amount is called the
A)dispersed medium.
B)emulsifying agent.
C)solute.
D)solvent.
A)dispersed medium.
B)emulsifying agent.
C)solute.
D)solvent.

Unlock Deck
Unlock for access to all 192 flashcards in this deck.
Unlock Deck
k this deck
16
For which case would ΔHsoln be expected to be negative?
A)if solute-solute interactions are much greater than solvent-solvent and solute-solvent interactions
B)if solvent-solvent interactions are much greater than solute-solvent and solute-solute interactions
C)if solute-solvent interactions are much greater than solvent-solvent and solute-solute interactions
D)if solute-solvent interactions are the same as solvent-solvent and solute-solute interactions
A)if solute-solute interactions are much greater than solvent-solvent and solute-solvent interactions
B)if solvent-solvent interactions are much greater than solute-solvent and solute-solute interactions
C)if solute-solvent interactions are much greater than solvent-solvent and solute-solute interactions
D)if solute-solvent interactions are the same as solvent-solvent and solute-solute interactions

Unlock Deck
Unlock for access to all 192 flashcards in this deck.
Unlock Deck
k this deck
17
Stainless steel is an example of a ________ solution.
A)gas/solid
B)liquid/liquid
C)solid/liquid
D)solid/solid
A)gas/solid
B)liquid/liquid
C)solid/liquid
D)solid/solid

Unlock Deck
Unlock for access to all 192 flashcards in this deck.
Unlock Deck
k this deck
18
The change in the Gibbs free energy for dissolving solute in a saturated solution is
A)negative.
B)zero.
C)positive.
D)positive at low temperatures and negative at high temperatures.
A)negative.
B)zero.
C)positive.
D)positive at low temperatures and negative at high temperatures.

Unlock Deck
Unlock for access to all 192 flashcards in this deck.
Unlock Deck
k this deck
19
The rubbing alcohol sold in drug stores often is composed of 70% isopropyl alcohol and 30% water.In this solution
A)isopropyl alcohol is the solvent.
B)water is the solvent.
C)both water and isopropyl alcohol are solvents.
D)neither water nor isopropyl alcohol is a solvent.
A)isopropyl alcohol is the solvent.
B)water is the solvent.
C)both water and isopropyl alcohol are solvents.
D)neither water nor isopropyl alcohol is a solvent.

Unlock Deck
Unlock for access to all 192 flashcards in this deck.
Unlock Deck
k this deck
20
Iodine,I2(s),is more soluble in dichloromethane,CH2Cl2(l),than in water because
A)both iodine and dichloromethane have strong ion-dipole interactions.
B)the dipole-dipole forces in dichloromethane are much stronger than the dispersion forces in iodine.
C)the intermolecular forces are similar in both iodine and dichloromethane.
D)iodine is polar and dichloromethane has a large number of hydrogen bonds.
A)both iodine and dichloromethane have strong ion-dipole interactions.
B)the dipole-dipole forces in dichloromethane are much stronger than the dispersion forces in iodine.
C)the intermolecular forces are similar in both iodine and dichloromethane.
D)iodine is polar and dichloromethane has a large number of hydrogen bonds.

Unlock Deck
Unlock for access to all 192 flashcards in this deck.
Unlock Deck
k this deck
21
Formaldehyde is a carcinogenic volatile organic compound with a permissible exposure level of 0.75 ppm.At this level,how many grams of formaldehyde are permissible in a 6.0-L breath of air having a density of 1.2 kg/m3?
A)3)8 × 10-2 g formaldehyde
B)5)4 × 10-6 g formaldehyde
C)3)8 g formaldehyde
D)5)4 g formaldehyde
A)3)8 × 10-2 g formaldehyde
B)5)4 × 10-6 g formaldehyde
C)3)8 g formaldehyde
D)5)4 g formaldehyde

Unlock Deck
Unlock for access to all 192 flashcards in this deck.
Unlock Deck
k this deck
22
The dose of amoxicillin given to a young child is 40 mg/kg of body weight/day.If the amoxicillin is administered as a suspension having a concentration of 400 mg/5 mL,how many mL of amoxicillin must be administered per dose for a child weighing 18 pounds?
A)2)0 mL
B)4)1 mL
C)4)5 mL
D)9)9 mL
A)2)0 mL
B)4)1 mL
C)4)5 mL
D)9)9 mL

Unlock Deck
Unlock for access to all 192 flashcards in this deck.
Unlock Deck
k this deck
23
Substances with high lattice energies tend to be less soluble than substances with low lattice energies.On that basis predict the relative aqueous solubility at 20°C,from highest to lowest,of the following ionic compounds: Ce2(SO4)3,K2SO4,KBr,NaCl.
A)Ce2(SO4)3 > K2SO4 > KBr > NaCl
B)Ce2(SO4)3 > K2SO4 > NaCl > KBr
C)KBr > NaCl > K2SO4 > Ce2(SO4)3
D)NaCl > KBr > K2SO4 > Ce2(SO4)3
A)Ce2(SO4)3 > K2SO4 > KBr > NaCl
B)Ce2(SO4)3 > K2SO4 > NaCl > KBr
C)KBr > NaCl > K2SO4 > Ce2(SO4)3
D)NaCl > KBr > K2SO4 > Ce2(SO4)3

Unlock Deck
Unlock for access to all 192 flashcards in this deck.
Unlock Deck
k this deck
24
What are the major solute-solvent interactions created when KBr dissolves in water?
A)dipole-dipole
B)dispersion
C)hydrogen bonding
D)ion-dipole
A)dipole-dipole
B)dispersion
C)hydrogen bonding
D)ion-dipole

Unlock Deck
Unlock for access to all 192 flashcards in this deck.
Unlock Deck
k this deck
25
For the process of dissolving a solid in a liquid,which of the following statements is true?
A)ΔHsoln is always negative and ΔSsoln is usually positive.
B)ΔHsoln is always positive and ΔSsoln is usually negative.
C)ΔHsoln is either positive or negative and ΔSsoln is usually positive.
D)ΔHsoln is either positive or negative and ΔSsoln is usually negative.
A)ΔHsoln is always negative and ΔSsoln is usually positive.
B)ΔHsoln is always positive and ΔSsoln is usually negative.
C)ΔHsoln is either positive or negative and ΔSsoln is usually positive.
D)ΔHsoln is either positive or negative and ΔSsoln is usually negative.

Unlock Deck
Unlock for access to all 192 flashcards in this deck.
Unlock Deck
k this deck
26
Which concentration becomes smaller as the temperature is increased from 20°C to 80°C?
A)mass %
B)molality
C)molarity
D)mole fraction
A)mass %
B)molality
C)molarity
D)mole fraction

Unlock Deck
Unlock for access to all 192 flashcards in this deck.
Unlock Deck
k this deck
27
What are the major solute-solvent interactions created when HOCH2CH2OH dissolves in water?
A)dipole-dipole
B)dispersion
C)hydrogen bonding
D)ion-dipole
A)dipole-dipole
B)dispersion
C)hydrogen bonding
D)ion-dipole

Unlock Deck
Unlock for access to all 192 flashcards in this deck.
Unlock Deck
k this deck
28
What is the mole fraction of ethanol in a solution made by dissolving 14.6 g of ethanol,C2H5OH,in 53.6 g of water?
A)0)0964
B)0)106
C)0)214
D)0)272
A)0)0964
B)0)106
C)0)214
D)0)272

Unlock Deck
Unlock for access to all 192 flashcards in this deck.
Unlock Deck
k this deck
29
A solution is prepared by dissolving 17.75 g sulfuric acid,H2SO4,in enough water to make 100.0 mL of solution.If the density of the solution is 1.1094 g/mL,what is the mole fraction H2SO4 in the solution?
A)0)0181
B)0)0338
C)0)0350
D)19.0
A)0)0181
B)0)0338
C)0)0350
D)19.0

Unlock Deck
Unlock for access to all 192 flashcards in this deck.
Unlock Deck
k this deck
30
Most gases become less soluble in water as the temperature increases.What can be concluded about the signs of ΔHsoln and ΔSsoln in this case?
A)ΔHsoln is negative and ΔSsoln is negative.
B)ΔHsoln is negative and ΔSsoln is positive.
C)ΔHsoln is positive and ΔSsoln is negative.
D)ΔHsoln is positive and ΔSsoln is positive.
A)ΔHsoln is negative and ΔSsoln is negative.
B)ΔHsoln is negative and ΔSsoln is positive.
C)ΔHsoln is positive and ΔSsoln is negative.
D)ΔHsoln is positive and ΔSsoln is positive.

Unlock Deck
Unlock for access to all 192 flashcards in this deck.
Unlock Deck
k this deck
31
What is the molality of ethanol in a solution made by dissolving 14.6 g of ethanol,C2H5OH,in 53.6 g of water?
A)0)00591 m
B)0)272 m
C)5)91 m
D)272 m
A)0)00591 m
B)0)272 m
C)5)91 m
D)272 m

Unlock Deck
Unlock for access to all 192 flashcards in this deck.
Unlock Deck
k this deck
32
What is the mole fraction of I2 in a solution made by dissolving 27.8 g of I2 in 245 g of hexane,C6H14?
A)0)0371
B)0)0385
C)0)0715
D)0)0770
A)0)0371
B)0)0385
C)0)0715
D)0)0770

Unlock Deck
Unlock for access to all 192 flashcards in this deck.
Unlock Deck
k this deck
33
A solution has a density of 1.023 g/mL and a concentration of 0.0800 g/dL.What is the concentration in parts per million?
A)700 ppm
B)782 ppm
C)800 ppm
D)818 ppm
A)700 ppm
B)782 ppm
C)800 ppm
D)818 ppm

Unlock Deck
Unlock for access to all 192 flashcards in this deck.
Unlock Deck
k this deck
34
Molarity is defined as moles of solute per
A)kilogram of solvent.
B)liter of solution.
C)mole of solvent.
D)total moles present.
A)kilogram of solvent.
B)liter of solution.
C)mole of solvent.
D)total moles present.

Unlock Deck
Unlock for access to all 192 flashcards in this deck.
Unlock Deck
k this deck
35
Fresh air contains approximately 400 ppm CO2,whereas the breath of an intoxicated person contains about 4 percent CO2.The amount of CO2 in the breath of an intoxicated person is ________ times the amount of CO2 in fresh air.
A)10-2
B)10-1
C)101
D)102
A)10-2
B)10-1
C)101
D)102

Unlock Deck
Unlock for access to all 192 flashcards in this deck.
Unlock Deck
k this deck
36
Molality is defined as moles of solute per
A)kilogram of solvent.
B)liter of solution.
C)mole of solvent.
D)total moles present.
A)kilogram of solvent.
B)liter of solution.
C)mole of solvent.
D)total moles present.

Unlock Deck
Unlock for access to all 192 flashcards in this deck.
Unlock Deck
k this deck
37
Which should be least soluble in water?
A)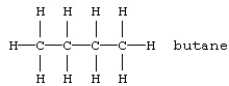
B)
C)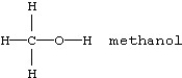
D)
A)

B)

C)
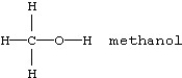
D)
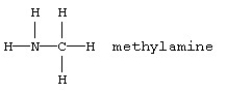

Unlock Deck
Unlock for access to all 192 flashcards in this deck.
Unlock Deck
k this deck
38
Which of the following should most favor the solubility of an ionic solid in water?
A)a low lattice energy for the solid and a low hydration energy for its ions
B)a low lattice energy for the solid and a high hydration energy for its ions
C)a high lattice energy for the solid and a low hydration energy for its ions
D)a high lattice energy for the solid and a high hydration energy for its ions
A)a low lattice energy for the solid and a low hydration energy for its ions
B)a low lattice energy for the solid and a high hydration energy for its ions
C)a high lattice energy for the solid and a low hydration energy for its ions
D)a high lattice energy for the solid and a high hydration energy for its ions

Unlock Deck
Unlock for access to all 192 flashcards in this deck.
Unlock Deck
k this deck
39
What is the mole fraction of oxygen in a gas mixture that is 22% oxygen and 78% nitrogen by volume?
A)0)20
B)0)22
C)0)25
D)0)28
A)0)20
B)0)22
C)0)25
D)0)28

Unlock Deck
Unlock for access to all 192 flashcards in this deck.
Unlock Deck
k this deck
40
Arrange the following compounds in order of their expected increasing solubility in water:
KCl,CH3CH2-O-CH2CH3,CH3CH2CH2CH2-OH,CH3CH2CH2CH2CH3.
A)CH3CH2CH2CH2CH3 < KCl < CH3CH2-O-CH2CH3 < CH3CH2CH2CH2-OH
B)KCl < CH3CH2-O-CH2CH3 < CH3CH2CH2CH2-OH < CH3CH2CH2CH2CH3
C)CH3CH2CH2CH2CH3 < CH3CH2-O-CH2CH3 < KCl < CH3CH2CH2CH2-OH
D)CH3CH2CH2CH2CH3 < CH3CH2-O-CH2CH3 < CH3CH2CH2CH2-OH < KCl
KCl,CH3CH2-O-CH2CH3,CH3CH2CH2CH2-OH,CH3CH2CH2CH2CH3.
A)CH3CH2CH2CH2CH3 < KCl < CH3CH2-O-CH2CH3 < CH3CH2CH2CH2-OH
B)KCl < CH3CH2-O-CH2CH3 < CH3CH2CH2CH2-OH < CH3CH2CH2CH2CH3
C)CH3CH2CH2CH2CH3 < CH3CH2-O-CH2CH3 < KCl < CH3CH2CH2CH2-OH
D)CH3CH2CH2CH2CH3 < CH3CH2-O-CH2CH3 < CH3CH2CH2CH2-OH < KCl

Unlock Deck
Unlock for access to all 192 flashcards in this deck.
Unlock Deck
k this deck
41
To make a 2.00 m solution,one could take 2.00 moles of solute and add
A)1)00 L of solvent.
B)1)00 kg of solvent.
C)enough solvent to make 1.00 L of solution.
D)enough solvent to make 1.00 kg of solution.
A)1)00 L of solvent.
B)1)00 kg of solvent.
C)enough solvent to make 1.00 L of solution.
D)enough solvent to make 1.00 kg of solution.

Unlock Deck
Unlock for access to all 192 flashcards in this deck.
Unlock Deck
k this deck
42
To make a 2.0 M solution,one could take 2.00 moles of solute and add
A)1)00 L of solvent.
B)1)00 kg of solvent.
C)enough solvent to make 1.00 L of solution.
D)enough solvent to make 1.00 kg of solution.
A)1)00 L of solvent.
B)1)00 kg of solvent.
C)enough solvent to make 1.00 L of solution.
D)enough solvent to make 1.00 kg of solution.

Unlock Deck
Unlock for access to all 192 flashcards in this deck.
Unlock Deck
k this deck
43
Aqueous solutions of 30% (by weight)hydrogen peroxide,H2O2,are used to oxidize metals or organic molecules in chemical reactions.Given that the density of the solution is 1.11 g/mL,calculate the molarity.
A)0)794 M
B)6)78 M
C)9)79 M
D)12.6 M
A)0)794 M
B)6)78 M
C)9)79 M
D)12.6 M

Unlock Deck
Unlock for access to all 192 flashcards in this deck.
Unlock Deck
k this deck
44
What molality of pentane is obtained by dissolving 5.0 g pentane,C5H12,in 245.0 g hexane,C6H14?
A)0)020 m
B)0)024 m
C)0)28 m
D)20.m
A)0)020 m
B)0)024 m
C)0)28 m
D)20.m

Unlock Deck
Unlock for access to all 192 flashcards in this deck.
Unlock Deck
k this deck
45
A solution is prepared by dissolving 171 g of CdCl2 in enough water to make exactly 250.0 mL of solution.If the density of the solution is 1.556 g/mL,what is the weight percent of CdCl2 in the solution?
A)7)17%
B)44.0%
C)56.0%
D)68.4%
A)7)17%
B)44.0%
C)56.0%
D)68.4%

Unlock Deck
Unlock for access to all 192 flashcards in this deck.
Unlock Deck
k this deck
46
A solution is prepared by dissolving 17.75 g sulfuric acid,H2SO4,in enough water to make exactly 100.0 mL of solution.If the density of the solution is 1.1094 g/mL,what is the weight % H2SO4 in the solution?
A)16.00%
B)18.00%
C)19.00%
D)84.00%
A)16.00%
B)18.00%
C)19.00%
D)84.00%

Unlock Deck
Unlock for access to all 192 flashcards in this deck.
Unlock Deck
k this deck
47
Sodium hydroxide is available commercially as a 50.0% by weight aqueous solution.Calculate the molality of this sodium hydroxide solution.
A)0)450 m
B)19.1 m
C)25.0 m
D)125.m
A)0)450 m
B)19.1 m
C)25.0 m
D)125.m

Unlock Deck
Unlock for access to all 192 flashcards in this deck.
Unlock Deck
k this deck
48
How much water must be added to 42.0 g of CaCl2 to produce a solution that is 35.0 wt% CaCl2?
A)56.7 g
B)78.0 g
C)83.3 g
D)120 g
A)56.7 g
B)78.0 g
C)83.3 g
D)120 g

Unlock Deck
Unlock for access to all 192 flashcards in this deck.
Unlock Deck
k this deck
49
Aqueous solutions of 30.0% (by weight)hydrogen peroxide,H2O2,are used to oxidize metals or organic molecules in chemical reactions.Calculate the molality of this solution.
A)0)974 m
B)6)78 m
C)9)79 m
D)12.6 m
A)0)974 m
B)6)78 m
C)9)79 m
D)12.6 m

Unlock Deck
Unlock for access to all 192 flashcards in this deck.
Unlock Deck
k this deck
50
A solution is prepared by dissolving 171 g of CdCl2 in enough water to make 250.0 mL of solution.If the density of the solution is 1.556 g/mL,what is the molarity of the solution?
A)0)440 M
B)0)684 M
C)0)933 M
D)3)73 M
A)0)440 M
B)0)684 M
C)0)933 M
D)3)73 M

Unlock Deck
Unlock for access to all 192 flashcards in this deck.
Unlock Deck
k this deck
51
What is the weight percent of a caffeine solution made by dissolving 4.35 g of caffeine,C8H10N4O2,in 75 g of benzene,C6H6?
A)0)055%
B)0)058%
C)5)5%
D)5)8%
A)0)055%
B)0)058%
C)5)5%
D)5)8%

Unlock Deck
Unlock for access to all 192 flashcards in this deck.
Unlock Deck
k this deck
52
What is the weight percent of vitamin C in a solution made by dissolving 1.30 g of vitamin C,C6H8O6,in 55.0 g of water?
A)0)195%
B)0)242%
C)2)31%
D)2)36%
A)0)195%
B)0)242%
C)2)31%
D)2)36%

Unlock Deck
Unlock for access to all 192 flashcards in this deck.
Unlock Deck
k this deck
53
Sodium hydroxide is available commercially as a 50.0% by weight aqueous solution.The density of the solution is 1.53 g/mL.Calculate the molarity of this sodium hydroxide solution.
A)0)450 M
B)19.1 M
C)25.0 M
D)125.M
A)0)450 M
B)19.1 M
C)25.0 M
D)125.M

Unlock Deck
Unlock for access to all 192 flashcards in this deck.
Unlock Deck
k this deck
54
How many grams of KBr are required to make 650.mL of a 0.115 M KBr solution?
A)0)628 g
B)5)65 g
C)8)90 g
D)74.8 g
A)0)628 g
B)5)65 g
C)8)90 g
D)74.8 g

Unlock Deck
Unlock for access to all 192 flashcards in this deck.
Unlock Deck
k this deck
55
What volume of 3.00 M CH3OH solution is needed to provide 0.270 mol of CH3OH?
A)1)23 mL
B)11.1 mL
C)90.0 mL
D)810 mL
A)1)23 mL
B)11.1 mL
C)90.0 mL
D)810 mL

Unlock Deck
Unlock for access to all 192 flashcards in this deck.
Unlock Deck
k this deck
56
What is the molality of a glucose solution prepared by dissolving 18.0 g of glucose,C6H12O6,in 125.9 g of water?
A)7)94 × 10-4 m
B)0)143 m
C)0)695 m
D)0)794 m
A)7)94 × 10-4 m
B)0)143 m
C)0)695 m
D)0)794 m

Unlock Deck
Unlock for access to all 192 flashcards in this deck.
Unlock Deck
k this deck
57
A solution is prepared by dissolving 17.75 g sulfuric acid,H2SO4,in enough water to make 100.0 mL of solution.If the density of the solution is 1.1094 g/mL,what is the molality?
A)0)1775 m H2SO4
B)0)1810 m H2SO4
C)1)810 m H2SO4
D)1)940 m H2SO4
A)0)1775 m H2SO4
B)0)1810 m H2SO4
C)1)810 m H2SO4
D)1)940 m H2SO4

Unlock Deck
Unlock for access to all 192 flashcards in this deck.
Unlock Deck
k this deck
58
What volume of 0.716 M KBr solution is needed to provide 10.5 g of KBr?
A)7)52 mL
B)14.7 mL
C)63.2 mL
D)123 mL
A)7)52 mL
B)14.7 mL
C)63.2 mL
D)123 mL

Unlock Deck
Unlock for access to all 192 flashcards in this deck.
Unlock Deck
k this deck
59
A solution is 2.25% by weight NaHCO3.How many grams of NaHCO3 are in 450.0 g of solution?
A)0)500 g
B)10.1 g
C)200 g
D)225 g
A)0)500 g
B)10.1 g
C)200 g
D)225 g

Unlock Deck
Unlock for access to all 192 flashcards in this deck.
Unlock Deck
k this deck
60
A solution is prepared by dissolving 17.75 g sulfuric acid,H2SO4,in enough water to make 100.0 mL of solution.If the density of the solution is 1.1094 g/mL,what is the molarity?
A)0)1775 M H2SO4
B)0)1810 M H2SO4
C)1)810 M H2SO4
D)1)940 M H2SO4
A)0)1775 M H2SO4
B)0)1810 M H2SO4
C)1)810 M H2SO4
D)1)940 M H2SO4

Unlock Deck
Unlock for access to all 192 flashcards in this deck.
Unlock Deck
k this deck
61
Which of the following does not affect the solubility of a solute in a given solvent?
A)polarity of the solute
B)polarity of the solvent
C)rate of stirring
D)temperature of the solvent and solute
A)polarity of the solute
B)polarity of the solvent
C)rate of stirring
D)temperature of the solvent and solute

Unlock Deck
Unlock for access to all 192 flashcards in this deck.
Unlock Deck
k this deck
62
At 25.0°C,a solution has a concentration of 3.179 M and a density of 1.260 g/mL.The density of the solution at 50.0°C is 1.249 g/mL.What is the molarity of the solution at 50.0°C?
A)2)545 M
B)3)151 M
C)3)179 M
D)3)230 M
A)2)545 M
B)3)151 M
C)3)179 M
D)3)230 M

Unlock Deck
Unlock for access to all 192 flashcards in this deck.
Unlock Deck
k this deck
63
A solution is prepared by dissolving 40.0 g of sucrose,C12H22O11,in 250.g of water at 25°C.What is the vapor pressure of the solution if the vapor pressure of water at 25°C is 23.76 mm Hg?
A)0)198 mm Hg
B)20.5 mm Hg
C)23.6 mm Hg
D)24.0 mm Hg
A)0)198 mm Hg
B)20.5 mm Hg
C)23.6 mm Hg
D)24.0 mm Hg

Unlock Deck
Unlock for access to all 192 flashcards in this deck.
Unlock Deck
k this deck
64
A 1.30 M solution of CaCl2 in water has a density of 1.11 g/mL.What is the molality?
A)1)17 m CaCl2
B)1)25 m CaCl2
C)1)35 m CaCl2
D)1)44 m CaCl2
A)1)17 m CaCl2
B)1)25 m CaCl2
C)1)35 m CaCl2
D)1)44 m CaCl2

Unlock Deck
Unlock for access to all 192 flashcards in this deck.
Unlock Deck
k this deck
65
The Henry's Law constant of methyl bromide,CH3Br,is k = 0.159 mol/(L ∙ atm)at 25°C.What is the solubility of methyl bromide in water at 25°C and at a partial pressure of 250.mm Hg?
A)0)0523 mol/L
B)0)329 mol/L
C)0)483 mol/L
D)39.8 mol/L
A)0)0523 mol/L
B)0)329 mol/L
C)0)483 mol/L
D)39.8 mol/L

Unlock Deck
Unlock for access to all 192 flashcards in this deck.
Unlock Deck
k this deck
66
A solution of LiCl in water has XLiCl = 0.0500.What is the molality?
A)2)60 m LiCl
B)2)77 m LiCl
C)2)92 m LiCl
D)5)26 m LiCl
A)2)60 m LiCl
B)2)77 m LiCl
C)2)92 m LiCl
D)5)26 m LiCl

Unlock Deck
Unlock for access to all 192 flashcards in this deck.
Unlock Deck
k this deck
67
In which case should CO2(g)be more soluble in water?
A)The total pressure is 5 atm and the partial pressure of CO2 is 1 atm.
B)The total pressure is 3 atm and the partial pressure of CO2 is 2 atm.
C)The total pressure is 1 atm and the partial pressure of CO2 is 0.03 atm.
D)The total pressure is 1 atm and the partial pressure of CO2 is 0.5 atm.
A)The total pressure is 5 atm and the partial pressure of CO2 is 1 atm.
B)The total pressure is 3 atm and the partial pressure of CO2 is 2 atm.
C)The total pressure is 1 atm and the partial pressure of CO2 is 0.03 atm.
D)The total pressure is 1 atm and the partial pressure of CO2 is 0.5 atm.

Unlock Deck
Unlock for access to all 192 flashcards in this deck.
Unlock Deck
k this deck
68
In general,as the temperature increases,the solubility of gases in water ________ and the solubility of most solids in water ________.
A)decreases,decreases
B)decreases,increases
C)increases,decreases
D)increases,increases
A)decreases,decreases
B)decreases,increases
C)increases,decreases
D)increases,increases

Unlock Deck
Unlock for access to all 192 flashcards in this deck.
Unlock Deck
k this deck
69
A saturated solution is defined as
A)a concentrated solution.
B)a solution that is in equilibrium with pure solvent.
C)a solution than is in equilibrium with undissolved solute.
D)a solution that is in equilibrium with both pure solvent and undissolved solute.
A)a concentrated solution.
B)a solution that is in equilibrium with pure solvent.
C)a solution than is in equilibrium with undissolved solute.
D)a solution that is in equilibrium with both pure solvent and undissolved solute.

Unlock Deck
Unlock for access to all 192 flashcards in this deck.
Unlock Deck
k this deck
70
At a given temperature the vapor pressures of benzene and toluene are 183 mm Hg and 59.2 mm Hg,respectively.Calculate the total vapor pressure over a solution of benzene and toluene with Xbenzene = 0.600.
A)110 mm Hg
B)121 mm Hg
C)133 mm Hg
D)242 mm Hg
A)110 mm Hg
B)121 mm Hg
C)133 mm Hg
D)242 mm Hg

Unlock Deck
Unlock for access to all 192 flashcards in this deck.
Unlock Deck
k this deck
71
At a given temperature the vapor pressures of benzene and toluene are 183 mm Hg and 59.2 mm Hg,respectively.Calculate the mole fraction of benzene in the vapor phase over a solution of benzene and toluene with Xbenzene = 0.600.
A)0)600
B)0)678
C)0)756
D)0)823
A)0)600
B)0)678
C)0)756
D)0)823

Unlock Deck
Unlock for access to all 192 flashcards in this deck.
Unlock Deck
k this deck
72
A solution of LiCl in water is 18.0 wt% LiCl.What is the mole fraction of LiCl?
A)0)0853
B)0)0933
C)0)425
D)4)56
A)0)0853
B)0)0933
C)0)425
D)4)56

Unlock Deck
Unlock for access to all 192 flashcards in this deck.
Unlock Deck
k this deck
73
A 3.17 m solution of CaCl2 in water has a density of 1.24 g/mL.What is the molarity?
A)2)56 M CaCl2
B)2)91 M CaCl2
C)3)50 M CaCl2
D)3)93 M CaCl2
A)2)56 M CaCl2
B)2)91 M CaCl2
C)3)50 M CaCl2
D)3)93 M CaCl2

Unlock Deck
Unlock for access to all 192 flashcards in this deck.
Unlock Deck
k this deck
74
How many grams of sucrose,C12H22O11,must be added to 500.g of water at 100°C to change the vapor pressure to 752 mm Hg?
A)0)295 g
B)5)32 g
C)10.6 g
D)101 g
A)0)295 g
B)5)32 g
C)10.6 g
D)101 g

Unlock Deck
Unlock for access to all 192 flashcards in this deck.
Unlock Deck
k this deck
75
The solubility of argon in water at 25°C is 0.0150 mol/L.What is the Henry's Law constant for argon if the partial pressure of argon in air is 0.00934 atm?
A)1)40 × 10-4 mol/(L ∙ atm)
B)0)623 mol/(L ∙ atm)
C)1)61 mol/(L ∙ atm)
D)4)10 mol/(L ∙ atm)
A)1)40 × 10-4 mol/(L ∙ atm)
B)0)623 mol/(L ∙ atm)
C)1)61 mol/(L ∙ atm)
D)4)10 mol/(L ∙ atm)

Unlock Deck
Unlock for access to all 192 flashcards in this deck.
Unlock Deck
k this deck
76
Which of the following is not an application of colligative properties?
A)adding silver to mercury to lower the vapor pressure of mercury
B)desalinating sea water by reverse osmosis
C)melting snow by application of salt
D)reduced boiling points of pure liquids at increased altitudes
A)adding silver to mercury to lower the vapor pressure of mercury
B)desalinating sea water by reverse osmosis
C)melting snow by application of salt
D)reduced boiling points of pure liquids at increased altitudes

Unlock Deck
Unlock for access to all 192 flashcards in this deck.
Unlock Deck
k this deck
77
Which of the following statements is true for a supersaturated solution?
A)The solute in the solution is at equilibrium with undissolved solute.
B)The solution contains more than the equilibrium amount of solute.
C)The solution is stable and the solute will not precipitate.
D)A supersaturated solution is more than 50% solute by mass.
A)The solute in the solution is at equilibrium with undissolved solute.
B)The solution contains more than the equilibrium amount of solute.
C)The solution is stable and the solute will not precipitate.
D)A supersaturated solution is more than 50% solute by mass.

Unlock Deck
Unlock for access to all 192 flashcards in this deck.
Unlock Deck
k this deck
78
The solubility of gaseous solutes in liquid solvents is greater when the
A)external pressure over the solution is increased.
B)external pressure is decreased.
C)partial pressure of the gas above the solution is increased.
D)partial pressure of the solvent is increased.
A)external pressure over the solution is increased.
B)external pressure is decreased.
C)partial pressure of the gas above the solution is increased.
D)partial pressure of the solvent is increased.

Unlock Deck
Unlock for access to all 192 flashcards in this deck.
Unlock Deck
k this deck
79
A KCl solution is prepared by dissolving 40.0 g KCl in 250.0 g of water at 25°C.What is the vapor pressure of the solution if the vapor pressure of water at 25°C is 23.76 mm Hg?
A)20.5 mm Hg
B)22.1 mm Hg
C)22.9 mm Hg
D)25.5 mm Hg
A)20.5 mm Hg
B)22.1 mm Hg
C)22.9 mm Hg
D)25.5 mm Hg

Unlock Deck
Unlock for access to all 192 flashcards in this deck.
Unlock Deck
k this deck
80
A 2.00 M solution of CaCl2 in water has a density of 1.17 g/mL.What is the mole fraction of CaCl2?
A)0)0348
B)0)0360
C)0)0366
D)0)0380
A)0)0348
B)0)0360
C)0)0366
D)0)0380

Unlock Deck
Unlock for access to all 192 flashcards in this deck.
Unlock Deck
k this deck


