Deck 15: Electric Forces and Electric Fields: Part A
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/42
Play
Full screen (f)
Deck 15: Electric Forces and Electric Fields: Part A
1
How many atoms are present in a sample of pure iron with a mass of 210 g? (The atomic mass of iron = 56 u and NA = 6.02 × 1023. )
A) 1.8 × 1019
B) 2.3 × 1028
C) 2.3 × 1024
D) 3.2 × 1028
A) 1.8 × 1019
B) 2.3 × 1028
C) 2.3 × 1024
D) 3.2 × 1028
2.3 × 1024
2
With volume and molar quantity held constant,by what factor does the absolute temperature change for an ideal gas when the pressure is three times bigger?
A) 0.3
B) 3.0
C) 5.0
D) 25.0
A) 0.3
B) 3.0
C) 5.0
D) 25.0
3.0
3
An ideal gas is confined to a container with adjustable volume.The pressure and mole number are constant.By what factor will volume change if absolute temperature doubles?
A) 4.0
B) 2.0
C) 1/2
D) 1/4
A) 4.0
B) 2.0
C) 1/2
D) 1/4
2.0
4
Four moles of nitrogen gas are contained in an enclosed cylinder with a movable piston.If the molecular mass of nitrogen is 28 u,how many grams of nitrogen are present?
A) 0.14
B) 56
C) 42
D) 112
A) 0.14
B) 56
C) 42
D) 112

Unlock Deck
Unlock for access to all 42 flashcards in this deck.
Unlock Deck
k this deck
5
In the relationship ΔP ∝ ΔT ,what scale is used for temperature?
A) Fahrenheit
B) Celsius
C) Kelvin
D) Both Celsius and Kelvin may be used since the degree size is the same for both.
A) Fahrenheit
B) Celsius
C) Kelvin
D) Both Celsius and Kelvin may be used since the degree size is the same for both.

Unlock Deck
Unlock for access to all 42 flashcards in this deck.
Unlock Deck
k this deck
6
The value 1.38 × 10−23 J/K is known as:
A) the universal gas constant.
B) Boltzmann's constant.
C) Avogadro's number.
D) the molar specific.
A) the universal gas constant.
B) Boltzmann's constant.
C) Avogadro's number.
D) the molar specific.

Unlock Deck
Unlock for access to all 42 flashcards in this deck.
Unlock Deck
k this deck
7
An ideal gas is confined to a container with adjustable volume.The number of moles and temperature are constant.By what factor will the volume change if pressure quadruples?
A) 16
B) 4
C) 1/4
D) 1/16
A) 16
B) 4
C) 1/4
D) 1/16

Unlock Deck
Unlock for access to all 42 flashcards in this deck.
Unlock Deck
k this deck
8
What is the mass of 2 moles of carbon dioxide?
A) 88 g
B) 80 g
C) 64 g
D) 56 g
A) 88 g
B) 80 g
C) 64 g
D) 56 g

Unlock Deck
Unlock for access to all 42 flashcards in this deck.
Unlock Deck
k this deck
9
What is the mass of a mole of oxygen gas?
A) 8.0 g
B) 16 g
C) 24 g
D) 32 g
A) 8.0 g
B) 16 g
C) 24 g
D) 32 g

Unlock Deck
Unlock for access to all 42 flashcards in this deck.
Unlock Deck
k this deck
10
A 4.00-L container holds half a mole of an ideal gas at a pressure of 18.5 atm.What is the gas temperature? (R = 0.0821 L · atm/mol · K)
A) 1800 K
B) 1190 K
C) 901 K
D) 450 K
A) 1800 K
B) 1190 K
C) 901 K
D) 450 K

Unlock Deck
Unlock for access to all 42 flashcards in this deck.
Unlock Deck
k this deck
11
What thermodynamic quantity is held constant in Boyle's law?
A) pressure
B) volume
C) temperature
D) Both pressure and volume are each held constant.
A) pressure
B) volume
C) temperature
D) Both pressure and volume are each held constant.

Unlock Deck
Unlock for access to all 42 flashcards in this deck.
Unlock Deck
k this deck
12
Which law is for a quantity of gas held at constant volume?
A) Boyle's law
B) Charles's law
C) Gay-Lussac's law
D) Avogadro's law
A) Boyle's law
B) Charles's law
C) Gay-Lussac's law
D) Avogadro's law

Unlock Deck
Unlock for access to all 42 flashcards in this deck.
Unlock Deck
k this deck
13
Avogadro's number is related to the universal gas constant R and the number of moles n by:
A) NA = Rn
B) NA = R/n
C) NA = n/R
D) Avogadro's number cannot be related to these two quantities alone.
A) NA = Rn
B) NA = R/n
C) NA = n/R
D) Avogadro's number cannot be related to these two quantities alone.

Unlock Deck
Unlock for access to all 42 flashcards in this deck.
Unlock Deck
k this deck
14
One way to heat a gas is to compress it.A gas at 1.00 atm at 25.0°C is compressed to one tenth of its original volume,and it reaches 30.0 atm pressure.What is its new temperature?
A) 1500 K
B) 621°C
C) 1192°C
D) 919°C
A) 1500 K
B) 621°C
C) 1192°C
D) 919°C

Unlock Deck
Unlock for access to all 42 flashcards in this deck.
Unlock Deck
k this deck
15
With molar quantity and temperature held constant,by what factor does the pressure of an ideal gas change when the volume is four times bigger?
A) 16
B) 4
C) 1/4
D) 1/16
A) 16
B) 4
C) 1/4
D) 1/16

Unlock Deck
Unlock for access to all 42 flashcards in this deck.
Unlock Deck
k this deck
16
For a quantity of ideal gas,which of the following is constant?
A) PTV
B) P/TV
C) PT/V
D) PV/T
A) PTV
B) P/TV
C) PT/V
D) PV/T

Unlock Deck
Unlock for access to all 42 flashcards in this deck.
Unlock Deck
k this deck
17
Two moles of an ideal gas at 3.0 atm and 10°C are heated up to 100°C.If the volume is held constant during this heating,what is the final pressure?
A) 4.5 atm
B) 4.0 atm
C) 0.14 atm
D) 1.0 atm
A) 4.5 atm
B) 4.0 atm
C) 0.14 atm
D) 1.0 atm

Unlock Deck
Unlock for access to all 42 flashcards in this deck.
Unlock Deck
k this deck
18
How many molecules are there in 14 grams of nitrogen gas?
A) NA/2
B) NA
C) 2 NA
D) The answer is not given.
A) NA/2
B) NA
C) 2 NA
D) The answer is not given.

Unlock Deck
Unlock for access to all 42 flashcards in this deck.
Unlock Deck
k this deck
19
The gas,C3H8,is used in outside grills,and several moles of this gas will burn during an afternoon's grilling.What is the mass of one molecule of this gas?
A) 4.4 × 10-23 g
B) 7.3 × 10-23 kg
C) 7.3 × 10-26 kg
D) 8.8 × 10-26 kg
A) 4.4 × 10-23 g
B) 7.3 × 10-23 kg
C) 7.3 × 10-26 kg
D) 8.8 × 10-26 kg

Unlock Deck
Unlock for access to all 42 flashcards in this deck.
Unlock Deck
k this deck
20
Four moles of nitrogen gas are contained in an enclosed cylinder with a movable piston.If the gas temperature is 298 K,and the pressure is 1.01 × 106 N/m2,what is the volume? (R = 8.31 J/mol·K)
A) 9.81 × 10−3 m3
B) 4.90 × 10−3 m3
C) 17.3 × 10−3 m3
D) 8.31 × 10−3 m3
A) 9.81 × 10−3 m3
B) 4.90 × 10−3 m3
C) 17.3 × 10−3 m3
D) 8.31 × 10−3 m3

Unlock Deck
Unlock for access to all 42 flashcards in this deck.
Unlock Deck
k this deck
21
A pressure of 2.0 × 10−7 mm of Hg is achieved in a vacuum system.How many gas molecules are present per liter volume if the temperature is 293 K? (760 mm of Hg = 1 atm,R = 0.0821 L · atm/mol · K,and NA = 6.02 × 1023)
A) 16 × 1018
B) 6.6 × 1012
C) 3.3 × 1012
D) 3.4 × 109
A) 16 × 1018
B) 6.6 × 1012
C) 3.3 × 1012
D) 3.4 × 109

Unlock Deck
Unlock for access to all 42 flashcards in this deck.
Unlock Deck
k this deck
22
The average kinetic energy of a molecule in a monatomic ideal gas is given by:
A)
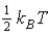
B)
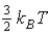
C)
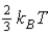
D) none of the these.
A)
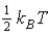
B)
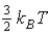
C)
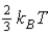
D) none of the these.

Unlock Deck
Unlock for access to all 42 flashcards in this deck.
Unlock Deck
k this deck
23
A quantity of a monatomic ideal gas expands to four times the volume while maintaining the same pressure.If the internal energy of the gas were U0 before the expansion,what is it after the expansion?
A) U0
B) 2U0
C) 4U0
D) The change in temperature must also be known to answer this question.
A) U0
B) 2U0
C) 4U0
D) The change in temperature must also be known to answer this question.

Unlock Deck
Unlock for access to all 42 flashcards in this deck.
Unlock Deck
k this deck
24
If the average kinetic energy of the molecules of a monatomic ideal gas at 60°C is doubled,what is the resulting temperature of the gas?
A) It becomes 60×
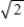 °C.
°C.
B) It becomes 666°C.
C) It becomes 120°C.
D) It becomes 393°C.
A) It becomes 60×
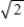 °C.
°C.B) It becomes 666°C.
C) It becomes 120°C.
D) It becomes 393°C.

Unlock Deck
Unlock for access to all 42 flashcards in this deck.
Unlock Deck
k this deck
25
The mass of a hot-air balloon and its cargo (not including the air inside)is 250 kg.The air outside is at a temperature of 10°C and a pressure of 1 atm = 105 N/m2.The volume of the balloon is 400 m3.Which temperature below of the air in the balloon will allow the balloon to just lift off? (Air density at 10°C is 1.25 kg/m3. )
A) 37°C
B) 293°C
C) 99°C
D) 200°C
A) 37°C
B) 293°C
C) 99°C
D) 200°C

Unlock Deck
Unlock for access to all 42 flashcards in this deck.
Unlock Deck
k this deck
26
What is the internal energy of 42 moles of Neon gas (molecular mass = 20 u)at 27°C? (R = 8.31 J/mol · K)
A) 1.9 × 105 J
B) 1.6 × 105 J
C) 3.8 × 103 J
D) It depends on the container size,which is not given.
A) 1.9 × 105 J
B) 1.6 × 105 J
C) 3.8 × 103 J
D) It depends on the container size,which is not given.

Unlock Deck
Unlock for access to all 42 flashcards in this deck.
Unlock Deck
k this deck
27
If the temperature of a gas triples,by what factor does the speed of a molecule with the average kinetic energy change?
A) It increases by a factor of 3.
B) It increases by a factor of
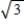 .
.
C) It increases by a factor of 3
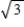 .
.
D) It increases by a factor of 1/3.
A) It increases by a factor of 3.
B) It increases by a factor of
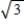 .
.C) It increases by a factor of 3
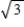 .
.D) It increases by a factor of 1/3.

Unlock Deck
Unlock for access to all 42 flashcards in this deck.
Unlock Deck
k this deck
28
What is the internal energy of a monatomic ideal gas with pressure 6 ×105 Pa and with volume 2 m3?
A) 6 × 105 J
B) 18 × 105 J
C) 9 × 105 J
D) More information is needed to find this value.
A) 6 × 105 J
B) 18 × 105 J
C) 9 × 105 J
D) More information is needed to find this value.

Unlock Deck
Unlock for access to all 42 flashcards in this deck.
Unlock Deck
k this deck
29
The volume holding 0.63 mole of an ideal gas is compressed to 1/4 of its original volume by a piston.At the same time the pressure of the gas triples.If the original absolute temperature of the gas was T,that is the new temperature after compression?
A) 4/3T
B) 3/4T
C) 0.63(3/4T)
D) 0.63(4/3T)
A) 4/3T
B) 3/4T
C) 0.63(3/4T)
D) 0.63(4/3T)

Unlock Deck
Unlock for access to all 42 flashcards in this deck.
Unlock Deck
k this deck
30
A spherical air bubble originating from a scuba diver at a depth of 30.0 m has a diameter of 1.0 cm.What will the bubble's diameter be when it reaches the surface? (Assume constant temperature. )
A) 0.7 cm
B) 1.0 cm
C) 1.4 cm
D) 1.6 cm
A) 0.7 cm
B) 1.0 cm
C) 1.4 cm
D) 1.6 cm

Unlock Deck
Unlock for access to all 42 flashcards in this deck.
Unlock Deck
k this deck
31
A tank with a volume of 0.200 m3 contains 27.0°C helium gas at a pressure of 63.5 atm.How many balloons can be blown up if each filled balloon is a sphere 30.0 cm in diameter at 27.0°C and absolute pressure of 1.20 atm? Assume all the helium is transferred to the balloons.
A) 1180 balloons
B) 884 balloons
C) 749 balloons
D) 375 balloons
A) 1180 balloons
B) 884 balloons
C) 749 balloons
D) 375 balloons

Unlock Deck
Unlock for access to all 42 flashcards in this deck.
Unlock Deck
k this deck
32
If the temperature of a volume of ideal gas increases for 100°C to 200°C,what happens to the average kinetic energy of the molecules?
A) It halves.
B) It doubles.
C) It increases but less that double.
D) It increases more than double.
A) It halves.
B) It doubles.
C) It increases but less that double.
D) It increases more than double.

Unlock Deck
Unlock for access to all 42 flashcards in this deck.
Unlock Deck
k this deck
33
How many moles of air must escape from a 10 m × 8.0 m × 5.0 m room when the temperature is raised from 0°C to 10°C? Assume the pressure remains unchanged at one atmosphere while the room is heated.
A) 1.3 × 103 moles
B) 1.2 × 103 moles
C) 6.5 × 102 moles
D) 3.7 × 102 moles
A) 1.3 × 103 moles
B) 1.2 × 103 moles
C) 6.5 × 102 moles
D) 3.7 × 102 moles

Unlock Deck
Unlock for access to all 42 flashcards in this deck.
Unlock Deck
k this deck
34
Estimate the volume of a helium-filled balloon if it is to lift a payload of 1000 kg.The density of air is 1.29 kg/m3 and helium has a density of 0.178 kg/m3.
A) 4410 m3
B) 900 m3
C) 450 m3
D) 225 m3
A) 4410 m3
B) 900 m3
C) 450 m3
D) 225 m3

Unlock Deck
Unlock for access to all 42 flashcards in this deck.
Unlock Deck
k this deck
35
A helium-filled weather balloon has a 0.90-m radius at liftoff where air pressure is 1.0 atm and the temperature is 298 K.When airborne,the temperature is 210 K,and its radius expands to 3.5 m.What is the pressure at the airborne location?
A) 0.50 atm
B) 0.012 atm
C) 0.019 atm
D) 0.38 atm
A) 0.50 atm
B) 0.012 atm
C) 0.019 atm
D) 0.38 atm

Unlock Deck
Unlock for access to all 42 flashcards in this deck.
Unlock Deck
k this deck
36
A storeroom has a volume of 30 m3 and is filled with air of an average molecular mass of 29 u.What is the mass of the air in the room at a pressure of 1.0 atm and temperature of 22°C? R = 0.082 L · atm/mol · K
A) 36 kg
B) 1240 kg
C) 72 kg
D) 700 kg
A) 36 kg
B) 1240 kg
C) 72 kg
D) 700 kg

Unlock Deck
Unlock for access to all 42 flashcards in this deck.
Unlock Deck
k this deck
37
Tricia puts 66 g of dry ice (solid CO2)into a 2.0-L container and seals the top.The dry ice turns to gas at room temperature (20°C).Find the pressure increase in the 2.0-L container.(One mole of CO2 has a mass of 44 g and R = 0.0821 L·atm/mol·K.Ignore the initial volume of the dry ice. )
A) 6.0 atm
B) 12 atm
C) 18 atm
D) 2.0 atm
A) 6.0 atm
B) 12 atm
C) 18 atm
D) 2.0 atm

Unlock Deck
Unlock for access to all 42 flashcards in this deck.
Unlock Deck
k this deck
38
The sulfur hexafluoride molecule consists of one sulfur atom and six fluorine atoms.The atomic masses of sulfur and fluorine are 32.0 u and 19.0 u,respectively.One-half mole of this very heavy gas has what mass?
A) 32 g
B) 73 g
C) 146 g
D) 608 g
A) 32 g
B) 73 g
C) 146 g
D) 608 g

Unlock Deck
Unlock for access to all 42 flashcards in this deck.
Unlock Deck
k this deck
39
Suppose there are 2 moles of a gas in a container of fixed size and the average spacing of molecules in the container is 4.0 nm.If an additional 14 moles of the gas are added to the container and thermal equilibrium is established,what is the new average spacing of the molecules?
A) 2.0 nm
B) 0.50 nm
C) 1.4 nm
D) 2.8 nm
A) 2.0 nm
B) 0.50 nm
C) 1.4 nm
D) 2.8 nm

Unlock Deck
Unlock for access to all 42 flashcards in this deck.
Unlock Deck
k this deck
40
9.0 g of water in a 2.0-L pressure vessel is heated to 300°C.What is the pressure inside the container? (R = 0.082 L · atm/mol · K,one mole of water has a mass of 18 grams)
A) 12 atm
B) 16 atm
C) 24 atm
D) 32 atm
A) 12 atm
B) 16 atm
C) 24 atm
D) 32 atm

Unlock Deck
Unlock for access to all 42 flashcards in this deck.
Unlock Deck
k this deck
41
For a monatomic gas,3R/2 is
A) the specific heat per mole at constant volume.
B) the specific heat per unit mass at constant volume.
C) NAkB.
D) none of the above.
A) the specific heat per mole at constant volume.
B) the specific heat per unit mass at constant volume.
C) NAkB.
D) none of the above.

Unlock Deck
Unlock for access to all 42 flashcards in this deck.
Unlock Deck
k this deck
42
The specific heat at constant volume per mole of 3R/2 is for what type of molecule?
A) monatomic
B) diatomic
C) polyatomic
D) any type
A) monatomic
B) diatomic
C) polyatomic
D) any type

Unlock Deck
Unlock for access to all 42 flashcards in this deck.
Unlock Deck
k this deck


