Deck 3: Chemical Bonds
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/82
Play
Full screen (f)
Deck 3: Chemical Bonds
1
Potassium tends to form a cation (K+).
True
2
The name for the compound formed between Co3+ and O2- is cobalt(II) oxide.
False
3
The compound represented below has the formula Na2S. 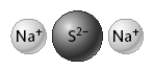

True
4
The element Co (cobalt) satisfies the octet rule.

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
5
Metals gain electrons to become ions in ionic compounds.

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
6
Examine the following image showing a "subatomic" level diagram of two atoms forming a bond. 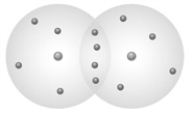 The type of bond formed is a single covalent bond.
The type of bond formed is a single covalent bond.
 The type of bond formed is a single covalent bond.
The type of bond formed is a single covalent bond.
Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
7
The Lewis structure for Cl2O could be written as: 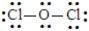
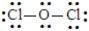

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
8
Aluminum and oxygen form a compound with the formula Al2O3. Oxygen and gallium (Ga) could from a compound with a formula unit containing two oxygen atoms and three gallium atoms.

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
9
The formula that corresponds to the IUPAC name dichlorine monoxide is Cl2O.

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
10
Using normal bonding requirements for atoms, the following correctly shows the bonding in a compound with the formula C2H3N. 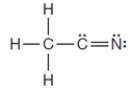
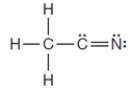

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
11
Phosphorus must gain three electrons to obey the octet rule.

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
12
A possible bonding pattern for an element in Group 5A would be to form one single bond and one double bond.

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
13
Sodium loses an electron in forming an ion which gives the ion a +1 charge.

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
14
Based on the number of valence electrons, sulfur (S) would normally form 1 bonds.

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
15
Sodium combines with oxygen to form Na2O. The expected formula between lithium and tellurium would be Li2Te.

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
16
The compound represented below contains two single covalent bonds. 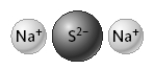


Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
17
Chloride ion (Cl-) is an anion because it has lost an electron.

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
18
Based on electronegativity values, in the bond shown below, nitrogen will have the negative charge.
H-N
H-N

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
19
Strontium forms an anion by losing two electrons.

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
20
In the following compound, phosphorus (P) would be at the center of the molecule.
PBrClF
PBrClF

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
21
Which of the following is an acceptable way to represent dinitrogen monoxide?
A)N2O
B)nitric oxide
C)NO2
D)both a and b
A)N2O
B)nitric oxide
C)NO2
D)both a and b

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
22
In which of the following cases is a polar covalent bond formed?
A)when an electron is transferred from one atom to another
B)when the electrons of a bond are shared equally by the two atoms
C)when the electrons of a bond are shared unequally by the two atoms
D)when a metallic element forms a bond with a non-metallic element
A)when an electron is transferred from one atom to another
B)when the electrons of a bond are shared equally by the two atoms
C)when the electrons of a bond are shared unequally by the two atoms
D)when a metallic element forms a bond with a non-metallic element

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
23
An ionic bond is associated with which of the following?
A)interactions between nuclei
B)equal sharing of electrons
C)unequal sharing of electrons
D)the transfer of electrons
A)interactions between nuclei
B)equal sharing of electrons
C)unequal sharing of electrons
D)the transfer of electrons

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
24
Which of the following occurs when an ionic bond is formed?
A)Electrons are transferred from the more electronegative element to the less electronegative element.
B)Electrons are transferred from the less electronegative element to the more electronegative element.
C)Electrons are shared equally.
D)Electrons are shared unequally.
A)Electrons are transferred from the more electronegative element to the less electronegative element.
B)Electrons are transferred from the less electronegative element to the more electronegative element.
C)Electrons are shared equally.
D)Electrons are shared unequally.

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
25
What is the correct IUPAC name for a compound with the formula N2O3?
A)nitrogen oxide
B)nitrogen trioxide
C)nitrogen ozide
D)dinitrogen trioxide
A)nitrogen oxide
B)nitrogen trioxide
C)nitrogen ozide
D)dinitrogen trioxide

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
26
How many bonds does a carbon atom typically form?
A)2
B)4
C)6
D)8
A)2
B)4
C)6
D)8

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
27
How many electrons are associated with a double bond?
A)1
B)2
C)4
D)6
A)1
B)2
C)4
D)6

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
28
When an ionic compound forms between aluminum (Al) and nitrogen (N) atoms,
A)nitrogen donates electrons to aluminum.
B)nitrogen attains a positive charge and aluminum a negative charge.
C)aluminum donates electrons to nitrogen.
D)both a and b
A)nitrogen donates electrons to aluminum.
B)nitrogen attains a positive charge and aluminum a negative charge.
C)aluminum donates electrons to nitrogen.
D)both a and b

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
29
When we name a molecular binary compound which of the following is true?
A)The element that has more atoms in the molecule is named first.
B)The elements are named in alphabetical order.
C)The less electronegative element is named first.
D)The more electronegative element is named first.
A)The element that has more atoms in the molecule is named first.
B)The elements are named in alphabetical order.
C)The less electronegative element is named first.
D)The more electronegative element is named first.

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
30
Neither O or F can from triple bonds.

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
31
Which of the following occurs when a sulfur atom is converted to S2-?
A)The sulfur atom gains two electrons and loses two protons.
B)The sulfur atom gains two electrons.
C)The sulfur atom loses two electrons and two protons.
D)The sulfur atom loses two electrons.
A)The sulfur atom gains two electrons and loses two protons.
B)The sulfur atom gains two electrons.
C)The sulfur atom loses two electrons and two protons.
D)The sulfur atom loses two electrons.

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
32
Which of the following statements is generally true about electronegativity?
A)Electronegativity decreases as we move left to right and decreases as we move top to bottom.
B)Electronegativity decreases as we move left to right and increases as we move top to bottom.
C)Electronegativity increases as we move left to right and decreases as we move top to bottom.
D)Electronegativity increases as we move left to right and increases as we move top to bottom.
A)Electronegativity decreases as we move left to right and decreases as we move top to bottom.
B)Electronegativity decreases as we move left to right and increases as we move top to bottom.
C)Electronegativity increases as we move left to right and decreases as we move top to bottom.
D)Electronegativity increases as we move left to right and increases as we move top to bottom.

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
33
An element with the following Lewis structure would most likely form how many covalent bonds? 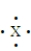
A)1
B)2
C)3
D)4
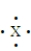
A)1
B)2
C)3
D)4

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
34
18.01 g of water (H2O) and 36.46 g of HCl contain the same number of molecules.

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
35
High electronegativities are associated with which type of elements?
A)metals
B)metalloids
C)noble gases
D)nonmetals
A)metals
B)metalloids
C)noble gases
D)nonmetals

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
36
Calcium carbonate is used as a phosphate binder to treat someone with too much phosphate, known as hyperphosphatemia, as can be seen in renal/kidney failure. The formula for this compound is CaCO3.

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
37
Which of the following elements could form more than one compound with bromine (Br)?
A)Fe
B)Cs
C)Li
D)Al
A)Fe
B)Cs
C)Li
D)Al

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
38
Using most common charges, the ionic compound that forms between calcium (Ca) and sulfur (S) has the formula
A)Ca2S
B)CaS
C)CaS2
D)Ca2S3
A)Ca2S
B)CaS
C)CaS2
D)Ca2S3

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
39
The following is a statement of the octet rule.
Representative elements normally form compounds in which each atom
has eight electrons in its outermost shell.
Representative elements normally form compounds in which each atom
has eight electrons in its outermost shell.

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
40
In order to satisfy the octet rule, an atom with the following electron arrangement can shell 1: 2 electrons shell 2: 8 electrons shell 3:5 electrons
A)gain three electrons.
B)gain two electrons.
C)gain one electron.
D)lose three electrons.
A)gain three electrons.
B)gain two electrons.
C)gain one electron.
D)lose three electrons.

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
41
How many bonding electron pairs are represented in the following Lewis structure? 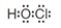
A)2
B)4
C)8
D)12
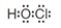
A)2
B)4
C)8
D)12

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
42
The following represents the formation of bond between atoms A and X. Based on this diagram which element is more electronegative? 
A)A
B)X
C)A and X have the same electronegativity.

A)A
B)X
C)A and X have the same electronegativity.

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
43
How many moles of oxygen are in 1.00 mol of carbon monoxide?
A)1 mol
B)2 mol
C)4 mol
D)3 mol
A)1 mol
B)2 mol
C)4 mol
D)3 mol

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
44
What is the name of the species formed when a bromine atom gains an electron?
A)bromate
B)bromide ion
C)bromine
D)bromine ion
A)bromate
B)bromide ion
C)bromine
D)bromine ion

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
45
The correct formula of an ionic compound containing Fe2+ and ClO  is
is
A)FeClO4
B)Fe2(ClO4)3
C)Fe(ClO4)2
D)Fe3(ClO4)2
 is
isA)FeClO4
B)Fe2(ClO4)3
C)Fe(ClO4)2
D)Fe3(ClO4)2

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
46
The correct name for K2SO4 is
A)potassium sulfate.
B)potassium sulfide.
C)potassium sulfite.
D)potassium tetrasulfoxygen.
A)potassium sulfate.
B)potassium sulfide.
C)potassium sulfite.
D)potassium tetrasulfoxygen.

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
47
The tin compound used to strengthen teeth has the formula SnF2. Which of the following is the correct IUPAC name for this compound?
A)tin difluoride
B)monotin difluoride
C)tin fluoride
D)tin(II) fluoride
A)tin difluoride
B)monotin difluoride
C)tin fluoride
D)tin(II) fluoride

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
48
What type of particles can atoms gain or lose when they become ions?
A)protons
B)neutrons
C)electrons
D)It depends on the atom involved.
A)protons
B)neutrons
C)electrons
D)It depends on the atom involved.

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
49
Which of the following metals would not use a Roman numeral as part of its name in a compound?
A)Cr
B)Mn
C)Co
D)Ca
A)Cr
B)Mn
C)Co
D)Ca

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
50
The anion in an ionic compound whose name ends in "-ide" is
A)always a monoatomic ion.
B)always a polyatomic ion.
C)either a monoatomic or polyatomic ion.
D)positively charged.
A)always a monoatomic ion.
B)always a polyatomic ion.
C)either a monoatomic or polyatomic ion.
D)positively charged.

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
51
Consider the molecule shown below. ClCFBrI
Which element in this molecule is the central atom?
A)Cl
B)C
C)F
D)Br
E)I
Which element in this molecule is the central atom?
A)Cl
B)C
C)F
D)Br
E)I

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
52
What is the mass of 1.00 mol of sulfur trioxide?
A)48.06 g
B)80.06 g
C)32.06 g
D)112.18 g
A)48.06 g
B)80.06 g
C)32.06 g
D)112.18 g

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
53
An ion of phosphorus has a -3 charge. Which of the following is true?
A)It is a cation.
B)It contains more protons than electrons.
C)It contains 18 electrons.
D)It contains 12 protons.
A)It is a cation.
B)It contains more protons than electrons.
C)It contains 18 electrons.
D)It contains 12 protons.

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
54
What is the formula for a compound named calcium nitride?
A)CaN
B)Ca3N2
C)Ca2N3
D)Ca3N
A)CaN
B)Ca3N2
C)Ca2N3
D)Ca3N

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
55
What is the correct name for BaI2?
A)barium iodine
B)barium iodide
C)barium diiodide
D)monobarium diiodide
A)barium iodine
B)barium iodide
C)barium diiodide
D)monobarium diiodide

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
56
What is the correct IUPAC name for the following compound? SBr2
A)sulfur dibromide
B)sulfur(II) bromide
C)monosulfur dibromide
D)sulfur bromide
A)sulfur dibromide
B)sulfur(II) bromide
C)monosulfur dibromide
D)sulfur bromide

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
57
Which of the following pairs of elements is most likely to form a covalent bond?
A)Fe and H
B)Cs and Br
C)Cl and O
D)Zn and S
A)Fe and H
B)Cs and Br
C)Cl and O
D)Zn and S

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
58
Which of the following has the highest electronegativity?
A)O
B)N
C)Si
D)P
A)O
B)N
C)Si
D)P

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
59
What is the formula for the compound named as tetraphosphorus decoxide?
Assume "deca" represents 10.
A)P4O10
B)P2O5
C)P10O4
D)PO4
Assume "deca" represents 10.
A)P4O10
B)P2O5
C)P10O4
D)PO4

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
60
Which is most likely true for an atom with one valence electron?
A)It will gain one electron.
B)It will gain two electrons.
C)It will lose one electron.
D)It will lose two electrons.
A)It will gain one electron.
B)It will gain two electrons.
C)It will lose one electron.
D)It will lose two electrons.

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
61
Phosgene is a compound used as a building block of some pharmaceuticals.It contains two chlorine atoms,one oxygen atom,and one carbon atom.Complete the following statement using the appropriate chemical symbol for the elements.
The central atom in phosgene is_____________________.
The central atom in phosgene is_____________________.

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
62
Which Group 5A element has the strongest attraction for electrons? Enter the chemical symbol in the blank.
________
________

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
63
Aluminum (Al)and sulfur (S)combine to form an ionic compound.Fill in the blank with the symbol for the appropriate element.
The formula for the ionic compound formed contains two_______________________ions and three ______________________ions.
The formula for the ionic compound formed contains two_______________________ions and three ______________________ions.

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
64
The following is the Lewis structure for the compound commonly known as acetylene 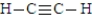
Fill in the blanks with the appropriate integer including zero if necessary (0,1,2,3,etc.).
__________________atoms in acetylene obey the octet rule.
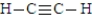
Fill in the blanks with the appropriate integer including zero if necessary (0,1,2,3,etc.).
__________________atoms in acetylene obey the octet rule.

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
65
Phosgene is a compound used as a building block of some pharmaceuticals.It contains two chlorine atoms,one oxygen atom,and one carbon atom.Complete the following statement using the appropriate chemical symbol for the elements.
Based on electronegativity values, the _________________________atom in phosgene would have a positive charge.
Based on electronegativity values, the _________________________atom in phosgene would have a positive charge.

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
66
Aluminum (Al)and sulfur (S)combine to form an ionic compound.Fill in the blank with the symbol for the appropriate element.
In the formation of this ionic compound, the _____________________atom attains a -2 charge.
In the formation of this ionic compound, the _____________________atom attains a -2 charge.

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
67
Which Period 3 element has the weakest attaction for electrons? Enter the chemical symbol in the blank.
________
________

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
68
Draw the Lewis structure for the following using lines to represent bonding electron pairs.
SiF4
SiF4

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
69
Draw the Lewis structure for the following using lines to represent bonding electron pairs.
CS2
CS2

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
70
Phosgene is a compound used as a building block of some pharmaceuticals.It contains two chlorine atoms,one oxygen atom,and one carbon atom.Complete the following statement using the appropriate chemical symbol for the elements.
In the Lewis structure for phosgene, the _____________________atom has four nonbonding electrons.
In the Lewis structure for phosgene, the _____________________atom has four nonbonding electrons.

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
71
Phosgene is a compound used as a building block of some pharmaceuticals.It contains two chlorine atoms,one oxygen atom,and one carbon atom.Complete the following statement using the appropriate chemical symbol for the elements.
In the Lewis structure for phosgene, two electrons are shared between the___________________ atom and the ____________________atom.
In the Lewis structure for phosgene, two electrons are shared between the___________________ atom and the ____________________atom.

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
72
Enter an integer number (1, 2, 3, ...) in the blank.
How many electrons are in shell 2 of a N3- ion?
____________________electrons
How many electrons are in shell 2 of a N3- ion?
____________________electrons

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
73
Which atom will be positively charged (if any) in the following bond? Enter the symbol for the element or none as appropriate in the blank.
P-P ________
P-P ________

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
74
The following is the Lewis structure for the compound commonly known as acetylene 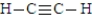
Fill in the blanks with the appropriate integer including zero if necessary (0,1,2,3,etc.).
There are____________________single bonds in acetlyene.
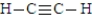
Fill in the blanks with the appropriate integer including zero if necessary (0,1,2,3,etc.).
There are____________________single bonds in acetlyene.

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
75
Redraw the following Lewis structure using lines to represent bonding electron pairs. 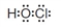
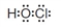

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
76
Phosgene is a compound used as a building block of some pharmaceuticals.It contains two chlorine atoms,one oxygen atom,and one carbon atom.Complete the following statement using the appropriate chemical symbol for the elements.
In the Lewis structure for phosgene, there is a double bond between_____________________and ____________________.
In the Lewis structure for phosgene, there is a double bond between_____________________and ____________________.

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
77
The following is the Lewis structure for the compound commonly known as acetylene 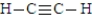
Fill in the blanks with the appropriate integer including zero if necessary (0,1,2,3,etc.).
Acetylene contains_____________________nonbonding electrons.
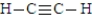
Fill in the blanks with the appropriate integer including zero if necessary (0,1,2,3,etc.).
Acetylene contains_____________________nonbonding electrons.

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
78
Draw the Lewis structure for the following using lines to represent bonding electron pairs.
H2O2
H2O2

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
79
N3- and O2- have the same number of _____________________.

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck
80
Aluminum (Al)and sulfur (S)combine to form an ionic compound.Fill in the blank with the symbol for the appropriate element.
In the formation of this compound _____________________gains electrons and __________________loses electrons.
In the formation of this compound _____________________gains electrons and __________________loses electrons.

Unlock Deck
Unlock for access to all 82 flashcards in this deck.
Unlock Deck
k this deck


