Deck 9: Ionic and Covalent Bonding
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/125
Play
Full screen (f)
Deck 9: Ionic and Covalent Bonding
1
Which one of the following has an enthalpy change that is equal to the lattice energy of SrCl2?
A)SrCl2(s)? Sr(g)+ 2Cl(g)
B)SrCl2(s)? Sr(g)+ Cl2(g)
C)SrCl2(s)? Sr2+(g)+ 2Cl(g)
D)SrCl2(s)? Sr(s)+ Cl2(g)
E)SrCl2(s)? Sr2+(g)+ 2Cl-(g)
A)SrCl2(s)? Sr(g)+ 2Cl(g)
B)SrCl2(s)? Sr(g)+ Cl2(g)
C)SrCl2(s)? Sr2+(g)+ 2Cl(g)
D)SrCl2(s)? Sr(s)+ Cl2(g)
E)SrCl2(s)? Sr2+(g)+ 2Cl-(g)
SrCl2(s)? Sr2+(g)+ 2Cl-(g)
2
The following representation of an atom is called

A)a Lewis dot structure.
B)an ion.
C)a structural formula.
D)an electrostatic potential map.
E)an ionic bond.

A)a Lewis dot structure.
B)an ion.
C)a structural formula.
D)an electrostatic potential map.
E)an ionic bond.
a Lewis dot structure.
3
Which of the following compounds has the most ionic bonding (has the highest percentage of ionic character)?
A)CaF2
B)LiI
C)OF2
D)CsF
E)LiF
A)CaF2
B)LiI
C)OF2
D)CsF
E)LiF
CsF
4
In the Born-Haber cycle for LiCl(s),which of the following processes corresponds to the first ionization energy of Li?
A)Li-(g)→ Li(g)+ e-
B)Li(s)→ Li(g)
C)Li+(g)+ Cl-(g)→ LiCl(s)
D)Li(g)→ Li+(g)+ e-
E)Li+(g)+ e- → Li(g)
A)Li-(g)→ Li(g)+ e-
B)Li(s)→ Li(g)
C)Li+(g)+ Cl-(g)→ LiCl(s)
D)Li(g)→ Li+(g)+ e-
E)Li+(g)+ e- → Li(g)

Unlock Deck
Unlock for access to all 125 flashcards in this deck.
Unlock Deck
k this deck
5
In which pair do both compounds exhibit predominantly ionic bonding?
A)RbCl and CaO
B)PCl5 and HF
C)KI and O3
D)Na2SO3 and BH3
E)NaF and H2O
A)RbCl and CaO
B)PCl5 and HF
C)KI and O3
D)Na2SO3 and BH3
E)NaF and H2O

Unlock Deck
Unlock for access to all 125 flashcards in this deck.
Unlock Deck
k this deck
6
All of the following have ground-state noble-gas electron configurations except
A)Ar
B)N3-
C)P3+
D)Mg2+
E)Cl-
A)Ar
B)N3-
C)P3+
D)Mg2+
E)Cl-

Unlock Deck
Unlock for access to all 125 flashcards in this deck.
Unlock Deck
k this deck
7
Atoms of an element X have the ground-state electron configuration 1s22s22p63s23p4.What type of ion is X most likely to form?
A)X6+
B)X3-
C)X4+
D)X-
E)X2-
A)X6+
B)X3-
C)X4+
D)X-
E)X2-

Unlock Deck
Unlock for access to all 125 flashcards in this deck.
Unlock Deck
k this deck
8
Which of the following compounds would be expected to have the lowest melting point?
A)AlF3
B)RbF
C)NaF
D)MgF2
E)CaF2
A)AlF3
B)RbF
C)NaF
D)MgF2
E)CaF2

Unlock Deck
Unlock for access to all 125 flashcards in this deck.
Unlock Deck
k this deck
9
When the cations Na+,K+,Rb+,Cs+ are combined with chloride ion in the gas phase to form ion pairs,which pair formation releases the greatest amount of energy?
A)KCl
B)All release the same amount of energy.
C)RbCl
D)NaCl
E)CsCl
A)KCl
B)All release the same amount of energy.
C)RbCl
D)NaCl
E)CsCl

Unlock Deck
Unlock for access to all 125 flashcards in this deck.
Unlock Deck
k this deck
10
Which of the following is the Lewis dot structure for the fluoride ion?
A)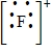
B)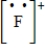
C)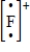
D)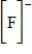
E)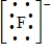
A)
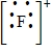
B)
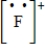
C)
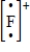
D)
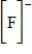
E)
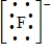

Unlock Deck
Unlock for access to all 125 flashcards in this deck.
Unlock Deck
k this deck
11
Which of the following compounds would be expected to have the highest melting point?
A)CsF
B)LiCl
C)LiF
D)NaBr
E)CsI
A)CsF
B)LiCl
C)LiF
D)NaBr
E)CsI

Unlock Deck
Unlock for access to all 125 flashcards in this deck.
Unlock Deck
k this deck
12
In the Born-Haber cycle for KI(s),which of the following processes corresponds to the electron affinity of I?
A)I(g)→ I+(g)+ e-
B)KI(s)→ K+(g)+ I-(g)
C)I-(g)→ I(g)+ e-
D)I2(g)→ 2I(g)
E)I(g)+ e- → I-(g)
A)I(g)→ I+(g)+ e-
B)KI(s)→ K+(g)+ I-(g)
C)I-(g)→ I(g)+ e-
D)I2(g)→ 2I(g)
E)I(g)+ e- → I-(g)

Unlock Deck
Unlock for access to all 125 flashcards in this deck.
Unlock Deck
k this deck
13
Which of the following processes is not exothermic?
A)Rb+(g)+ e- → Rb(g)
B)Rb+(g)+ Cl-(g)→ RbCl(s)
C)Cl(g)+ e- → Cl-(g)
D)Rb(g)→ Rb(s)
E) Cl2(g)→ Cl(g)
Cl2(g)→ Cl(g)
A)Rb+(g)+ e- → Rb(g)
B)Rb+(g)+ Cl-(g)→ RbCl(s)
C)Cl(g)+ e- → Cl-(g)
D)Rb(g)→ Rb(s)
E)
 Cl2(g)→ Cl(g)
Cl2(g)→ Cl(g)
Unlock Deck
Unlock for access to all 125 flashcards in this deck.
Unlock Deck
k this deck
14
Which of the following is a correct description of lattice energy?
A)The energy change that occurs when electrons are removed from a lattice.
B)The energy change that occurs when a gas condenses to a liquid.
C)The energy change that occurs when a liquid freezes.
D)The energy change that occurs when an ionic solid is separated into its ions in the gas phase.
E)The lattice energy of a substance is identical to the ionic bond energy determined from coulombs law.
A)The energy change that occurs when electrons are removed from a lattice.
B)The energy change that occurs when a gas condenses to a liquid.
C)The energy change that occurs when a liquid freezes.
D)The energy change that occurs when an ionic solid is separated into its ions in the gas phase.
E)The lattice energy of a substance is identical to the ionic bond energy determined from coulombs law.

Unlock Deck
Unlock for access to all 125 flashcards in this deck.
Unlock Deck
k this deck
15
Which of the following compounds would be expected to have the highest melting point?
A)NCl3
B)OCl2
C)MgCl2
D)LiCl
E)CCl4
A)NCl3
B)OCl2
C)MgCl2
D)LiCl
E)CCl4

Unlock Deck
Unlock for access to all 125 flashcards in this deck.
Unlock Deck
k this deck
16
Which of the following is the Lewis dot structure for the potassium ion?
A)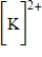
B)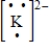
C)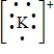
D)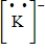
E)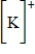
A)
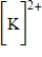
B)
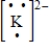
C)
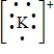
D)
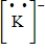
E)
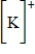

Unlock Deck
Unlock for access to all 125 flashcards in this deck.
Unlock Deck
k this deck
17
Which of the following statements concerning lattice energy is false?
A)MgO has a larger lattice energy than NaF.
B)The lattice energy for a solid with 2+ and 2- ions should be two times that for a solid with 1+ and 1- ions.
C)MgO has a larger lattice energy than LiF.
D)Lattice energy is often defined as the change in energy that occurs when an ionic solid is separated into isolated ions in the gas phase.
E)All of these are true.
A)MgO has a larger lattice energy than NaF.
B)The lattice energy for a solid with 2+ and 2- ions should be two times that for a solid with 1+ and 1- ions.
C)MgO has a larger lattice energy than LiF.
D)Lattice energy is often defined as the change in energy that occurs when an ionic solid is separated into isolated ions in the gas phase.
E)All of these are true.

Unlock Deck
Unlock for access to all 125 flashcards in this deck.
Unlock Deck
k this deck
18
In the Born-Haber cycle for NaCl(s),which of the following processes corresponds to the enthalpy of formation of NaCl(s)?
A)Na(s)+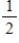 Cl2(g)→ NaCl(s)
Cl2(g)→ NaCl(s)
B)Na+(aq)+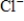 (aq)→ NaCl(s)
(aq)→ NaCl(s)
C)NaCl(g)→ NaCl(s)
D)2Na(g)+ Cl2(g)→ 2NaCl(g)
E)NaCl(aq)→ NaCl(s)
A)Na(s)+
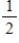 Cl2(g)→ NaCl(s)
Cl2(g)→ NaCl(s)B)Na+(aq)+
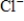 (aq)→ NaCl(s)
(aq)→ NaCl(s)C)NaCl(g)→ NaCl(s)
D)2Na(g)+ Cl2(g)→ 2NaCl(g)
E)NaCl(aq)→ NaCl(s)

Unlock Deck
Unlock for access to all 125 flashcards in this deck.
Unlock Deck
k this deck
19
Which of the following is the Lewis dot structure for one formula unit of calcium sulfide?
A)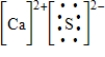
B)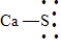
C)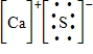
D)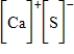
E)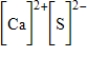
A)
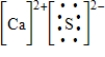
B)
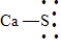
C)
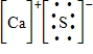
D)
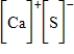
E)
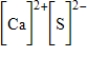

Unlock Deck
Unlock for access to all 125 flashcards in this deck.
Unlock Deck
k this deck
20
All of the following species have ground-state noble-gas electron configurations except
A)Ge4+
B)K+
C)Kr
D)I-
E)P3-
A)Ge4+
B)K+
C)Kr
D)I-
E)P3-

Unlock Deck
Unlock for access to all 125 flashcards in this deck.
Unlock Deck
k this deck
21
What is the ground-state electron configuration of the phosphide ion?
A)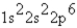
B)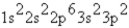
C)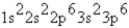
D)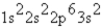
E)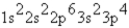
A)
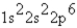
B)
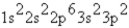
C)
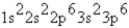
D)
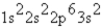
E)
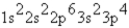

Unlock Deck
Unlock for access to all 125 flashcards in this deck.
Unlock Deck
k this deck
22
The following species, 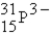 ,
, 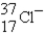
,and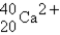
,all have the same number of
A)electrons.
B)nucleons.
C)neutrons.
D)protons.
E)isotopes.
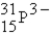 ,
, 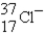
,and
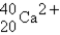
,all have the same number of
A)electrons.
B)nucleons.
C)neutrons.
D)protons.
E)isotopes.

Unlock Deck
Unlock for access to all 125 flashcards in this deck.
Unlock Deck
k this deck
23
What is the electron configuration for ? Mn3+
A)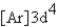
B)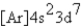
C)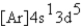
D)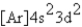
E)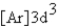
A)
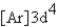
B)
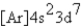
C)
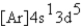
D)
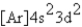
E)
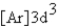

Unlock Deck
Unlock for access to all 125 flashcards in this deck.
Unlock Deck
k this deck
24
Which two species are isoelectronic?
A)Na+ and K+
B)Al3+ and Ne
C)P- and Ca+
D)Cl- and F-
E)Ca2+ and Mg2+
A)Na+ and K+
B)Al3+ and Ne
C)P- and Ca+
D)Cl- and F-
E)Ca2+ and Mg2+

Unlock Deck
Unlock for access to all 125 flashcards in this deck.
Unlock Deck
k this deck
25
What is the electron configuration of Mn2+?
A)[Ar]3d5
B)[Ar]3d34s2
C)[Ar]3d44s1
D)[Ar]3d54s2
E)[Ar]3d54s1
A)[Ar]3d5
B)[Ar]3d34s2
C)[Ar]3d44s1
D)[Ar]3d54s2
E)[Ar]3d54s1

Unlock Deck
Unlock for access to all 125 flashcards in this deck.
Unlock Deck
k this deck
26
All of the following species are isoelectronic except
A)O-
B)Ne
C)N3-
D)Mg2+
E)F-
A)O-
B)Ne
C)N3-
D)Mg2+
E)F-

Unlock Deck
Unlock for access to all 125 flashcards in this deck.
Unlock Deck
k this deck
27
All of the following ions have the ground-state electron configuration of a noble gas except which one?
A)Ca2+
B)Cl-
C)Ga3+
D)Al3+
E)H-
A)Ca2+
B)Cl-
C)Ga3+
D)Al3+
E)H-

Unlock Deck
Unlock for access to all 125 flashcards in this deck.
Unlock Deck
k this deck
28
What is the ground-state electron configuration of Fe3+?
A)[Ar]3d64s2
B)[Ar]3d34s2
C)[Ar]3d44s1
D)[Ar]3d5
E)[Ar]3d6
A)[Ar]3d64s2
B)[Ar]3d34s2
C)[Ar]3d44s1
D)[Ar]3d5
E)[Ar]3d6

Unlock Deck
Unlock for access to all 125 flashcards in this deck.
Unlock Deck
k this deck
29
Which of the following species is isoelectronic with Ar?
A)Na+
B)Ca2+
C)Ga3+
D)O2-
E)Ne
A)Na+
B)Ca2+
C)Ga3+
D)O2-
E)Ne

Unlock Deck
Unlock for access to all 125 flashcards in this deck.
Unlock Deck
k this deck
30
The Cr2+ ion would be expected to have ____ unpaired electrons.
A)4
B)2
C)3
D)0
E)1
A)4
B)2
C)3
D)0
E)1

Unlock Deck
Unlock for access to all 125 flashcards in this deck.
Unlock Deck
k this deck
31
The formation of which monatomic ion of oxygen is the most energetically favorable?
A)O4+
B)O2-
C)O-
D)O6+
E)O2+
A)O4+
B)O2-
C)O-
D)O6+
E)O2+

Unlock Deck
Unlock for access to all 125 flashcards in this deck.
Unlock Deck
k this deck
32
All of the following species are isoelectronic except
A)S2-
B)K+
C)Na+
D)Ar
E)Cl-
A)S2-
B)K+
C)Na+
D)Ar
E)Cl-

Unlock Deck
Unlock for access to all 125 flashcards in this deck.
Unlock Deck
k this deck
33
Which pair of species is isoelectronic?
A)Na+ and K+
B)K+ and Cl-
C)Be2+ and Na+
D)Ne and Ar
E)Li+ and Ne
A)Na+ and K+
B)K+ and Cl-
C)Be2+ and Na+
D)Ne and Ar
E)Li+ and Ne

Unlock Deck
Unlock for access to all 125 flashcards in this deck.
Unlock Deck
k this deck
34
Which set of ions are isoelectronic in their ground-state electron configurations?
A)N,O,F,Ne
B)Na+,K+,Rb+,Cs+
C)F-,Cl-,Br-,I-
D)Mg2+,Ca2+,Sr2+,Ba2+
E)N3-,O2-,Mg2+,Al3+
A)N,O,F,Ne
B)Na+,K+,Rb+,Cs+
C)F-,Cl-,Br-,I-
D)Mg2+,Ca2+,Sr2+,Ba2+
E)N3-,O2-,Mg2+,Al3+

Unlock Deck
Unlock for access to all 125 flashcards in this deck.
Unlock Deck
k this deck
35
What is the ground-state electron configuration of the zinc ion,Zn2+?
A)1s22s22p63s23p63d104s24p2
B)1s22s22p63s23p63d104s2
C)1s22s22p63s23p63d84s2
D)1s22s22p63s23p63d10
E)1s22s22p63s23p64s2
A)1s22s22p63s23p63d104s24p2
B)1s22s22p63s23p63d104s2
C)1s22s22p63s23p63d84s2
D)1s22s22p63s23p63d10
E)1s22s22p63s23p64s2

Unlock Deck
Unlock for access to all 125 flashcards in this deck.
Unlock Deck
k this deck
36
What is the ground-state electron configuration of Co3+?
A)[Ar]3d5
B)[Ar]3d44s2
C)[Ar]3d6
D)[Ar]3d64s1
E)[Ar]3d54s2
A)[Ar]3d5
B)[Ar]3d44s2
C)[Ar]3d6
D)[Ar]3d64s1
E)[Ar]3d54s2

Unlock Deck
Unlock for access to all 125 flashcards in this deck.
Unlock Deck
k this deck
37
All of the following species are isoelectronic except
A)Ar.
B)Ca2+.
C)Mg2+.
D)Cl-.
E)S2-.
A)Ar.
B)Ca2+.
C)Mg2+.
D)Cl-.
E)S2-.

Unlock Deck
Unlock for access to all 125 flashcards in this deck.
Unlock Deck
k this deck
38
What is the ground-state electron configuration of Ni2+?
A)[Ar]3d64s2
B)[Ar]3d74s2
C)[Ar]3d74s1
D)[Ar]3d64s1
E)[Ar]3d8
A)[Ar]3d64s2
B)[Ar]3d74s2
C)[Ar]3d74s1
D)[Ar]3d64s1
E)[Ar]3d8

Unlock Deck
Unlock for access to all 125 flashcards in this deck.
Unlock Deck
k this deck
39
What is the ground-state electron configuration of the Mg2+ ion?
A)1s22s22p63s23p2
B)1s22s22p3
C)1s22s22p6
D)1s22s22p1
E)1s22s22p63s2
A)1s22s22p63s23p2
B)1s22s22p3
C)1s22s22p6
D)1s22s22p1
E)1s22s22p63s2

Unlock Deck
Unlock for access to all 125 flashcards in this deck.
Unlock Deck
k this deck
40
Rank the following ions in order of decreasing atomic radii: Os4+,Os2+,Os5+.
A)Os2+ > Os4+ > Os5+
B)Os5+ > Os4+ > Os2+
C)Os4+ > Os2+ > Os5+
D)Os5+ > Os2+ > Os4+
E)Os2+ > Os5+ > Os4+
A)Os2+ > Os4+ > Os5+
B)Os5+ > Os4+ > Os2+
C)Os4+ > Os2+ > Os5+
D)Os5+ > Os2+ > Os4+
E)Os2+ > Os5+ > Os4+

Unlock Deck
Unlock for access to all 125 flashcards in this deck.
Unlock Deck
k this deck
41
Which of the following atoms is the most electronegative?
A)B
B)N
C)Al
D)Cs
E)Na
A)B
B)N
C)Al
D)Cs
E)Na

Unlock Deck
Unlock for access to all 125 flashcards in this deck.
Unlock Deck
k this deck
42
Which of the following is the best explanation for a covalent bond?
A)electrons simultaneously attracted by more than one nucleus
B)an interaction between outer electrons
C)the overlapping of unoccupied orbitals of two or more atoms
D)the overlapping of two electron-filled orbitals having different energies
E)a positive ion attracting negative ions
A)electrons simultaneously attracted by more than one nucleus
B)an interaction between outer electrons
C)the overlapping of unoccupied orbitals of two or more atoms
D)the overlapping of two electron-filled orbitals having different energies
E)a positive ion attracting negative ions

Unlock Deck
Unlock for access to all 125 flashcards in this deck.
Unlock Deck
k this deck
43
In which of the following lists do the ions not appear in order of increasing ionic radius?
A)S2- < Cl- < K+
B)Na+ < F- < O2-
C)Cl- < Br- < I-
D)Li+ < Na+ < K+
E)Al3+ < Mg2+ < Na+
A)S2- < Cl- < K+
B)Na+ < F- < O2-
C)Cl- < Br- < I-
D)Li+ < Na+ < K+
E)Al3+ < Mg2+ < Na+

Unlock Deck
Unlock for access to all 125 flashcards in this deck.
Unlock Deck
k this deck
44
Rank the following ions in order of decreasing ionic radius: S2-,O2-,F-,Na+,Mg2+.
A)S2-,O2-,F-,Na+,Mg2+
B)O2-,F-,Na+,Mg2+,S2-
C)O2-,S2-,F-,Na+,Mg2+
D)Mg2+,Na+,F-,O2-,S2-
E)Mg2+,S2-,Na+,F-,O2-
A)S2-,O2-,F-,Na+,Mg2+
B)O2-,F-,Na+,Mg2+,S2-
C)O2-,S2-,F-,Na+,Mg2+
D)Mg2+,Na+,F-,O2-,S2-
E)Mg2+,S2-,Na+,F-,O2-

Unlock Deck
Unlock for access to all 125 flashcards in this deck.
Unlock Deck
k this deck
45
In which of the following species is there the greatest unequal sharing of the bonding electrons?
A)SO3
B)SO32-
C)H2S
D)H2O
E)NH4+
A)SO3
B)SO32-
C)H2S
D)H2O
E)NH4+

Unlock Deck
Unlock for access to all 125 flashcards in this deck.
Unlock Deck
k this deck
46
Which pair of elements would form a covalent bond that is the least polar?
A)S and Li
B)Al and N
C)O and H
D)O and F
E)S and Cs
A)S and Li
B)Al and N
C)O and H
D)O and F
E)S and Cs

Unlock Deck
Unlock for access to all 125 flashcards in this deck.
Unlock Deck
k this deck
47
Which of the following bonds would be the least polar yet still be considered polar covalent?
A)Mg-O
B)C-O
C)Si-O
D)O-O
E)N-O
A)Mg-O
B)C-O
C)Si-O
D)O-O
E)N-O

Unlock Deck
Unlock for access to all 125 flashcards in this deck.
Unlock Deck
k this deck
48
The measure of the attraction that an atom has for the electrons in a chemical bond is called
A)electronegativity.
B)lattice energy.
C)resonance energy.
D)ionization energy.
E)electron affinity.
A)electronegativity.
B)lattice energy.
C)resonance energy.
D)ionization energy.
E)electron affinity.

Unlock Deck
Unlock for access to all 125 flashcards in this deck.
Unlock Deck
k this deck
49
For which of the following pairs of species is the difference in radius the greatest?
A)C and F
B)K+ and Br-
C)Li+ and I-
D)Na and Mg
E)O2- and F-
A)C and F
B)K+ and Br-
C)Li+ and I-
D)Na and Mg
E)O2- and F-

Unlock Deck
Unlock for access to all 125 flashcards in this deck.
Unlock Deck
k this deck
50
Rank the following covalent bonds in order of decreasing polarity: C-H,N-H,O-H,F-H.
A)F-H,O-H,N-H,C-H
B)O-H,F-H,N-H,C-H
C)N-H,F-H,O-H,C-H
D)C-H,N-H,O-H,F-H
E)C-H,F-H,O-H,N-H
A)F-H,O-H,N-H,C-H
B)O-H,F-H,N-H,C-H
C)N-H,F-H,O-H,C-H
D)C-H,N-H,O-H,F-H
E)C-H,F-H,O-H,N-H

Unlock Deck
Unlock for access to all 125 flashcards in this deck.
Unlock Deck
k this deck
51
Rank the following ions in order of decreasing atomic radii: Te2-,Te4+,Te6+.
A)Te2- > Te4+ > Te6+
B)Te6+ > Te4+ > Te2-
C)Te4+ > Te2- > Te6+
D)Te2- > Te6+ > Te4+
E)Te4+ > Te6+ > Te2-
A)Te2- > Te4+ > Te6+
B)Te6+ > Te4+ > Te2-
C)Te4+ > Te2- > Te6+
D)Te2- > Te6+ > Te4+
E)Te4+ > Te6+ > Te2-

Unlock Deck
Unlock for access to all 125 flashcards in this deck.
Unlock Deck
k this deck
52
An atom of which of the following elements has the highest electronegativity?
A)K
B)As
C)Ba
D)Si
E)Br
A)K
B)As
C)Ba
D)Si
E)Br

Unlock Deck
Unlock for access to all 125 flashcards in this deck.
Unlock Deck
k this deck
53
During the formation of a chemical bond between two hydrogen atoms,which of the following statements is always true?
A)Energy is released during the formation of the bond.
B)A polar covalent bond is formed.
C)Electrons always are between the nuclei of the atoms.
D)One of the hydrogen atoms is ionized.
E)Resonance stabilizes the bond.
A)Energy is released during the formation of the bond.
B)A polar covalent bond is formed.
C)Electrons always are between the nuclei of the atoms.
D)One of the hydrogen atoms is ionized.
E)Resonance stabilizes the bond.

Unlock Deck
Unlock for access to all 125 flashcards in this deck.
Unlock Deck
k this deck
54
Rank the following species in order of decreasing radii: Al3+,Mg2+,Al3+,Al3+.
A)Al3+ > Al3+ > Mg2+ > Al
B)Al3+ > Al3+ > Mg2+ > Al3+
C)Al3+> Mg2+ > Al3+ > Al3+
D)Al3+ > Mg2+ > Al3+ > Al3+
E)Mg2+ > Al3+ > Al3+ > Al3+
A)Al3+ > Al3+ > Mg2+ > Al
B)Al3+ > Al3+ > Mg2+ > Al3+
C)Al3+> Mg2+ > Al3+ > Al3+
D)Al3+ > Mg2+ > Al3+ > Al3+
E)Mg2+ > Al3+ > Al3+ > Al3+

Unlock Deck
Unlock for access to all 125 flashcards in this deck.
Unlock Deck
k this deck
55
A bond in which an electron pair is unequally shared by two atoms is
A)polar covalent.
B)coordinate covalent.
C)ionic.
D)nonpolar covalent.
E)metallic.
A)polar covalent.
B)coordinate covalent.
C)ionic.
D)nonpolar covalent.
E)metallic.

Unlock Deck
Unlock for access to all 125 flashcards in this deck.
Unlock Deck
k this deck
56
The Lewis formula for phosphine,PH3,has
A)four lone pairs.
B)four bonding pairs.
C)two bonding pairs and two lone pairs.
D)three bonding pairs and one lone pair.
E)one bonding pair and three lone pairs.
A)four lone pairs.
B)four bonding pairs.
C)two bonding pairs and two lone pairs.
D)three bonding pairs and one lone pair.
E)one bonding pair and three lone pairs.

Unlock Deck
Unlock for access to all 125 flashcards in this deck.
Unlock Deck
k this deck
57
Which of the following species would you expect to have the largest radius?
A)S2-
B)P
C)Na+
D)Se2-
E)Ca2+
A)S2-
B)P
C)Na+
D)Se2-
E)Ca2+

Unlock Deck
Unlock for access to all 125 flashcards in this deck.
Unlock Deck
k this deck
58
The formulas of many binary covalent compounds can be predicted on the basis
A)that a bond is formed by the overlapping of two filled orbitals.
B)that the number of bonds an atom can have is equal to the number of empty valence orbitals it has.
C)that a bond is formed by the overlapping of atomic orbitals.
D)that the number of bonds an atom can have is equal to the number of half-filled valence orbitals it can have.
E)that bonding electrons are simultaneously attracted by more than one nucleus.
A)that a bond is formed by the overlapping of two filled orbitals.
B)that the number of bonds an atom can have is equal to the number of empty valence orbitals it has.
C)that a bond is formed by the overlapping of atomic orbitals.
D)that the number of bonds an atom can have is equal to the number of half-filled valence orbitals it can have.
E)that bonding electrons are simultaneously attracted by more than one nucleus.

Unlock Deck
Unlock for access to all 125 flashcards in this deck.
Unlock Deck
k this deck
59
A bond in which both electrons of the bond are donated by one atom is called ____.
A)a coordinate covalent bond
B)a polar covalent bond
C)an ionic bond
D)a double bond
E)a triple bond
A)a coordinate covalent bond
B)a polar covalent bond
C)an ionic bond
D)a double bond
E)a triple bond

Unlock Deck
Unlock for access to all 125 flashcards in this deck.
Unlock Deck
k this deck
60
What is the total number of valence electrons in N2O?
A)16
B)17
C)34
D)11
E)22
A)16
B)17
C)34
D)11
E)22

Unlock Deck
Unlock for access to all 125 flashcards in this deck.
Unlock Deck
k this deck
61
How many valence electrons are present in the Lewis formula for the chlorate ion,C1O3-?
A)32
B)24
C)30
D)26
E)28
A)32
B)24
C)30
D)26
E)28

Unlock Deck
Unlock for access to all 125 flashcards in this deck.
Unlock Deck
k this deck
62
What is the total number of valence electrons in the monohydrogen phosphate ion,HPO42-?
A)30
B)28
C)32
D)34
E)36
A)30
B)28
C)32
D)34
E)36

Unlock Deck
Unlock for access to all 125 flashcards in this deck.
Unlock Deck
k this deck
63
How many valence electrons does a carbonate ion have?
A)30
B)28
C)24
D)32
E)22
A)30
B)28
C)24
D)32
E)22

Unlock Deck
Unlock for access to all 125 flashcards in this deck.
Unlock Deck
k this deck
64
In the Lewis formula for difluorodiazine,N2F2,the total number of lone electron pairs around the two nitrogen atoms is
A)4.
B)0.
C)3.
D)1.
E)2.
A)4.
B)0.
C)3.
D)1.
E)2.

Unlock Deck
Unlock for access to all 125 flashcards in this deck.
Unlock Deck
k this deck
65
Which of the following is a correct Lewis electron-dot formula for CO?
A)
B)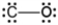
C)
D)
E)
A)

B)
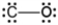
C)

D)

E)


Unlock Deck
Unlock for access to all 125 flashcards in this deck.
Unlock Deck
k this deck
66
What is the total number of valence electrons in the ammonium ion,NH4+ ?
A)9
B)11
C)8
D)10
E)12
A)9
B)11
C)8
D)10
E)12

Unlock Deck
Unlock for access to all 125 flashcards in this deck.
Unlock Deck
k this deck
67
What is the total number of valence electrons in the cyanate ion,CNO- ion,?
A)20
B)12
C)16
D)22
E)18
A)20
B)12
C)16
D)22
E)18

Unlock Deck
Unlock for access to all 125 flashcards in this deck.
Unlock Deck
k this deck
68
How many valence electrons are there in the tetramethylammonium ion,(CH3)4N+?
A)32
B)18
C)8
D)33
E)24
A)32
B)18
C)8
D)33
E)24

Unlock Deck
Unlock for access to all 125 flashcards in this deck.
Unlock Deck
k this deck
69
The Lewis structure for each of the following except ____contains at least one double bond.
A)O2
B)CS2
C)C2H4
D)NO+
E)N2H2
A)O2
B)CS2
C)C2H4
D)NO+
E)N2H2

Unlock Deck
Unlock for access to all 125 flashcards in this deck.
Unlock Deck
k this deck
70
Which of the following Lewis formulas is incorrect?
A)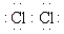
B)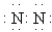
C)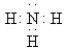
D)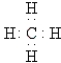
E)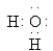
A)
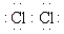
B)
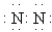
C)
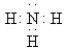
D)
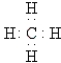
E)

Unlock Deck
Unlock for access to all 125 flashcards in this deck.
Unlock Deck
k this deck
71
The total number of valence electrons in a peroxide ion,O22-,is
A)2.
B)12.
C)14.
D)13.
E)15.
A)2.
B)12.
C)14.
D)13.
E)15.

Unlock Deck
Unlock for access to all 125 flashcards in this deck.
Unlock Deck
k this deck
72
The number of valence electrons in the nitrite ion is
A)22.
B)16.
C)23.
D)18.
E)24.
A)22.
B)16.
C)23.
D)18.
E)24.

Unlock Deck
Unlock for access to all 125 flashcards in this deck.
Unlock Deck
k this deck
73
Which of the following is a correct Lewis electron-dot formula for H2SO4?
A)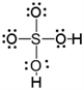
B)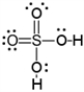
C)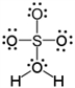
D)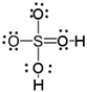
E)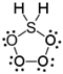
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 125 flashcards in this deck.
Unlock Deck
k this deck
74
The Lewis structure for each of the following species except ____ contains a triple bond.
A)N3-
B)N2
C)HCCH
D)NO+
E)O22+
A)N3-
B)N2
C)HCCH
D)NO+
E)O22+

Unlock Deck
Unlock for access to all 125 flashcards in this deck.
Unlock Deck
k this deck
75
Which one of the following has a Lewis formula most similar to that of NO-?
A)O2
B)O22-
C)O2-
D)NO+
E)NO
A)O2
B)O22-
C)O2-
D)NO+
E)NO

Unlock Deck
Unlock for access to all 125 flashcards in this deck.
Unlock Deck
k this deck
76
What is the total number of valence electrons in the sulfite ion?
A)30
B)26
C)24
D)8
E)32
A)30
B)26
C)24
D)8
E)32

Unlock Deck
Unlock for access to all 125 flashcards in this deck.
Unlock Deck
k this deck
77
How many valence electrons are there in the trifluoroacetate ion,CF3COO-?
A)41
B)42
C)54
D)56
E)40
A)41
B)42
C)54
D)56
E)40

Unlock Deck
Unlock for access to all 125 flashcards in this deck.
Unlock Deck
k this deck
78
The number of valence electrons in the propionate ion,CH3CH2COO-,is
A)30.
B)32.
C)36.
D)50.
E)28.
A)30.
B)32.
C)36.
D)50.
E)28.

Unlock Deck
Unlock for access to all 125 flashcards in this deck.
Unlock Deck
k this deck
79
The total number of valence electrons in the tetrathionate ion,S4O62-,is
A)58.
B)60.
C)56.
D)54.
E)62.
A)58.
B)60.
C)56.
D)54.
E)62.

Unlock Deck
Unlock for access to all 125 flashcards in this deck.
Unlock Deck
k this deck
80
The total number of valence electrons in the phosphate ion is
A)32.
B)30.
C)24.
D)28.
E)26.
A)32.
B)30.
C)24.
D)28.
E)26.

Unlock Deck
Unlock for access to all 125 flashcards in this deck.
Unlock Deck
k this deck


