Deck 18: Thermodynamics and Equilibrium
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/94
Play
Full screen (f)
Deck 18: Thermodynamics and Equilibrium
1
What is the change in internal energy of the system (ΔU)if 82 kJ of heat energy is absorbed by the system and 40 kJ of work is done on the system for a certain process?
A)122 kJ
B)42 kJ
C)82 kJ
D)-122 kJ
E)-42 kJ
A)122 kJ
B)42 kJ
C)82 kJ
D)-122 kJ
E)-42 kJ
122 kJ
2
The enthalpy of vaporization (ΔH°vap)of benzene is 30.7 kJ/mol at its normal boiling point of 353.3 K.What is ΔS°vap at this temperature?
A)86.9 J/(mol·K)
B)0.087 J/(mol·K)
C)11.5 J/(mol·K)
D)0.0115 J/(mol·K)
E)383 J/(mol·K)
A)86.9 J/(mol·K)
B)0.087 J/(mol·K)
C)11.5 J/(mol·K)
D)0.0115 J/(mol·K)
E)383 J/(mol·K)
86.9 J/(mol·K)
3
Which of the following is true for the condensation of a gaseous substance?
A)ΔS = 0 and ΔH = 0.
B)ΔS > 0 and ΔH > 0.
C)ΔS < 0 and ΔH > 0.
D)ΔS < 0 and ΔH < 0.
E)ΔS > 0 and ΔH < 0.
A)ΔS = 0 and ΔH = 0.
B)ΔS > 0 and ΔH > 0.
C)ΔS < 0 and ΔH > 0.
D)ΔS < 0 and ΔH < 0.
E)ΔS > 0 and ΔH < 0.
ΔS < 0 and ΔH < 0.
4
The standard enthalpy of vaporization of Freon-11,CFCl3,is 25.21 kJ/mol at its normal boiling point of 17°C.What is the change of entropy for 1 mol of liquid Freon-11 when it vaporizes at its normal boiling point?
A)8.69 × 10-2 J/K
B)7.31 × 103 J/K
C)86.9 J/K
D)1.48J/K
E)1.48× 103 J/K
A)8.69 × 10-2 J/K
B)7.31 × 103 J/K
C)86.9 J/K
D)1.48J/K
E)1.48× 103 J/K

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
5
The standard enthalpy of fusion of iodobenzene is 9.75 kJ/mol at its melting point,241.8 K.What is the standard change in entropy for the melting of iodobenzene at its melting point?
A)0.0189 J/(mol ∙ K)
B)40.3 J/(mol ∙ K)
C)9.75 J/(mol ∙ K)
D)311 J/(mol ∙ K)
E)0.0403 J/(mol ∙ K)
A)0.0189 J/(mol ∙ K)
B)40.3 J/(mol ∙ K)
C)9.75 J/(mol ∙ K)
D)311 J/(mol ∙ K)
E)0.0403 J/(mol ∙ K)

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
6
The total entropy of a system and its surroundings always increases for a spontaneous process.This is a statement of
A)the third law of thermodynamics.
B)the law of constant composition.
C)the second law of thermodynamics.
D)the law of conservation of matter.
E)the first law of thermodynamics.
A)the third law of thermodynamics.
B)the law of constant composition.
C)the second law of thermodynamics.
D)the law of conservation of matter.
E)the first law of thermodynamics.

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
7
Assuming ΔH and ΔS are constant with respect to temperature,under what conditions will a chemical reaction be spontaneous at all temperatures?
A)ΔH is negative,and ΔS is positive.
B)ΔH is positive,and ΔS is negative.
C)ΔS = 0,and ΔH is positive.
D)ΔH = 0,and ΔS is negative.
E)none of these
A)ΔH is negative,and ΔS is positive.
B)ΔH is positive,and ΔS is negative.
C)ΔS = 0,and ΔH is positive.
D)ΔH = 0,and ΔS is negative.
E)none of these

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
8
For the isothermal (constant-temperature)expansion of an ideal gas,
A)w > 0 and q < 0.
B)w = 0 and q > 0.
C)w < 0 and q = 0.
D)w < 0 and q > 0.
E)w > 0 and q > 0.
A)w > 0 and q < 0.
B)w = 0 and q > 0.
C)w < 0 and q = 0.
D)w < 0 and q > 0.
E)w > 0 and q > 0.

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
9
Which of the following compounds has the highest standard entropy per mole at 298 K?
A)H2O(l)
B)CaCO3(s)
C)CO(g)
D)SiO2(s)
E)CH3OH(l)
A)H2O(l)
B)CaCO3(s)
C)CO(g)
D)SiO2(s)
E)CH3OH(l)

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
10
In which of the following scenarios is no change in the internal energy of the system possible?
A)q < 0,w > 0
B)q > 0,w > 0
C)q = 0,w > 0
D)q < 0,w = 0
E)q < 0,w < 0
A)q < 0,w > 0
B)q > 0,w > 0
C)q = 0,w > 0
D)q < 0,w = 0
E)q < 0,w < 0

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
11
A gas absorbs 0.0 J of heat and then performs 99.5 J of work.What is the change in internal energy of the gas?
A)-99.5 J
B)59.5 J
C)139.5 J
D)99.1 J
E)none of these
A)-99.5 J
B)59.5 J
C)139.5 J
D)99.1 J
E)none of these

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
12
For a particular process,q = 20 kJ and w = 15 kJ.Which of the following statements is true?
A)ΔU = 35 kJ.
B)The system does work on the surroundings.
C)Heat flows from the system to the surroundings.
D)All of the above are true.
E)None of the above are true.
A)ΔU = 35 kJ.
B)The system does work on the surroundings.
C)Heat flows from the system to the surroundings.
D)All of the above are true.
E)None of the above are true.

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
13
Which of the following is not a state function?
A)w
B)H
C)P
D)U
E)T
A)w
B)H
C)P
D)U
E)T

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
14
The standard enthalpy of vaporization of methanol is 35.2 kJ/mol at its normal boiling point,64.6°C.What is the standard change in entropy for the vaporization of methanol at its normal boiling point?
A)104 J/(mol ∙ K)
B)35.2 J/(mol ∙ K)
C)0.544 J/(mol ∙ K)
D)0.104 J/(mol ∙ K)
E)544 J/(mol ∙ K)
A)104 J/(mol ∙ K)
B)35.2 J/(mol ∙ K)
C)0.544 J/(mol ∙ K)
D)0.104 J/(mol ∙ K)
E)544 J/(mol ∙ K)

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
15
A system under constant external pressure undergoes a decrease in volume.What is the effect on the surroundings?
A)Energy is transferred as pressure-volume work done by the surroundings on the system.
B)Energy is transferred as pressure-volume work done on the surroundings.
C)Energy is transferred as heat from the system to the surroundings.
D)Energy is transferred as heat from the surroundings to the system.
E)None of the above.
A)Energy is transferred as pressure-volume work done by the surroundings on the system.
B)Energy is transferred as pressure-volume work done on the surroundings.
C)Energy is transferred as heat from the system to the surroundings.
D)Energy is transferred as heat from the surroundings to the system.
E)None of the above.

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
16
According to the first law of thermodynamics,the energy of the universe is constant.Does this mean that ΔE is always equal to zero?
A)No,ΔE does not always equal zero,but this is due only to factors such as friction and heat.
B)No,ΔE never equals zero because energy is always flowing between the system and the surroundings.
C)No,ΔE does not always equal zero because it refers to the system's internal energy,which is affected by heat and work.
D)Yes,ΔE = 0 at all times,which is why q = -w.
E)No,ΔE never equals zero because work is always being done on the system or by the system.
A)No,ΔE does not always equal zero,but this is due only to factors such as friction and heat.
B)No,ΔE never equals zero because energy is always flowing between the system and the surroundings.
C)No,ΔE does not always equal zero because it refers to the system's internal energy,which is affected by heat and work.
D)Yes,ΔE = 0 at all times,which is why q = -w.
E)No,ΔE never equals zero because work is always being done on the system or by the system.

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
17
Which of the following has the lowest entropy per mole?
A)liquid sodium at 100°C
B)gaseous sodium at 900°C and 0.5 atm
C)a solid solution of sodium in potassium at 30°C
D)gaseous sodium at 900°C and 1 atm
E)solid sodium at 30°C
A)liquid sodium at 100°C
B)gaseous sodium at 900°C and 0.5 atm
C)a solid solution of sodium in potassium at 30°C
D)gaseous sodium at 900°C and 1 atm
E)solid sodium at 30°C

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
18
At the normal boiling point of benzene,ΔH°vap = 30.7 kJ/mol and ΔS°vap = 86.9 J/(mol ∙ K).What is the normal boiling point of benzene?
A)267 K
B)373 K
C)115 K
D)869 K
E)353 K
A)267 K
B)373 K
C)115 K
D)869 K
E)353 K

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
19
ΔH and ΔU are nearly the same in all the following processes except
A)F2(g)+ H2(g)→ 2HF(g).
B)CH4(g)+ Cl2(g)→ CH3Cl(g)+ HCl(g).
C)C6H6(s)→ C6H6(l).
D)CuO(s)+ H2(g)→ Cu(s)+ H2O(g).
E)3O2(g)→ 2O3(g).
A)F2(g)+ H2(g)→ 2HF(g).
B)CH4(g)+ Cl2(g)→ CH3Cl(g)+ HCl(g).
C)C6H6(s)→ C6H6(l).
D)CuO(s)+ H2(g)→ Cu(s)+ H2O(g).
E)3O2(g)→ 2O3(g).

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
20
What is the change in internal energy (ΔU)of the system if q = -8 kJ and w = -1 kJ for a certain process?
A)-9 kJ
B)-7 kJ
C)7 kJ
D)9 kJ
E)-8 kJ
A)-9 kJ
B)-7 kJ
C)7 kJ
D)9 kJ
E)-8 kJ

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
21
For which of the following reactions is ΔS° at 25°C closest to zero?
A)N2(g)+ O2(g)→ 2NO(g)
B)H2(g)+ I2(s)→ 2HI(g)
C)CH3CHO(g)+ 5/2O2(g)→ 2CO2(g)+ 2H2O(g)
D)2NO(g)+ O2(g)→ 2NO2(g)
E)C2H4(g)+ Br2(l)→ C2H4Br2(l)
A)N2(g)+ O2(g)→ 2NO(g)
B)H2(g)+ I2(s)→ 2HI(g)
C)CH3CHO(g)+ 5/2O2(g)→ 2CO2(g)+ 2H2O(g)
D)2NO(g)+ O2(g)→ 2NO2(g)
E)C2H4(g)+ Br2(l)→ C2H4Br2(l)

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
22
For the reaction N2(g)→ 2N(g),
A)ΔH < 0 and ΔS < 0.
B)ΔH > 0 and ΔS < 0.
C)ΔH < 0 and ΔS > 0.
D)ΔH = 0 and ΔS > 0.
E)ΔH > 0 and ΔS > 0.
A)ΔH < 0 and ΔS < 0.
B)ΔH > 0 and ΔS < 0.
C)ΔH < 0 and ΔS > 0.
D)ΔH = 0 and ΔS > 0.
E)ΔH > 0 and ΔS > 0.

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
23
For the process Cl2(g)→ 2Cl(g),
A)ΔH is + and ΔS is + for the reaction.
B)ΔH is + and ΔS = 0 for the reaction.
C)ΔH is - and ΔS is - for the reaction.
D)ΔH is - and ΔS is + for the reaction.
E)ΔH is + and ΔS is - for the reaction.
A)ΔH is + and ΔS is + for the reaction.
B)ΔH is + and ΔS = 0 for the reaction.
C)ΔH is - and ΔS is - for the reaction.
D)ΔH is - and ΔS is + for the reaction.
E)ΔH is + and ΔS is - for the reaction.

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
24
For which of the following reactions is ΔS° > 0 at 25°C?
A)2H2(g)+ O2(g)→ 2H2O(g)
B)2ClBr(g)→ Cl2(g)+ Br2(g)
C)I2(g)→ I2(s)
D)2NO(g)+ O2(g)→ 2NO2(g)
E)NH4HS(s)→ NH3(g)+ H2S(g)
A)2H2(g)+ O2(g)→ 2H2O(g)
B)2ClBr(g)→ Cl2(g)+ Br2(g)
C)I2(g)→ I2(s)
D)2NO(g)+ O2(g)→ 2NO2(g)
E)NH4HS(s)→ NH3(g)+ H2S(g)

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
25
What is the thermodynamic quantity that provides the criterion for the spontaneity of a chemical reaction?
A)TΔS
B)ΔU
C)ΔS
D)ΔG
E)ΔH
A)TΔS
B)ΔU
C)ΔS
D)ΔG
E)ΔH

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
26
The third law of thermodynamics states that
A)the entropy of the universe is increasing.
B)the entropy of the universe is constant.
C)the entropy of the universe equals the sum of the entropy of system and that of the surroundings.
D)the absolute entropy of a substance decreases with increasing temperature.
E)the entropy is zero at 0 K for a perfect crystal.
A)the entropy of the universe is increasing.
B)the entropy of the universe is constant.
C)the entropy of the universe equals the sum of the entropy of system and that of the surroundings.
D)the absolute entropy of a substance decreases with increasing temperature.
E)the entropy is zero at 0 K for a perfect crystal.

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
27
Which of the following equations is correct?
A)G = S - TH
B)G = H - PV
C)G = H - TS
D)ΔG = Ginitial - Gfinal
E)G = S - PV
A)G = S - TH
B)G = H - PV
C)G = H - TS
D)ΔG = Ginitial - Gfinal
E)G = S - PV

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
28
What is the change in entropy when 0.802 g of silicon is burned in excess oxygen to yield silicon dioxide at 298 K?
Si(s)+ O2(g)→ SiO2(s); ΔS° = -182.4 J/K at 298 K
A)182J/K
B)146 J/K
C)-1.75 × 10-2 J/K
D)-6.39 × 103 J/K
E)5.2 J/K
Si(s)+ O2(g)→ SiO2(s); ΔS° = -182.4 J/K at 298 K
A)182J/K
B)146 J/K
C)-1.75 × 10-2 J/K
D)-6.39 × 103 J/K
E)5.2 J/K

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
29
In which reaction is ΔS° expected to be negative?
A)2C2H6(g)+ 7O2(g)→ 4CO2(g)+ 6H2O(l)
B)Ga(l)→ Ga(s)
C)H2O(l)+ 2SO2(g)→ H2SO4(l)
D)CO2(g)→ CO2(s)
E)all of above
A)2C2H6(g)+ 7O2(g)→ 4CO2(g)+ 6H2O(l)
B)Ga(l)→ Ga(s)
C)H2O(l)+ 2SO2(g)→ H2SO4(l)
D)CO2(g)→ CO2(s)
E)all of above

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
30
Given the following,determine S° at 298 K for one mole of NO(g).
2NO(g)+ O2(g)→ 2NO2(g); ΔS° = −146.7 J/K at 298K

A)210.9 J/K
B)−90.85 J/K
C)421.7 J/K
D)-421.7 J/K
E)+90.85 J/K
2NO(g)+ O2(g)→ 2NO2(g); ΔS° = −146.7 J/K at 298K

A)210.9 J/K
B)−90.85 J/K
C)421.7 J/K
D)-421.7 J/K
E)+90.85 J/K

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
31
Which of the following reactions has the smallest value of ΔS° at 25°C?
A)C6H6(l)+ 9/2O2(g)→ 6CO(g)+ 3H2O(g)
B)C6H6(s)→ C6H6(l)
C)C6H6(l)+ Br2(l)→ C6H5Br(l)+ HBr(g)
D)C6H6(s)→ C6H6(g)
E)C6H6(l)+ 15/2O2(g)→ 6CO2(g)+ 3H2O(g)
A)C6H6(l)+ 9/2O2(g)→ 6CO(g)+ 3H2O(g)
B)C6H6(s)→ C6H6(l)
C)C6H6(l)+ Br2(l)→ C6H5Br(l)+ HBr(g)
D)C6H6(s)→ C6H6(g)
E)C6H6(l)+ 15/2O2(g)→ 6CO2(g)+ 3H2O(g)

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
32
The free-energy change of a reaction is a measure of
A)the excess entropy given off to the reaction system.
B)the increased molecular disorder that occurs in the system.
C)the energy given off to the surroundings.
D)the direction in which a net reaction occurs.
E)the excess entropy given off to the surroundings.
A)the excess entropy given off to the reaction system.
B)the increased molecular disorder that occurs in the system.
C)the energy given off to the surroundings.
D)the direction in which a net reaction occurs.
E)the excess entropy given off to the surroundings.

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
33
For which of the following reactions is ΔS° < 0 at 25°C?
A)2KClO3(s)→ 2KCl(s)+ 3O2(g)
B)2HgO(s)→ 2Hg(l)+ O2(g)
C)Br2(l)→ Br2(g)
D)P4(s)+ 5O2(g)→ P4O10(s)
E)(NH4)2Cr2O7(s)→ N2(g)+ 4H2O(l)+ Cr2O3(s)
A)2KClO3(s)→ 2KCl(s)+ 3O2(g)
B)2HgO(s)→ 2Hg(l)+ O2(g)
C)Br2(l)→ Br2(g)
D)P4(s)+ 5O2(g)→ P4O10(s)
E)(NH4)2Cr2O7(s)→ N2(g)+ 4H2O(l)+ Cr2O3(s)

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
34
What is the change in entropy when 7.61 mL of liquid benzene (C6H6,d = 0.879 g/mL)is combusted in the presence of 22.3 L of oxygen gas,measured at 298 K and 1 atm pressure? (R = 0.0821 L · atm/(K · mol))
2C6H6(l)+ 15O2(g)→ 12CO2(g)+ 6H2O(l); ΔS° = -437.7 J/K at 298 K
A)398 J/K
B)45.3 J/K
C)37.4 J/K
D)18.7 J/K
E)436 J/K
2C6H6(l)+ 15O2(g)→ 12CO2(g)+ 6H2O(l); ΔS° = -437.7 J/K at 298 K
A)398 J/K
B)45.3 J/K
C)37.4 J/K
D)18.7 J/K
E)436 J/K

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
35
What is the change in entropy when 0.517 g of water decomposes to form hydrogen gas and oxygen gas at 298 K?
2H2O(l)→ 2H2(g)+ O2(g); ΔS° = 326.3 J/K at 298 K
A)4.68 J/K
B)0.0628 J/K
C)18.7 J/K
D)9.36 J/K
E)168 J/K
2H2O(l)→ 2H2(g)+ O2(g); ΔS° = 326.3 J/K at 298 K
A)4.68 J/K
B)0.0628 J/K
C)18.7 J/K
D)9.36 J/K
E)168 J/K

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
36
Which of the following is the best criterion for determining the spontaneity of a chemical reaction?
A)ΔH
B)ΔH°
C)ΔG
D)ΔG°
E)TΔS
A)ΔH
B)ΔH°
C)ΔG
D)ΔG°
E)TΔS

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
37
Which of the following is not a spontaneous process at 25°C and 1 atm pressure?
A)salt dissolving
B)ice melting
C)water boiling
D)iron rusting
E)steam condensing
A)salt dissolving
B)ice melting
C)water boiling
D)iron rusting
E)steam condensing

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
38
For which of the following reactions is ΔS° > 0 at 25°C?
A)MgO(s)+ CO2(g)→ MgCO3(s)
B)2H2(g)+ O2(l)→ 2H2O(l)
C)2Li(s)+ O2(g)→ Li2O(s)
D)2CO(g)+ O2(g)→ 2CO2(g)
E)F3BNH3(s)→ BF3(g)+ NH3(g)
A)MgO(s)+ CO2(g)→ MgCO3(s)
B)2H2(g)+ O2(l)→ 2H2O(l)
C)2Li(s)+ O2(g)→ Li2O(s)
D)2CO(g)+ O2(g)→ 2CO2(g)
E)F3BNH3(s)→ BF3(g)+ NH3(g)

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
39
For which of the following processes would ΔS° be expected to be most positive?
A)H2O(l)→ H2O(s)
B)NH3(g)+ HCl(g)→ NH4Cl(s)
C)O2(g)+ 2H2(g)→ 2H2O(g)
D)N2O4(g)→ 2NO2(g)
E)2NH4NO3(s)→ 2N2(g)+ O2(g)+ 4H2O(g)
A)H2O(l)→ H2O(s)
B)NH3(g)+ HCl(g)→ NH4Cl(s)
C)O2(g)+ 2H2(g)→ 2H2O(g)
D)N2O4(g)→ 2NO2(g)
E)2NH4NO3(s)→ 2N2(g)+ O2(g)+ 4H2O(g)

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
40
Which of the following reactions has the largest positive value of ΔS° per mole of O2 at 25°C?
A)2H2(g)+ O2(g)→ 2H2O(g)
B)2C(s)+ O2(g)→ 2CO(g)
C)2Mg(s)+ O2(g)→ 2MgO(s)
D)C(s)+ O2(g)→ CO2(g)
E)2NO(g)+ O2(g)→ 2NO2(g)
A)2H2(g)+ O2(g)→ 2H2O(g)
B)2C(s)+ O2(g)→ 2CO(g)
C)2Mg(s)+ O2(g)→ 2MgO(s)
D)C(s)+ O2(g)→ CO2(g)
E)2NO(g)+ O2(g)→ 2NO2(g)

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
41
Based on the following data,what is the standard Gibbs free energy of formation of the sulfate ion at 298 K? (R = 8.31 J/(K ∙ mol))
PbSO4(s) Pb2+(aq)+ SO42-(aq); Ksp = 1.7 × 10-8
Pb2+(aq)+ SO42-(aq); Ksp = 1.7 × 10-8
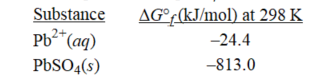
A)-788.6 kJ/mol
B)-793.1 kJ/mol
C)-837.4 kJ/mol
D)-744.3 kJ/mol
E)832.9 kJ/mol
PbSO4(s)
 Pb2+(aq)+ SO42-(aq); Ksp = 1.7 × 10-8
Pb2+(aq)+ SO42-(aq); Ksp = 1.7 × 10-8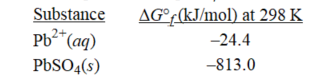
A)-788.6 kJ/mol
B)-793.1 kJ/mol
C)-837.4 kJ/mol
D)-744.3 kJ/mol
E)832.9 kJ/mol

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
42
Consider the following reaction:
CaO(s)+ CO2(g)→ CaCO3(s); ΔG° = -130.9 kJ at 298 K
At what partial pressure of CO2(g)will the reaction no longer be spontaneous at 298 K? (R = 0.0821 L ∙ atm/(K ∙ mol)= 8.31 J/(K ∙ mol))
A)1.59 × 108 atm
B)1.00 atm
C)6.28 × 10-9 atm
D)8.77 × 1022 atm
E)1.14 × 10-23 atm
CaO(s)+ CO2(g)→ CaCO3(s); ΔG° = -130.9 kJ at 298 K
At what partial pressure of CO2(g)will the reaction no longer be spontaneous at 298 K? (R = 0.0821 L ∙ atm/(K ∙ mol)= 8.31 J/(K ∙ mol))
A)1.59 × 108 atm
B)1.00 atm
C)6.28 × 10-9 atm
D)8.77 × 1022 atm
E)1.14 × 10-23 atm

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
43
For a reaction system that is at equilibrium,which of the following must always be true?
A)ΔH = 0
B)ΔS = 0
C)ΔG = 0
D)q = 0
E)ΔU = 0
A)ΔH = 0
B)ΔS = 0
C)ΔG = 0
D)q = 0
E)ΔU = 0

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
44
Given the following,determine ΔG°f at 298 K for SnO.
Sn(s)+ SnO2(s)→ 2SnO(s); ΔG° = 12.0 kJ at 298K
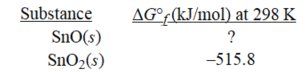
A)-251.9 kJ/mol
B)-503.8 kJ/mol
C)527.8 kJ/mol
D)263.9 kJ/mol
E)1055.6 kJ/mol
Sn(s)+ SnO2(s)→ 2SnO(s); ΔG° = 12.0 kJ at 298K
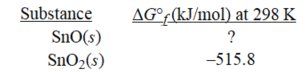
A)-251.9 kJ/mol
B)-503.8 kJ/mol
C)527.8 kJ/mol
D)263.9 kJ/mol
E)1055.6 kJ/mol

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
45
For which of the following substances is the standard free energy of formation not equal to zero at 298 K?
A)Xe(g)
B)2.7182818285(s)
C)H2(g)
D)Ca(g)
E)Zn(s)
A)Xe(g)
B)2.7182818285(s)
C)H2(g)
D)Ca(g)
E)Zn(s)

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
46
Determine ΔG° for the following reaction:
CH4(g)+ 2O2(g)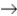
CO2(g)+ 2H2O(l)
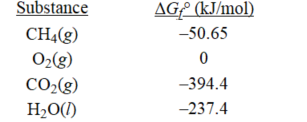
A)-581.2 kJ
B)-818.6 kJ
C)131.1 kJ
D)-682.5 kJ
E)-919.9 kJ
CH4(g)+ 2O2(g)
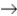
CO2(g)+ 2H2O(l)
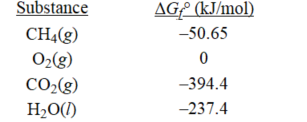
A)-581.2 kJ
B)-818.6 kJ
C)131.1 kJ
D)-682.5 kJ
E)-919.9 kJ

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
47
What is ΔS° at 298 K for the following reaction?
CH4(g)+ N2(g)→ HCN(g)+ NH3(g); ΔH° = 164.1 kJ; ΔG° = 159.1 kJ at 298 K
A)2.0 J/K
B)5.5 × 102 J/K
C)1.1 × 103 J/K
D)5.3 × 102 J/K
E)17 J/K
CH4(g)+ N2(g)→ HCN(g)+ NH3(g); ΔH° = 164.1 kJ; ΔG° = 159.1 kJ at 298 K
A)2.0 J/K
B)5.5 × 102 J/K
C)1.1 × 103 J/K
D)5.3 × 102 J/K
E)17 J/K

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
48
Which of the following is correct for the condensation of gaseous ammonia at -38°C? The normal boiling point of ammonia is -33°C.
A)ΔH < 0,ΔS > 0,and ΔG > 0.
B)ΔH < 0,ΔS < 0,and ΔG < 0.
C)ΔH > 0,ΔS < 0,and ΔG < 0.
D)ΔH = 0,ΔS = 0,and ΔG < 0.
E)ΔH > 0,ΔS > 0,and ΔG > 0.
A)ΔH < 0,ΔS > 0,and ΔG > 0.
B)ΔH < 0,ΔS < 0,and ΔG < 0.
C)ΔH > 0,ΔS < 0,and ΔG < 0.
D)ΔH = 0,ΔS = 0,and ΔG < 0.
E)ΔH > 0,ΔS > 0,and ΔG > 0.

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
49
-Given the following,determine ΔG° at 298 K for the precipitation reaction,
Ag+(aq)+I−(aq)→ AgI(s)
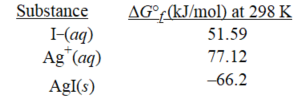
A)-91.7 kJ/mol
B)-40.7 kJ/mol
C)91.7 kJ/mol
D)40.7 kJ/mol
E)62.5 kJ/mol
Ag+(aq)+I−(aq)→ AgI(s)
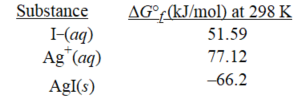
A)-91.7 kJ/mol
B)-40.7 kJ/mol
C)91.7 kJ/mol
D)40.7 kJ/mol
E)62.5 kJ/mol

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
50
Consider the following hypothetical reaction at 310 K.Standard free energies of formation are given in parentheses.
B C ΔG° = -25.0kJ/mol
C ΔG° = -25.0kJ/mol
(?) (176.4 kJ/mol)
Calculate the standard free energy of formation of compound B.
A)151.4 kJ/mol
B)-201.4 kJ/mol
C)-151.4kJ/mol
D)201.4 kJ/mol
E)none of these
B
 C ΔG° = -25.0kJ/mol
C ΔG° = -25.0kJ/mol(?) (176.4 kJ/mol)
Calculate the standard free energy of formation of compound B.
A)151.4 kJ/mol
B)-201.4 kJ/mol
C)-151.4kJ/mol
D)201.4 kJ/mol
E)none of these

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
51
oWhat is the change in free energy at 298 K when 70.0 mL of 0.704 M calcium chloride is combined with 47.9 mL of 0.859 M sodium carbonate? (R = 8.31 J/(K ∙ mol))
Ca2+(aq)+ CO32-(aq)→ CaCO3(s)
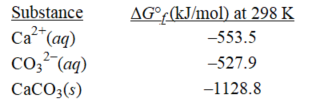
A)42.6 kJ
B)-2.21 × 103 kJ
C)46.1 kJ
D)47.4 kJ
E)2.56 × 103 kJ
Ca2+(aq)+ CO32-(aq)→ CaCO3(s)
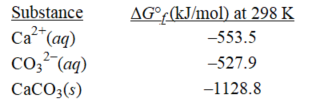
A)42.6 kJ
B)-2.21 × 103 kJ
C)46.1 kJ
D)47.4 kJ
E)2.56 × 103 kJ

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
52
Which of the following is true for the reaction NH3(l)  NH3(g)at -33°C and 1 atm pressure? (The normal boiling point for NH3 is -33°C.)
NH3(g)at -33°C and 1 atm pressure? (The normal boiling point for NH3 is -33°C.)
A)ΔH = TΔS
B)ΔH = 0
C)ΔH = ΔnRT
D)ΔS = 0
E)ΔH = PΔV
 NH3(g)at -33°C and 1 atm pressure? (The normal boiling point for NH3 is -33°C.)
NH3(g)at -33°C and 1 atm pressure? (The normal boiling point for NH3 is -33°C.)A)ΔH = TΔS
B)ΔH = 0
C)ΔH = ΔnRT
D)ΔS = 0
E)ΔH = PΔV

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
53
For a certain process,at 300.K,ΔG = -37.2 kJ and ΔH = -7.0 kJ.If the process is carried out reversibly,what is the amount of useful work that can be performed?
A)-7.0 kJ
B)-44.2 kJ
C)-30.2 kJ
D)30.2 kJ
E)-37.2 kJ
A)-7.0 kJ
B)-44.2 kJ
C)-30.2 kJ
D)30.2 kJ
E)-37.2 kJ

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
54
What is ΔG° at 298 K for the following reaction?
H2(g)+ Br2(g)→ 2HBr(g); ΔH° = -103.8 kJ; ΔS° = 21.3 J/K at 298 K
A)97.45 kJ
B)111 kJ
C)110.1 kJ
D)6.451 × 103 kJ
E)-6.451× 103 kJ
H2(g)+ Br2(g)→ 2HBr(g); ΔH° = -103.8 kJ; ΔS° = 21.3 J/K at 298 K
A)97.45 kJ
B)111 kJ
C)110.1 kJ
D)6.451 × 103 kJ
E)-6.451× 103 kJ

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
55
For a reversible phase change at constant temperature and pressure,
A)ΔG = 0.
B)ΔU = 0.
C)w = 0.
D)ΔH = 0.
E)q = 0.
A)ΔG = 0.
B)ΔU = 0.
C)w = 0.
D)ΔH = 0.
E)q = 0.

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
56
If a process is both endothermic and spontaneous,then
A)ΔH = 0.
B)ΔG > 0.
C)ΔS > 0.
D)ΔU < 0.
E)ΔH < 0.
A)ΔH = 0.
B)ΔG > 0.
C)ΔS > 0.
D)ΔU < 0.
E)ΔH < 0.

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
57
Which of the following has a value of zero for the standard free energy of formation at 298 K?
A)I(l)
B)I2(g)
C)I2(l)
D)I2(s)
E)I(g)
A)I(l)
B)I2(g)
C)I2(l)
D)I2(s)
E)I(g)

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
58
An ideal fuel for the control jet of a space vehicle should decompose with
A)ΔG = 0 and ΔH = 0.
B)ΔG < 0 and ΔH < 0.
C)ΔG > 0 and ΔH > 0.
D)ΔG < 0 and ΔH > 0.
E)ΔG > 0 and ΔH < 0.
A)ΔG = 0 and ΔH = 0.
B)ΔG < 0 and ΔH < 0.
C)ΔG > 0 and ΔH > 0.
D)ΔG < 0 and ΔH > 0.
E)ΔG > 0 and ΔH < 0.

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
59
From these two reactions at 298 K,
V2O3(s)+ 3CO(g)→ 2V(s)+ 3CO2(g); ΔH° = 369.8 kJ; ΔS° = 8.3 J/K
V2O5(s)+ 2CO(g)→ V2O3(s)+ 2CO2(g); ΔH° = -234.2 kJ; ΔS° = 0.2 J/K
Calculate ΔG° for the following at 298 K:
2V(s)+ 5CO2(g)→ V2O5(s)+ 5CO(g)
A)+133.1 kJ
B)+601.6 kJ
C)-601.6 kJ
D)-133.1 kJ
E)+1.6 kJ
V2O3(s)+ 3CO(g)→ 2V(s)+ 3CO2(g); ΔH° = 369.8 kJ; ΔS° = 8.3 J/K
V2O5(s)+ 2CO(g)→ V2O3(s)+ 2CO2(g); ΔH° = -234.2 kJ; ΔS° = 0.2 J/K
Calculate ΔG° for the following at 298 K:
2V(s)+ 5CO2(g)→ V2O5(s)+ 5CO(g)
A)+133.1 kJ
B)+601.6 kJ
C)-601.6 kJ
D)-133.1 kJ
E)+1.6 kJ

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
60
Given the following,determine K at 298K for the reaction,
AgI(s)→ Ag+(aq)+I−(aq)
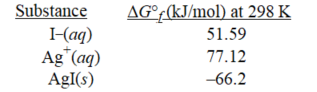
A)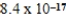
B)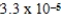
C)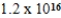
D)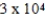
E)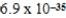
AgI(s)→ Ag+(aq)+I−(aq)
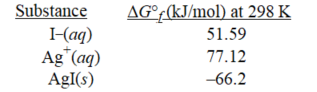
A)
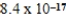
B)
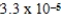
C)
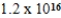
D)
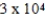
E)
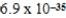

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
61
A 0.0333 M solution of a particular weak base,B,has a pH of 8.30 at 298 K.What is ΔG° for the following equilibrium?
B(aq)+ H2O(l) BH+(aq)+ OH-(aq)
BH+(aq)+ OH-(aq)
A)32.5 kJ
B)47.3 kJ
C)8.42 kJ
D)56.5 kJ
E)86.2 kJ
B(aq)+ H2O(l)
 BH+(aq)+ OH-(aq)
BH+(aq)+ OH-(aq)A)32.5 kJ
B)47.3 kJ
C)8.42 kJ
D)56.5 kJ
E)86.2 kJ

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
62
A certain reaction has negative values for both ΔH and ΔS.Therefore,the reaction
A)can be spontaneous if the temperature is low enough.
B)cannot be spontaneous at any temperature.
C)must be spontaneous at all temperatures.
D)can be spontaneous if the temperature is high enough.
E)has a positive free energy at any temperature.
A)can be spontaneous if the temperature is low enough.
B)cannot be spontaneous at any temperature.
C)must be spontaneous at all temperatures.
D)can be spontaneous if the temperature is high enough.
E)has a positive free energy at any temperature.

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
63
For a reaction,if ΔG° = 0,then
A)ΔS° = 0.
B)K = 0.
C)ΔH° = 0.
D)K = 1.
E)ΔG = 0.
A)ΔS° = 0.
B)K = 0.
C)ΔH° = 0.
D)K = 1.
E)ΔG = 0.

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
64
Consider the following hypothetical reaction (at 316.8 K).Standard free energies,in kJ/mol,are given in parentheses.
A B+C
B+C
ΔG° = ?
(-32.2)
(207.8)
(-237.0)
What is the value of the equilibrium constant for the reaction at 316.8 K?
A)0.42
B)1.0
C)273
D)6.5× 104
E)0.32
A
 B+C
B+CΔG° = ?
(-32.2)
(207.8)
(-237.0)
What is the value of the equilibrium constant for the reaction at 316.8 K?
A)0.42
B)1.0
C)273
D)6.5× 104
E)0.32

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
65
For the reaction 2SO2(g)+ O2(g)→ 2SO3(g),ΔH° and ΔS° are both negative at 298 K,and the process is spontaneous at 298 K.Which of the following statements must also be true?
A)The change in entropy is the driving force of the reaction.
B)ΔG is positive for the reaction at 298 K.
C)The direction of the reaction may be reversed at high temperatures.
D)ΔG is temperature independent.
E)At high temperature,ΔH becomes positive.
A)The change in entropy is the driving force of the reaction.
B)ΔG is positive for the reaction at 298 K.
C)The direction of the reaction may be reversed at high temperatures.
D)ΔG is temperature independent.
E)At high temperature,ΔH becomes positive.

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
66
For the reaction MgSO3(s)→ MgO(s)+ SO2(g),which is spontaneous only at high temperatures,one would predict that
A)ΔH is negative and ΔS is negative at room temperature.
B)ΔH is positive and ΔS is positive at room temperature.
C)ΔH is positive and ΔS is negative at room temperature.
D)ΔG is positive at high temperatures.
E)ΔH is negative and ΔS is positive at room temperature.
A)ΔH is negative and ΔS is negative at room temperature.
B)ΔH is positive and ΔS is positive at room temperature.
C)ΔH is positive and ΔS is negative at room temperature.
D)ΔG is positive at high temperatures.
E)ΔH is negative and ΔS is positive at room temperature.

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
67
The following reaction is spontaneous at all temperatures:
CaC2(s)+ 2H2O(l)→ Ca(OH)2(s)+ C2H2(g)
Which of the following statements is true?
A)ΔH is negative and ΔS is positive.
B)ΔH is negative and ΔS is negative.
C)ΔH is positive and ΔS is negative.
D)ΔH is positive and ΔS is positive.
E)ΔG is positive at all temperatures.
CaC2(s)+ 2H2O(l)→ Ca(OH)2(s)+ C2H2(g)
Which of the following statements is true?
A)ΔH is negative and ΔS is positive.
B)ΔH is negative and ΔS is negative.
C)ΔH is positive and ΔS is negative.
D)ΔH is positive and ΔS is positive.
E)ΔG is positive at all temperatures.

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
68
The reaction Br2(g)→ 2Br(g)is spontaneous only at temperatures in excess of 1600°C.We can conclude that
A)ΔH is + and ΔS is + for the reaction.
B)ΔH is - and ΔS is + for the reaction.
C)ΔG is + for all temperatures.
D)ΔH is - and ΔS is - for the reaction.
E)ΔH is + and ΔS is - for the reaction.
A)ΔH is + and ΔS is + for the reaction.
B)ΔH is - and ΔS is + for the reaction.
C)ΔG is + for all temperatures.
D)ΔH is - and ΔS is - for the reaction.
E)ΔH is + and ΔS is - for the reaction.

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
69
A 0.0835 M solution of a particular monoprotic weak acid,HA,has a pH of 5.00 at 298 K.What is ΔG° for the following equilibrium?
HA(aq)+ H2O(l) H3O+(aq)+ A-(aq)
H3O+(aq)+ A-(aq)
A)22.3 kJ
B)28.5 kJ
C)502 kJ
D)6.14 kJ
E)50.8 kJ
HA(aq)+ H2O(l)
 H3O+(aq)+ A-(aq)
H3O+(aq)+ A-(aq)A)22.3 kJ
B)28.5 kJ
C)502 kJ
D)6.14 kJ
E)50.8 kJ

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
70
For the reaction 4Ag(s)+ O2(g)→ 2Ag2O(s),ΔH° = -61.14 kJ and ΔS° = -132 J/K at 25°C.Which of the following statements is true? Assume that ΔH° and ΔS° are essentially temperature independent.
A)The change in entropy is the driving force at low temperatures.
B)The reaction will be spontaneous at high temperatures,and the reverse reaction will be spontaneous at low temperatures.
C)The reaction will not be spontaneous at any temperature.
D)The reaction will be spontaneous at low temperatures,and the reverse reaction will be spontaneous at high temperatures.
E)The reaction will be spontaneous at all temperatures.
A)The change in entropy is the driving force at low temperatures.
B)The reaction will be spontaneous at high temperatures,and the reverse reaction will be spontaneous at low temperatures.
C)The reaction will not be spontaneous at any temperature.
D)The reaction will be spontaneous at low temperatures,and the reverse reaction will be spontaneous at high temperatures.
E)The reaction will be spontaneous at all temperatures.

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
71
What is ΔG° at 298 K for the following equilibrium?
Ag+(aq)+ 2NH3(aq) Ag(NH3)2+(aq); Kf = 1.7 × 107 at 298 K
Ag(NH3)2+(aq); Kf = 1.7 × 107 at 298 K
A)-41 kJ
B)41 kJ
C)-18 kJ
D)0
E)18 kJ
Ag+(aq)+ 2NH3(aq)
 Ag(NH3)2+(aq); Kf = 1.7 × 107 at 298 K
Ag(NH3)2+(aq); Kf = 1.7 × 107 at 298 KA)-41 kJ
B)41 kJ
C)-18 kJ
D)0
E)18 kJ

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
72
For a reaction that has an equilibrium constant of 6 × 109,which of the following statements must be true?
A)ΔS° is positive.
B)ΔG° is negative.
C)ΔG° is positive.
D)ΔH° is negative.
E)ΔH° is positive.
A)ΔS° is positive.
B)ΔG° is negative.
C)ΔG° is positive.
D)ΔH° is negative.
E)ΔH° is positive.

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
73
A certain reaction is found to be reactant favored.Which of the following is a correct description of the reaction?
A)ΔG° > 0,K < 1
B)ΔG° < 0,K > 1
C)ΔG° > 0,K > 1
D)ΔG° < 0,K < 1
E)ΔG° = 0,K < 1
A)ΔG° > 0,K < 1
B)ΔG° < 0,K > 1
C)ΔG° > 0,K > 1
D)ΔG° < 0,K < 1
E)ΔG° = 0,K < 1

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
74
The standard free energy of formation of nitric oxide,NO,at 1000.K (roughly the temperature in an automobile engine during ignition)is 77.7 kJ/mol.Calculate the equilibrium constant for the reaction 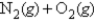

2NO(g)
At 1000.K.(R = 8.31 J/(K ∙ mol))
A)0.95
B)7.6 × 10-9
C)1.6 × 105
D)-15
E)8.7 × 10-5
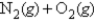

2NO(g)
At 1000.K.(R = 8.31 J/(K ∙ mol))
A)0.95
B)7.6 × 10-9
C)1.6 × 105
D)-15
E)8.7 × 10-5

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
75
For the reaction CaCO3(s)→ CaO(s)+ O2(g)at 1 atm pressure,the values of ΔH and ΔS are both positive,and the process is spontaneous at high temperatures.Which of the following statements about this reaction is true?
A)The change in entropy is the driving force for the reaction.
B)The process is exothermic at high temperatures and endothermic at room temperature.
C)The reverse reaction is endothermic.
D)The reverse reaction is nonspontaneous at room temperature.
E)ΔG at room temperature is negative.
A)The change in entropy is the driving force for the reaction.
B)The process is exothermic at high temperatures and endothermic at room temperature.
C)The reverse reaction is endothermic.
D)The reverse reaction is nonspontaneous at room temperature.
E)ΔG at room temperature is negative.

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
76
The reaction CaO(s)+ SO3(g)→ CaSO4(s)is nonspontaneous at 2200 K,whereas it is spontaneous at room temperature.Which of the following statements is false?
A)The change in enthalpy is the main driving force of the reaction.
B)Both ΔH and ΔS are negative for the reaction.
C)ΔG is negative at room temperature.
D)The change in entropy is the main driving force of the reaction.
E)ΔG becomes zero at a temperature between 300 and 2200 K.
A)The change in enthalpy is the main driving force of the reaction.
B)Both ΔH and ΔS are negative for the reaction.
C)ΔG is negative at room temperature.
D)The change in entropy is the main driving force of the reaction.
E)ΔG becomes zero at a temperature between 300 and 2200 K.

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
77
Water gas,a commercial fuel,is made by the reaction of hot coke carbon with steam: 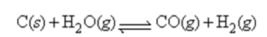
When equilibrium is established at 832°C,the concentrations of CO,H2,and H2O are 4.00 × 10-2,4.00 × 10-2,and 1.00 × 10-2 mol/L,respectively.Calculate the value of ΔG° for this reaction at 832°C.
A)55.0 kJ
B)12.7 kJ
C)16.8 kJ
D)-12.7 kJ
E)none of these
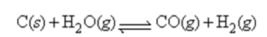
When equilibrium is established at 832°C,the concentrations of CO,H2,and H2O are 4.00 × 10-2,4.00 × 10-2,and 1.00 × 10-2 mol/L,respectively.Calculate the value of ΔG° for this reaction at 832°C.
A)55.0 kJ
B)12.7 kJ
C)16.8 kJ
D)-12.7 kJ
E)none of these

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
78
Consider the following reaction:
2AgCl(s)→ 2Ag(s)+ Cl2(g); ΔH° = 127.1 kJ; ΔS° = 115.7 J/K at 298 K
Suppose 61.4 g of silver(I)chloride is placed in a 50.0 L vessel at 298 K.What is the equilibrium partial pressure of chlorine gas? (R = 0.0821 L ∙ atm/(K ∙ mol)= 8.31 J/(K ∙ mol))
A)0.100 atm
B)5.1 × 10-23 atm
C)0.950 atm
D)0.210 atm
E)5.7 × 10-17 atm
2AgCl(s)→ 2Ag(s)+ Cl2(g); ΔH° = 127.1 kJ; ΔS° = 115.7 J/K at 298 K
Suppose 61.4 g of silver(I)chloride is placed in a 50.0 L vessel at 298 K.What is the equilibrium partial pressure of chlorine gas? (R = 0.0821 L ∙ atm/(K ∙ mol)= 8.31 J/(K ∙ mol))
A)0.100 atm
B)5.1 × 10-23 atm
C)0.950 atm
D)0.210 atm
E)5.7 × 10-17 atm

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
79
What is ΔG° at 500.0 K for the following reaction?
Zn(s)+ H2O(g)→ ZnO(s)+ H2(g)

A)80.7 kJ
B)-80.7 kJ
C)92.0 kJ
D)-92.0 kJ
E)-136.7 kJ
Zn(s)+ H2O(g)→ ZnO(s)+ H2(g)

A)80.7 kJ
B)-80.7 kJ
C)92.0 kJ
D)-92.0 kJ
E)-136.7 kJ

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
80
Consider the following reaction:
2C(s)+ 2H2(g)→ C2H4(g); ΔH° = 52.47 kJ; ΔS° = -53.5 J/K at 298 K
What is the equilibrium constant at 298 K for this reaction?
A)1.0 × 10-12
B)1.0
C)1.6 × 10-3
D)9.8 × 1011
E)6.4 × 10-10
2C(s)+ 2H2(g)→ C2H4(g); ΔH° = 52.47 kJ; ΔS° = -53.5 J/K at 298 K
What is the equilibrium constant at 298 K for this reaction?
A)1.0 × 10-12
B)1.0
C)1.6 × 10-3
D)9.8 × 1011
E)6.4 × 10-10

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck


