Deck 22: The Transition Elements and Coordination Compounds
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/75
Play
Full screen (f)
Deck 22: The Transition Elements and Coordination Compounds
1
Which of the following statements is incorrect concerning the 3d,4d,and 5d transition series?
A)There is a large increase in radius in going from the 3d to the 4d metals.
B)There is a general increase in size going left to right across each series due to an increasing number of electrons.
C)Hafnium through mercury exhibit what is referred to as the lanthanide contraction.
D)The separation of hafnium and zirconium found together in nature is difficult due to their similar chemistries,which are attributed to their virtually identical sizes.
E)The transition metal from a period 6 group is similar in size to the peroid 5 transition metal sitting above it on the periodic table.
A)There is a large increase in radius in going from the 3d to the 4d metals.
B)There is a general increase in size going left to right across each series due to an increasing number of electrons.
C)Hafnium through mercury exhibit what is referred to as the lanthanide contraction.
D)The separation of hafnium and zirconium found together in nature is difficult due to their similar chemistries,which are attributed to their virtually identical sizes.
E)The transition metal from a period 6 group is similar in size to the peroid 5 transition metal sitting above it on the periodic table.
There is a general increase in size going left to right across each series due to an increasing number of electrons.
2
What is the maximum oxidation state for niobium,Nb?
A)4
B)3
C)2
D)6
E)5
A)4
B)3
C)2
D)6
E)5
5
3
What is the maximum oxidation state expected for manganese?
A)+7
B)+4
C)+5
D)+8
E)+6
A)+7
B)+4
C)+5
D)+8
E)+6
+7
4
In which compound is cobalt in the highest oxidation state?
A)Co2(CO)8
B)[Co(NH3)6]Cl2
C)[Co(NH3)4Cl2]Cl
D)Na2[CoCl4]
E)K4[CoF6]
A)Co2(CO)8
B)[Co(NH3)6]Cl2
C)[Co(NH3)4Cl2]Cl
D)Na2[CoCl4]
E)K4[CoF6]

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
5
Which of the following first-row transition elements is not able to have a +3 oxidation state?
A)Sc
B)Cr
C)V
D)Zn
E)Fe
A)Sc
B)Cr
C)V
D)Zn
E)Fe

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
6
A property that many representative nonmetals and many transition elements share is that they both have
A)color in many compounds.
B)many paramagnetic compounds.
C)high electronegativities.
D)many compounds that observe the octet rule.
E)many oxidation states.
A)color in many compounds.
B)many paramagnetic compounds.
C)high electronegativities.
D)many compounds that observe the octet rule.
E)many oxidation states.

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
7
The oxidation number of which of the following elements is often +2 but can be as great as +7?
A)manganese
B)cobalt
C)aluminum
D)bismuth
E)chromium
A)manganese
B)cobalt
C)aluminum
D)bismuth
E)chromium

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
8
In which of the following compounds is the metal atom not in a common oxidation state?
A)CrOCl2
B)NiF2
C)CuI
D)Zn(NO3)2
E)MnSO4
A)CrOCl2
B)NiF2
C)CuI
D)Zn(NO3)2
E)MnSO4

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
9
Which of the following is not a transition-metal?
A)cobalt
B)iridium
C)vanadium
D)rhodium
E)rubidium
A)cobalt
B)iridium
C)vanadium
D)rhodium
E)rubidium

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
10
What is the maximum oxidation state for rhenium,Re?
A)6
B)3
C)2
D)7
E)4
A)6
B)3
C)2
D)7
E)4

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
11
All of the following elements have the oxidation states listed except
A)Ti,+3.
B)Zn,+3.
C)V,+3.
D)Co,+3.
E)Fe,+3.
A)Ti,+3.
B)Zn,+3.
C)V,+3.
D)Co,+3.
E)Fe,+3.

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
12
Which of the following is not a characteristic property of transition-element compounds?
A)Most of them are colored.
B)Most of them are paramagnetic.
C)Most of the metals exhibit multiple oxidation states.
D)Most of the elements form many complexes.
E)Most of the elements,upon ionizing,lose the d electrons first.
A)Most of them are colored.
B)Most of them are paramagnetic.
C)Most of the metals exhibit multiple oxidation states.
D)Most of the elements form many complexes.
E)Most of the elements,upon ionizing,lose the d electrons first.

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
13
Transition metals can be distinguished from main-group metals by the fact that
A)main-group metals have only +1 or +2 oxidation states.
B)transition metals have higher relative atomic weights than main-group metals.
C)transition metals have a greater tendency to form colored compounds than main-group metals.
D)main-group metals have higher relative atomic weights than transition metals.
E)only the main-group metals can form complex ions.
A)main-group metals have only +1 or +2 oxidation states.
B)transition metals have higher relative atomic weights than main-group metals.
C)transition metals have a greater tendency to form colored compounds than main-group metals.
D)main-group metals have higher relative atomic weights than transition metals.
E)only the main-group metals can form complex ions.

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
14
The phenomenon called __________ contraction is responsible for the great similarity in atomic size and chemistry of 4d and 5d elements.
A)coordination
B)isomeric
C)lanthanide
D)transition
E)none of these
A)coordination
B)isomeric
C)lanthanide
D)transition
E)none of these

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
15
Which of the following is not a characteristic of the transition metals?
A)variable oxidation state
B)the ability to form many colored compounds
C)valence electrons in a d subshell
D)high electronegativity
E)the ability to form complex ions
A)variable oxidation state
B)the ability to form many colored compounds
C)valence electrons in a d subshell
D)high electronegativity
E)the ability to form complex ions

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
16
1s22s22p63s23p64s23d2 is the correct electron configuration for which of the following atoms?
A)Ti
B)Ca
C)Ge
D)Zr
E)none of these
A)Ti
B)Ca
C)Ge
D)Zr
E)none of these

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
17
Fe has __________ that is(are)unpaired in its d orbitals.
A)3 electrons
B)4 electrons
C)1 electron
D)2 electrons
E)5 electrons
A)3 electrons
B)4 electrons
C)1 electron
D)2 electrons
E)5 electrons

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
18
An element with the electron configuration [Xe]6s24f145d7 would belong to which class on the periodic table?
A)rare-earth elements
B)transition elements
C)alkaline-earth elements
D)halogens
E)none of the above
A)rare-earth elements
B)transition elements
C)alkaline-earth elements
D)halogens
E)none of the above

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
19
Ti has __________ in its d orbitals.
A)1 electron
B)2 electrons
C)3 electrons
D)4 electrons
E)none of these
A)1 electron
B)2 electrons
C)3 electrons
D)4 electrons
E)none of these

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
20
What is the maximum oxidation number for titanium?
A)5
B)2
C)4
D)3
E)6
A)5
B)2
C)4
D)3
E)6

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
21
Which of the following Lewis bases may not serve as a bidentate ligand?
A)C2O42-
B)NH2CH2CH2NH2
C)HOCH2CH2NH2
D)HOCH2CH2OH
E)CH3NH2
A)C2O42-
B)NH2CH2CH2NH2
C)HOCH2CH2NH2
D)HOCH2CH2OH
E)CH3NH2

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
22
What is the principal ore of chromium?
A)chromite
B)chromate
C)chrome alum
D)stibnite
E)hematite
A)chromite
B)chromate
C)chrome alum
D)stibnite
E)hematite

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
23
The freezing point of an aqueous 0.050 m solution of a platinum(IV)complex having the molecular formula Pt(NH3)3Cl4 is -0.19°C.Which of the following equations best represents what happens when Pt(NH3)3Cl4 dissociates in water? (Kf for water is 1.86°C/m.)
A)Pt(NH3)3Cl4(s)→ [Pt(NH3)3Cl2]2+(aq)+ 2Cl-(aq)
B)Pt(NH3)3Cl4(s)→ [Pt(NH3)3Cl3]+(aq)+ Cl-(aq)
C)Pt(NH3)3Cl4(s)→ [Pt(NH3)3Cl]3+(aq)+ 3Cl-(aq)
D)Pt(NH3)3Cl4(s)→ [Pt(NH3)2Cl2]2+(aq)+ 2Cl-(aq)+ NH3(aq)
E)Pt(NH3)3Cl4(s)→ Pt4+(aq)+ 4Cl-(aq)+ 3NH3(aq)
A)Pt(NH3)3Cl4(s)→ [Pt(NH3)3Cl2]2+(aq)+ 2Cl-(aq)
B)Pt(NH3)3Cl4(s)→ [Pt(NH3)3Cl3]+(aq)+ Cl-(aq)
C)Pt(NH3)3Cl4(s)→ [Pt(NH3)3Cl]3+(aq)+ 3Cl-(aq)
D)Pt(NH3)3Cl4(s)→ [Pt(NH3)2Cl2]2+(aq)+ 2Cl-(aq)+ NH3(aq)
E)Pt(NH3)3Cl4(s)→ Pt4+(aq)+ 4Cl-(aq)+ 3NH3(aq)

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
24
Which of the following liquids could best act as a bidentate ligand for a metal ion?
A)NH2Cl
B)NH3
C)ClCH2CH2NH2
D)N2H5+
E)H2NCH2CH2NH2
A)NH2Cl
B)NH3
C)ClCH2CH2NH2
D)N2H5+
E)H2NCH2CH2NH2

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
25
Copper metal can be oxidized by which of the following acids?
A)H3PO4
B)HBr
C)H2SO4
D)CH3COOH
E)HCl
A)H3PO4
B)HBr
C)H2SO4
D)CH3COOH
E)HCl

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
26
What is the oxidation state of iron in K4[Fe(CN)6]?
A)3
B)2
C)4
D)−4
E)6
A)3
B)2
C)4
D)−4
E)6

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
27
What is the correct IUPAC name for [AuCl4(H2O)2]-?
A)diaquatetrachloroaurate(I)ion
B)diaquatetrachloroaurate(III)ion
C)diaquatetrachloroaurate(0)ion
D)diaquatetrachlorogold(III)ion
E)diaquatetrachlorogold(I)ion
A)diaquatetrachloroaurate(I)ion
B)diaquatetrachloroaurate(III)ion
C)diaquatetrachloroaurate(0)ion
D)diaquatetrachlorogold(III)ion
E)diaquatetrachlorogold(I)ion

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
28
Which of the following species is least likely to function as a ligand in a transition-metal coordination compound?
A)NH3
B)CO32-
C)H2O
D)BF3
E)Cl-
A)NH3
B)CO32-
C)H2O
D)BF3
E)Cl-

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
29
Which of the following is a necessary characteristic of a ligand?
A)A ligand must be an anion.
B)A ligand must be an ion.
C)A ligand must be a Lewis base.
D)A ligand must be bidentate.
E)A ligand must be a Lewis acid.
A)A ligand must be an anion.
B)A ligand must be an ion.
C)A ligand must be a Lewis base.
D)A ligand must be bidentate.
E)A ligand must be a Lewis acid.

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
30
Which of the following coordination compounds will immediately form a precipitate when combined with an AgNO3 solution?
A)K[Cr(NH3)2Cl4]
B)K3[Cr(CN)6]
C)[Cr(NH3)6]Cl3
D)[Cr(NH3)2(H2O)Cl3]
E)[Cr(NH3)3Cl3]
A)K[Cr(NH3)2Cl4]
B)K3[Cr(CN)6]
C)[Cr(NH3)6]Cl3
D)[Cr(NH3)2(H2O)Cl3]
E)[Cr(NH3)3Cl3]

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
31
In which of the following ions does the metal ion have a 3d7 electron configuration?
A)[NiCl4]2-
B)[Mn(H2O)6]2+
C)[CoCl4]2-
D)[Co(NH3)5Cl]2+
E)[Co(NH3)4Cl2]+
A)[NiCl4]2-
B)[Mn(H2O)6]2+
C)[CoCl4]2-
D)[Co(NH3)5Cl]2+
E)[Co(NH3)4Cl2]+

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
32
Which of the following is a dark green solid?
A)CrO3
B)KCr(SO4)2·12H2O
C)Na2Cr2O7
D)Na2CrO4
E)Cr2O3
A)CrO3
B)KCr(SO4)2·12H2O
C)Na2Cr2O7
D)Na2CrO4
E)Cr2O3

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
33
Ag+ forms many complexes with a coordination number of 2.Some of these are [Ag(NH3)2]+,[Ag(CN)2]-,and [Ag(S2O3)2]3-.Which of the following statements is true?
A)The hybridization of Ag+ is sp2.
B)In these complexes,Ag+ is a Lewis base.
C)In these complexes,the ligands are monodentate.
D)The hybridization of Ag+ is dsp.
E)The Ag+ complexes are good reducing agents.
A)The hybridization of Ag+ is sp2.
B)In these complexes,Ag+ is a Lewis base.
C)In these complexes,the ligands are monodentate.
D)The hybridization of Ag+ is dsp.
E)The Ag+ complexes are good reducing agents.

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
34
In which of these pairs of elements can both elements normally exhibit multiple oxidation states?
A)K and O
B)Cs and Cl
C)Mg and Se
D)Co and Cu
E)Sc and Al
A)K and O
B)Cs and Cl
C)Mg and Se
D)Co and Cu
E)Sc and Al

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
35
What is the coordination number of cobalt in [Co(en)2Cl2]Cl?
En =![<strong>What is the coordination number of cobalt in [Co(en)<sub>2</sub>Cl<sub>2</sub>]Cl? En = </strong> A)6 B)3 C)4 D)5 E)7](https://storage.examlex.com/TB2288/11ea7a3a_9f58_2fbf_a82d_bb2ff91bd099_TB2288_00.jpg)
A)6
B)3
C)4
D)5
E)7
En =
![<strong>What is the coordination number of cobalt in [Co(en)<sub>2</sub>Cl<sub>2</sub>]Cl? En = </strong> A)6 B)3 C)4 D)5 E)7](https://storage.examlex.com/TB2288/11ea7a3a_9f58_2fbf_a82d_bb2ff91bd099_TB2288_00.jpg)
A)6
B)3
C)4
D)5
E)7

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
36
In complexes of platinum(IV),the coordination number is usually
A)3.
B)4.
C)6.
D)2.
E)1.
A)3.
B)4.
C)6.
D)2.
E)1.

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
37
Which of the following is a product of the acidification of sodium chromate?
A)CrO42-(aq)
B)Cr2O72-(aq)
C)Cr3+(aq)
D)CrO3(s)
E)Cr(s)
A)CrO42-(aq)
B)Cr2O72-(aq)
C)Cr3+(aq)
D)CrO3(s)
E)Cr(s)

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
38
The formula for a platinum(IV)complex is [Pt(NH3)2Br2]Cl2.In aqueous solution,it will dissociate into
A)5 ions.
B)4 ions.
C)6 ions.
D)2 ions.
E)3 ions.
A)5 ions.
B)4 ions.
C)6 ions.
D)2 ions.
E)3 ions.

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
39
The bond between a metal cation and a ligand is best classified as a(n)
A)polar covalent bond.
B)ionic bond.
C)nonpolar covalent bond.
D)polydentate bond.
E)coordinate covalent bond.
A)polar covalent bond.
B)ionic bond.
C)nonpolar covalent bond.
D)polydentate bond.
E)coordinate covalent bond.

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
40
What is the coordination number of iron in K3[Fe(ox)3]?
Ox =![<strong>What is the coordination number of iron in K<sub>3</sub>[Fe(ox)<sub>3</sub>]? Ox = </strong> A)6 B)3 C)4 D)5 E)2](https://storage.examlex.com/TB2288/11ea7a3a_9f58_2fbe_a82d_e5f764afaaa4_TB2288_00.jpg)
A)6
B)3
C)4
D)5
E)2
Ox =
![<strong>What is the coordination number of iron in K<sub>3</sub>[Fe(ox)<sub>3</sub>]? Ox = </strong> A)6 B)3 C)4 D)5 E)2](https://storage.examlex.com/TB2288/11ea7a3a_9f58_2fbe_a82d_e5f764afaaa4_TB2288_00.jpg)
A)6
B)3
C)4
D)5
E)2

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
41
What is the formula for the hexaaquavanadium(II)ion?
A)[V(H2O)6]2+
B)[V(H2O)4]2+
C)[V2(H2O)6]4-
D)[V2(H2O)6]2+
E)[V2(H2O)6]4+
A)[V(H2O)6]2+
B)[V(H2O)4]2+
C)[V2(H2O)6]4-
D)[V2(H2O)6]2+
E)[V2(H2O)6]4+

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
42
What bond hybridization is associated with octahedral complexes?
A)d2sp3
B)sp3
C)sp
D)dsp3
E)dsp2
A)d2sp3
B)sp3
C)sp
D)dsp3
E)dsp2

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
43
What is the total number of geometric isomers possible for the octahedral complex [Co(NH3)3(H2O)3]?
A)1
B)4
C)5
D)2
E)3
A)1
B)4
C)5
D)2
E)3

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
44
Which of the following describes a d6 metal-ion complex with ligands that bond strongly to the metal ion?
A)Pairing energy is less than crystal field splitting,giving a low-spin complex.
B)Pairing energy is greater than crystal field splitting,giving a low-spin complex.
C)Pairing energy is less than crystal field splitting,giving a high-spin complex.
D)A d6 ion has only one possible electron arrangement regardless of the ligands.
E)Pairing energy is greater than crystal field splitting,giving a high-spin complex.
A)Pairing energy is less than crystal field splitting,giving a low-spin complex.
B)Pairing energy is greater than crystal field splitting,giving a low-spin complex.
C)Pairing energy is less than crystal field splitting,giving a high-spin complex.
D)A d6 ion has only one possible electron arrangement regardless of the ligands.
E)Pairing energy is greater than crystal field splitting,giving a high-spin complex.

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
45
Which complex is capable of forming geometric isomers?
A)K3[FeCl6]
B)[Co(NH3)5Br]Br2
C)[Co(NH3)6]Br3
D)[Pd(NH3)2Br2]
E)[Pt(NH3)4]Br2
A)K3[FeCl6]
B)[Co(NH3)5Br]Br2
C)[Co(NH3)6]Br3
D)[Pd(NH3)2Br2]
E)[Pt(NH3)4]Br2

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
46
Which combination leads to a high-spin octahedral complex?
A)low pairing energy and a weak-bonding ligand
B)high pairing energy and a weak-bonding ligand
C)moderate pairing energy and a strong-bonding ligand
D)high pairing energy and a strong-bonding ligand
E)low pairing energy and a strong-bonding ligand
A)low pairing energy and a weak-bonding ligand
B)high pairing energy and a weak-bonding ligand
C)moderate pairing energy and a strong-bonding ligand
D)high pairing energy and a strong-bonding ligand
E)low pairing energy and a strong-bonding ligand

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
47
Which of the metal ions in the following complex ions has a d5 electron configuration?
A)V(H2O)62+
B)RhCl64-
C)Co(CN)4-
D)Fe(CN)63-
E)Mo(NH3)63+
A)V(H2O)62+
B)RhCl64-
C)Co(CN)4-
D)Fe(CN)63-
E)Mo(NH3)63+

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
48
According to valence bond theory,what hybrid orbitals are occupied by the ligands in the complex [Mn(H2O)6]2+?
A)dsp3
B)sp3
C)d2sp3
D)sp2
E)dsp2
A)dsp3
B)sp3
C)d2sp3
D)sp2
E)dsp2

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
49
How many optical isomers do the cis and trans isomers of [Co(en)2Br2]+ have?
A)cis,1 and trans,1
B)cis,2 and trans,0
C)cis,0 and trans,0
D)cis,0 and trans,2
E)cis,2 and trans,2
A)cis,1 and trans,1
B)cis,2 and trans,0
C)cis,0 and trans,0
D)cis,0 and trans,2
E)cis,2 and trans,2

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
50
Which of the following best describes the complex [Mn(H2O)6]2+?
A)sp3 hybridization; diamagnetic
B)d2sp3 hybridization; diamagnetic
C)d2sp3 hybridization; paramagnetic
D)dsp3 hybridization; diamagnetic
E)sp3 hybridization; paramagnetic
A)sp3 hybridization; diamagnetic
B)d2sp3 hybridization; diamagnetic
C)d2sp3 hybridization; paramagnetic
D)dsp3 hybridization; diamagnetic
E)sp3 hybridization; paramagnetic

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
51
Which of the following compounds can exhibit optical activity?
A)[Co(NH3)4Cl2]Cl
B)[Co(NH2CH2CH2NH2)3]Cl3
C)Na2[CoCl4]
D)[Co(NH3)6]Cl3
E)Cr(CO)6
A)[Co(NH3)4Cl2]Cl
B)[Co(NH2CH2CH2NH2)3]Cl3
C)Na2[CoCl4]
D)[Co(NH3)6]Cl3
E)Cr(CO)6

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
52
Which of the following are constitutional isomers?
I.coordination isomers
II.linkage isomers
III.geometric isomers
IV.optical isomers
A)I,III,and IV
B)II and III
C)I and II
D)I and III only
E)II and IV only
I.coordination isomers
II.linkage isomers
III.geometric isomers
IV.optical isomers
A)I,III,and IV
B)II and III
C)I and II
D)I and III only
E)II and IV only

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
53
Which of the following high-spin complexes has the most unpaired electrons?
A)[Ti(H2O)6]2+
B)[V(H2O)6]3+
C)[Fe(H2O)6]3+
D)[Cr(H2O)6]3+
E)[Sc(H2O)]63+
A)[Ti(H2O)6]2+
B)[V(H2O)6]3+
C)[Fe(H2O)6]3+
D)[Cr(H2O)6]3+
E)[Sc(H2O)]63+

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
54
What is the correct formula for sodium tetrachlorocuprate(II)?
A)Na3[CuCl4]
B)Na2[CuCl4]
C)Na[CuCl4]
D)Na4[CuCl4]
E)Na2[CuCl6]
A)Na3[CuCl4]
B)Na2[CuCl4]
C)Na[CuCl4]
D)Na4[CuCl4]
E)Na2[CuCl6]

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
55
In which of the following complexes does the transition metal have a d8 configuration?
A)Cu(H2O)62+
B)Fe(CN)63-
C)Ni(CO)4
D)PtCl42-
E)Zn(NH3)42+
A)Cu(H2O)62+
B)Fe(CN)63-
C)Ni(CO)4
D)PtCl42-
E)Zn(NH3)42+

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
56
The complexes [Ni(NH3)5Cl]Br and [Ni(NH3)5Br]Cl are what type of isomers?
A)geometric isomers
B)constitutional isomers
C)stereoisomers
D)linkage isomers
E)optical isomers
A)geometric isomers
B)constitutional isomers
C)stereoisomers
D)linkage isomers
E)optical isomers

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
57
How many geometric isomers are possible for the square planar complex [Pt(NH3)2BrCl]?
A)2
B)4
C)5
D)1
E)3
A)2
B)4
C)5
D)1
E)3

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
58
Molecules that have nonsuperimposable mirror images are
A)multidentate.
B)racemic.
C)dextrorotatory.
D)chiral.
E)levorotatory.
A)multidentate.
B)racemic.
C)dextrorotatory.
D)chiral.
E)levorotatory.

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
59
What is the formula for the pentaamminehydroxochromium(II)ion?
A)[Cr(NH3)5(OH)5]2+
B)[Cr(NH3)5(OH)5]3-
C)[Cr(NH3)5(OH)]2+
D)[Cr(NH3)5(OH)]+
E)[Cr(NH3)(OH)5]3-
A)[Cr(NH3)5(OH)5]2+
B)[Cr(NH3)5(OH)5]3-
C)[Cr(NH3)5(OH)]2+
D)[Cr(NH3)5(OH)]+
E)[Cr(NH3)(OH)5]3-

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
60
Which of the following complexes can exhibit optical isomerism? (en = H2N-CH2-CH2-NH2 and is bidentate)
A)Co(NH3)3Cl3
B)cis-Co(en)2Cl2
C)trans-Co(en)2Br2
D)cis-Co(NH3)4Cl2
E)none of these
A)Co(NH3)3Cl3
B)cis-Co(en)2Cl2
C)trans-Co(en)2Br2
D)cis-Co(NH3)4Cl2
E)none of these

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
61
Which of the following compounds is paramagnetic?
A)Li4[Fe(NO2)6]
B)K3[Co(CN)6]
C)K3[ScCl6]
D)Na2[TiBr6]
E)[Mn(H2O)6]Cl2
A)Li4[Fe(NO2)6]
B)K3[Co(CN)6]
C)K3[ScCl6]
D)Na2[TiBr6]
E)[Mn(H2O)6]Cl2

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
62
Which of the following statements concerning crystal field theory is incorrect?
A)In an isolated metal ion the d-orbitals have identical energies.
B)In low spin octahedral complexes the electrons are concentrated in the dxy,dxz,and dyz orbitals.
C)High spin complexes have the minimum of unpaired electrons.
D)Strong bonding ligands favor a larger crystal field splitting (Δ)than weak bonding ligands.
E)Low spin complexes are favored when the electron pairing energy (P)is less than the crystal field splitting (Δ).
A)In an isolated metal ion the d-orbitals have identical energies.
B)In low spin octahedral complexes the electrons are concentrated in the dxy,dxz,and dyz orbitals.
C)High spin complexes have the minimum of unpaired electrons.
D)Strong bonding ligands favor a larger crystal field splitting (Δ)than weak bonding ligands.
E)Low spin complexes are favored when the electron pairing energy (P)is less than the crystal field splitting (Δ).

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
63
Which of the following salts would not be expected to be colored in aqueous solution?
A)CoCl2
B)FeCl2
C)CuCl2
D)CuCl
E)CrCl3
A)CoCl2
B)FeCl2
C)CuCl2
D)CuCl
E)CrCl3

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
64
Which of the following salts would not be expected to be colored in aqueous solution?
A)CrF3
B)NiF2
C)TiF3
D)ScF3
E)MnF3
A)CrF3
B)NiF2
C)TiF3
D)ScF3
E)MnF3

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
65
Which of the following salts would not be expected to be colored in aqueous solution?
A)NiCl2
B)CuCl2
C)CrCl2
D)TiCl3
E)CdCl2
A)NiCl2
B)CuCl2
C)CrCl2
D)TiCl3
E)CdCl2

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
66
How many unpaired electrons are there in an octahedral cobalt(III)complex with weak-bonding ligands?
A)1
B)2
C)4
D)3
E)0
A)1
B)2
C)4
D)3
E)0

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
67
The octahedral complex hexacyanoferrate(III)ion,[Fe(CN)6]3-,is a low-spin complex.How many unpaired electrons does the complex have?
A)5
B)1
C)4
D)0
E)3
A)5
B)1
C)4
D)0
E)3

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
68
The spectrochemical series is an arrangement of
A)ligands in order of their tendency to split the d orbitals of a transition metal ion.
B)coordination compounds in order of increasing ligand field splitting (Δ).
C)complex ions in order of the wavelength of light absorbed.
D)ligands in the order of electronegativity.
E)ligands in order of Lewis basicity.
A)ligands in order of their tendency to split the d orbitals of a transition metal ion.
B)coordination compounds in order of increasing ligand field splitting (Δ).
C)complex ions in order of the wavelength of light absorbed.
D)ligands in the order of electronegativity.
E)ligands in order of Lewis basicity.

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
69
The complex ion NiCl42- is tetrahedral.The number of unpaired electrons in the complex is
A)2.
B)3.
C)0.
D)4.
E)1.
A)2.
B)3.
C)0.
D)4.
E)1.

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
70
How many unpaired electrons are there in the tetrahedral complex ion [FeCl4]-?
A)3
B)2
C)1
D)4
E)5
A)3
B)2
C)1
D)4
E)5

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
71
_____ is a model of the electronic structure of transition-metal complexes that considers how the energies of the d orbitals of a metal ion are affected by the electric field of the ligands.
A)Valence bond theory
B)Mixed potential theory
C)Toba catastrophe theory
D)Crystal field theory
E)Lorentz ether theory
A)Valence bond theory
B)Mixed potential theory
C)Toba catastrophe theory
D)Crystal field theory
E)Lorentz ether theory

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
72
Which of the following salts would be expected to be colored in aqueous solution?
A)AlI3
B)CuI
C)TiCl4
D)CrCl2
E)ScI3
A)AlI3
B)CuI
C)TiCl4
D)CrCl2
E)ScI3

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
73
How is pure chromium metal prepared?
A)By exothermic reaction of chromium (VI)oxide,obtained from aluminum ore,with steel
B)By endothermic reaction of chromium (III)oxide,obtained from chromite ore,with aluminum
C)By endothermic reaction of chromium (II)oxide,obtained from iron ore,with copper
D)By exothermic reaction of chromium (III)oxide,obtained from chromite ore,with aluminum
E)By endothermic reaction of chromium (VI)oxide,obtained from aluminum ore,with brass
A)By exothermic reaction of chromium (VI)oxide,obtained from aluminum ore,with steel
B)By endothermic reaction of chromium (III)oxide,obtained from chromite ore,with aluminum
C)By endothermic reaction of chromium (II)oxide,obtained from iron ore,with copper
D)By exothermic reaction of chromium (III)oxide,obtained from chromite ore,with aluminum
E)By endothermic reaction of chromium (VI)oxide,obtained from aluminum ore,with brass

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
74
If an octahedral iron(II)complex is paramagnetic,which of the following sets of conditions best describes the complex?
A)low spin,Δ small
B)low spin,Δ large
C)high spin,Δ small
D)high spin,Δ large
E)none of the above
A)low spin,Δ small
B)low spin,Δ large
C)high spin,Δ small
D)high spin,Δ large
E)none of the above

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck
75
A complex ion is a square planar complex.It has a d8 electron configuration.What is the most reasonable d orbital scheme for this complex?
A)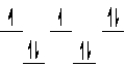
B)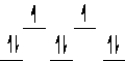
C)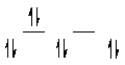
D)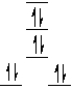
E)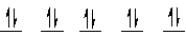
A)
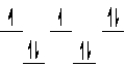
B)
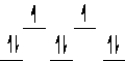
C)
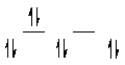
D)
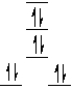
E)
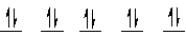

Unlock Deck
Unlock for access to all 75 flashcards in this deck.
Unlock Deck
k this deck


