Deck 6: Chemical Reactions
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/68
Play
Full screen (f)
Deck 6: Chemical Reactions
1
An aqueous solution of sodium nitrate (NaNO3) is used in a reaction. The following should be used in the chemical equation for the reaction.
NaNO3(l)
NaNO3(l)
False
2
Overall the reactions that occur during respiration can be classified as combustion reactions.
True
3
During the carbon cycle plants produce the oxygen that is consumed by animals.
True
4
Based on the following energy diagram, the activation energy for the reaction represented is approximately 40 kcal. 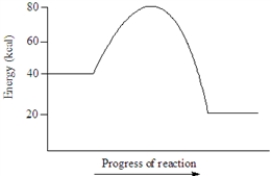


Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
5
When magnesium reacts with nitrogen, the reaction container becomes very hot. The ΔH for this reaction will have a positive sign.

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
6
When you perspire, your body is cooled because the evaporation of moisture from you skin is an exothermic process.

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
7
Photosynthesis and respiration are linked to each other in the nitrogen cycle.

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
8
The following represents a typical energy diagram. 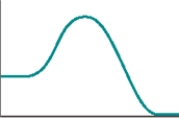 The reaction represented by this energy diagram is endothermic.
The reaction represented by this energy diagram is endothermic.
 The reaction represented by this energy diagram is endothermic.
The reaction represented by this energy diagram is endothermic.
Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
9
The combustion of glucose per gram produces more energy than the combustion of an equal mass of triolein (a fat).

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
10
One Hawaiian sweet roll contains:
2.5 g fat
18 g carbohydrate
4.0 g protein
The total energy content of four rolls (to two significant figures) is 440 Calories.
2.5 g fat
18 g carbohydrate
4.0 g protein
The total energy content of four rolls (to two significant figures) is 440 Calories.

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
11
Consider the following image representing an equilibrium mixture. The black spheres represent the reactant and the gray spheres the product.  If this reaction was started with only reactants, at equilibrium about 67% of the reactant was converted to product.
If this reaction was started with only reactants, at equilibrium about 67% of the reactant was converted to product.
 If this reaction was started with only reactants, at equilibrium about 67% of the reactant was converted to product.
If this reaction was started with only reactants, at equilibrium about 67% of the reactant was converted to product.
Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
12
The following reaction could be classified as a combustion reaction.
2Al2O3 (s) → 4Al(s) + 3O2(g)
2Al2O3 (s) → 4Al(s) + 3O2(g)

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
13
Decreasing the temperature will decrease the number of collisions that result in product formation.

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
14
Based on the following energy diagram, the heat of reaction for the reaction represented is approximately 20 kcal. 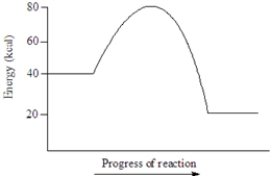


Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
15
The process that converts the energy stored in molecules to a form usable by an animal is respiration.

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
16
Reactions that have a low energy of activation tend to proceed at a faster rate.

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
17
The following represents a balanced chemical equation.
Al(NO3)3(aq) + Na 3PO4 (aq) → AlPO4(s) + 3NaNO3(aq)
Al(NO3)3(aq) + Na 3PO4 (aq) → AlPO4(s) + 3NaNO3(aq)

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
18
Consider the following image representing an equilibrium mixture. The black spheres represent the reactant and the gray spheres the product.  This reaction was started with only reactants present. If the reaction had been started with only product present, the equilibrium mixture would contain 4 gray spheres and 8 black spheres.
This reaction was started with only reactants present. If the reaction had been started with only product present, the equilibrium mixture would contain 4 gray spheres and 8 black spheres.
 This reaction was started with only reactants present. If the reaction had been started with only product present, the equilibrium mixture would contain 4 gray spheres and 8 black spheres.
This reaction was started with only reactants present. If the reaction had been started with only product present, the equilibrium mixture would contain 4 gray spheres and 8 black spheres.
Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
19
Consider the following reaction. 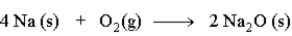 The mass relationship between sodium (Na) and oxygen (O2) is:
The mass relationship between sodium (Na) and oxygen (O2) is: 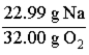
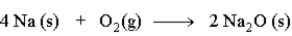 The mass relationship between sodium (Na) and oxygen (O2) is:
The mass relationship between sodium (Na) and oxygen (O2) is: 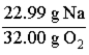

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
20
Consider the following image. 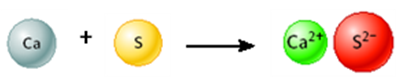
This is a representation of a chemical reaction.
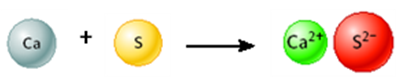
This is a representation of a chemical reaction.

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
21
The Haber Process is a method for the production of ammonia, an important chemical intermediate. The reaction for this process is:
N2 + 3H2 → 2NH3
When the equation is correctly interpreted in terms of moles, how many moles of H2 will react with one mole of N2?
A)1
B)2
C)3
D)6
N2 + 3H2 → 2NH3
When the equation is correctly interpreted in terms of moles, how many moles of H2 will react with one mole of N2?
A)1
B)2
C)3
D)6

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
22
Which of the following most closely defines the term "reaction rate"?
A)the temperature needed to initiate a reaction
B)the position of a reaction at equilibrium
C)the speed of a reaction
D)the activation energy in the presence of a catalyst
A)the temperature needed to initiate a reaction
B)the position of a reaction at equilibrium
C)the speed of a reaction
D)the activation energy in the presence of a catalyst

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
23
According to the law of mass conservation, which of the following may NEVER occur as a result of a chemical reaction?
A)creation of new chemical species
B)reaction of starting materials
C)rearranged to form new substances
D)reduction in total mass
A)creation of new chemical species
B)reaction of starting materials
C)rearranged to form new substances
D)reduction in total mass

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
24
Consider the following reaction: 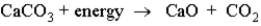 This reaction:
This reaction:
A)is endothermic.
B)has a negative ΔH.
C)makes the surroundings warmer.
D)converts chemical energy into thermal energy.
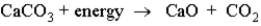 This reaction:
This reaction:A)is endothermic.
B)has a negative ΔH.
C)makes the surroundings warmer.
D)converts chemical energy into thermal energy.

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
25
When the equation below is properly balanced, what coefficient is of KClO3?
KClO3 → KCl + O2
A)1
B)2
C)3
D)4
KClO3 → KCl + O2
A)1
B)2
C)3
D)4

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
26
Consider the following reaction.
NaOH(aq) + H2SO4(aq) → Na2SO4(aq) + H2O(l)
Which of the following represents the correct mass relationships, respectively, across the balanced equation?
A)40.00 g NaOH, 98.08 g H2SO4, 142.04 g Na2SO4, 18.02 g H2O
B)40.00 g NaOH, 40.04 g H2SO4, 142.04 g Na2SO4, 9.010 g H2O
C)80.00 g NaOH, 98.08 g H2SO4, 142.04 g Na2SO4, 36.04 g H2O
D)80.00 g NaOH, 98.08 g H2SO4, 142.04 g Na2SO4, 18.02 g H2O
NaOH(aq) + H2SO4(aq) → Na2SO4(aq) + H2O(l)
Which of the following represents the correct mass relationships, respectively, across the balanced equation?
A)40.00 g NaOH, 98.08 g H2SO4, 142.04 g Na2SO4, 18.02 g H2O
B)40.00 g NaOH, 40.04 g H2SO4, 142.04 g Na2SO4, 9.010 g H2O
C)80.00 g NaOH, 98.08 g H2SO4, 142.04 g Na2SO4, 36.04 g H2O
D)80.00 g NaOH, 98.08 g H2SO4, 142.04 g Na2SO4, 18.02 g H2O

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
27
Which of the following will influence all reaction rates?
A)the presence of catalysts
B)the temperature of reactants
C)the concentration of reactants
D)More than one response is correct.
A)the presence of catalysts
B)the temperature of reactants
C)the concentration of reactants
D)More than one response is correct.

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
28
Which of the following reactions is correctly balanced?
A)N2 + H → 2NH3
B)2H2O + C → CO + 2H2
C)Zn + 2HCl → H2 + ZnCl2
D)CO + O2 → CO2
A)N2 + H → 2NH3
B)2H2O + C → CO + 2H2
C)Zn + 2HCl → H2 + ZnCl2
D)CO + O2 → CO2

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
29
When you inhale (breathe in), oxygen is entering your respiratory system and when you exhale (breathe out), carbon dioxide is leaving your respiratory system.

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
30
If the reaction given below occurs and pure A and B were mixed, which of the following would take place as equilibrium was established?
A + B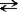 C
C
A)The concentration of C would increase for a time, then remain constant.
B)The concentration of A would increase for a time, then decrease.
C)The concentration of B would increase for a time, then remain constant.
D)Both a and c will occur.
A + B
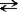 C
CA)The concentration of C would increase for a time, then remain constant.
B)The concentration of A would increase for a time, then decrease.
C)The concentration of B would increase for a time, then remain constant.
D)Both a and c will occur.

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
31
In the equation for an exothermic reaction, the word "energy" appears
A)on the right side.
B)on the left side.
C)on both sides.
D)on neither side.
A)on the right side.
B)on the left side.
C)on both sides.
D)on neither side.

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
32
A block of iron forming a pool of the liquid metal is correctly classified as
A)a chemical change.
B)a physical change.
C)both a chemical and a physical change.
D)neither a chemical nor a physical change.
A)a chemical change.
B)a physical change.
C)both a chemical and a physical change.
D)neither a chemical nor a physical change.

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
33
Which of the following is a physical property of matter?
A)does not burn.
B)produces a gas when placed in an acid
C)freezes at −10°F
D)the surface of a solid turns black in air
A)does not burn.
B)produces a gas when placed in an acid
C)freezes at −10°F
D)the surface of a solid turns black in air

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
34
Consider the following reaction.
NaOH(aq) + HCl(aq) → NaCl(aq) + H2O(l)
If 25.0 mL of a 0.100 M solution of NaOH reacted with excess HCl, how many moles of NaCl could form?
A)25.0 mol
B)0.100 mol
C)0.00250 mol
D)2.50 mol
NaOH(aq) + HCl(aq) → NaCl(aq) + H2O(l)
If 25.0 mL of a 0.100 M solution of NaOH reacted with excess HCl, how many moles of NaCl could form?
A)25.0 mol
B)0.100 mol
C)0.00250 mol
D)2.50 mol

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
35
How many moles of N2 are required to completely react with 3.53 grams of H2 for the following balanced chemical equation?
N2 + 3H2 → 2NH3
A)16.4 mol
B)1.17 mol
C)0.584 mol
D)5.25 mol
N2 + 3H2 → 2NH3
A)16.4 mol
B)1.17 mol
C)0.584 mol
D)5.25 mol

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
36
When natural gas (predominantly methane, CH4) burns in air. The following reaction occurs. How much energy is involved in the combustion of 13.0 g of methane? 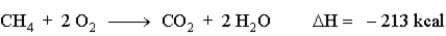
A)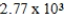 kcal
kcal
B)16.4 kcal
C)173 kcal
D)0.979 kcal
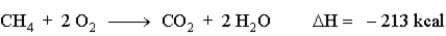
A)
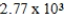 kcal
kcalB)16.4 kcal
C)173 kcal
D)0.979 kcal

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
37
Consider the following generic reaction.
2 A(s) + 3 B(l) → C(g)
If 58.0 g of A reacted completely with B to produce 100.8 g of C, what mass of B reacted?
A)58.0 g
B)174 g
C)87.0 g
D)42.8 g
E)The mass cannot be determined without knowing the identities of reactants and products.
2 A(s) + 3 B(l) → C(g)
If 58.0 g of A reacted completely with B to produce 100.8 g of C, what mass of B reacted?
A)58.0 g
B)174 g
C)87.0 g
D)42.8 g
E)The mass cannot be determined without knowing the identities of reactants and products.

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
38
Which of the following statements is not true about the reaction as written below? 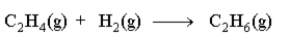
A)Hydrogen is a reactant.
B)C2H4 is a reactant.
C)The reaction is balanced.
D)The reaction is at equilibrium.
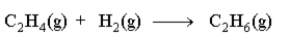
A)Hydrogen is a reactant.
B)C2H4 is a reactant.
C)The reaction is balanced.
D)The reaction is at equilibrium.

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
39
Ethanol is produced industrially by the acid catalyzed reaction of ethylene with water. The balanced equation for this reaction is:
C2H4(g) + H2O(g) → C2H5OH(l)
If water is present in excess, how many grams of ethanol can be produced from 5.75 g of ethylene?
A)265 g
B)0.205 g
C)3.50 g
D)9.44 g
C2H4(g) + H2O(g) → C2H5OH(l)
If water is present in excess, how many grams of ethanol can be produced from 5.75 g of ethylene?
A)265 g
B)0.205 g
C)3.50 g
D)9.44 g

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
40
The reactants in a chemical reaction are analogous to which of the following?
A)a baked cake
B)the flour, eggs, chocolate used to bake a cake
C)the cake pan.
D)the heat of the oven
A)a baked cake
B)the flour, eggs, chocolate used to bake a cake
C)the cake pan.
D)the heat of the oven

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
41
The following would be classified as a _______________________reaction. 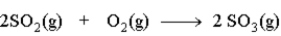
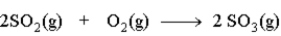

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
42
Which of the following symbols is used to denote a reversible reaction?
A)→
B)←
C)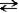
D)↔
A)→
B)←
C)
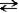
D)↔

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
43
If a reaction occurs very rapidly, even at a relatively low temperature, which of the following is probably true?
A)The reaction is endothermic.
B)The reaction is exothermic.
C)The reaction has a low activation energy.
D)The reaction is at equilibrium.
A)The reaction is endothermic.
B)The reaction is exothermic.
C)The reaction has a low activation energy.
D)The reaction is at equilibrium.

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
44
Consider the following reaction. 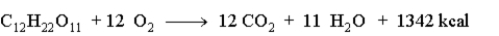 How many grams of sucrose would produce 2546 kcal?
How many grams of sucrose would produce 2546 kcal?
A)649.4 g
B)1342 g
C)180.4 g
D)1.897 g
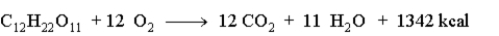 How many grams of sucrose would produce 2546 kcal?
How many grams of sucrose would produce 2546 kcal?A)649.4 g
B)1342 g
C)180.4 g
D)1.897 g

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
45
Which of the following is the best definition of a catalyst?
A)A catalyst is a material that speeds up the rate of a chemical reaction.
B)A catalyst is a material that speeds up the rate of a chemical reaction without participating in the reaction.
C)A catalyst is a material that speeds up the rate of a chemical reaction without being consumed during the reaction.
D)All of the above are equally good definitions.
A)A catalyst is a material that speeds up the rate of a chemical reaction.
B)A catalyst is a material that speeds up the rate of a chemical reaction without participating in the reaction.
C)A catalyst is a material that speeds up the rate of a chemical reaction without being consumed during the reaction.
D)All of the above are equally good definitions.

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
46
What is the name of a branch on the principal carbon chain that contains three carbon atoms?
A)pentyl
B)propyl
C)ethyl
D)methyl
A)pentyl
B)propyl
C)ethyl
D)methyl

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
47
Consider the following energy diagram showing a reaction with and without a catalyst.. 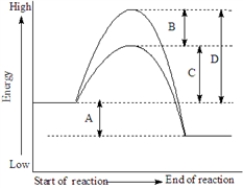
Fill in the blank with the appropriate letter (A,B,C,etc.).
The activation energy of the catalyzed reaction is represented by_____________________.

Fill in the blank with the appropriate letter (A,B,C,etc.).
The activation energy of the catalyzed reaction is represented by_____________________.

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
48
Copper reacts with nitric acid as given in the following reaction. 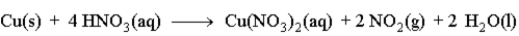 Predict the effect of the given change on the rate of the reaction by filling in the blank with one of the following terms.
Predict the effect of the given change on the rate of the reaction by filling in the blank with one of the following terms.
increase
decrease
no effect
The concentration of the nitric acid is increased. ______________________
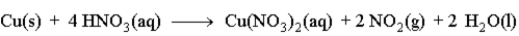 Predict the effect of the given change on the rate of the reaction by filling in the blank with one of the following terms.
Predict the effect of the given change on the rate of the reaction by filling in the blank with one of the following terms.increase
decrease
no effect
The concentration of the nitric acid is increased. ______________________

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
49
Consider the following energy diagram showing a reaction with and without a catalyst.. 
Fill in the blank with the appropriate letter (A,B,C,etc.).
The heat of reaction would be represented by _____________________.

Fill in the blank with the appropriate letter (A,B,C,etc.).
The heat of reaction would be represented by _____________________.

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
50
Consider a sample of water in a closed container. When the reaction H2O(l)  H2O(g)
H2O(g)
Has reached equilibrium, what can we say about any specific water molecule?
A)If it was present in the liquid it will always remain in the liquid.
B)If it was present in the gas it will always remain in the gas.
C)It will sometimes be in the liquid phase and sometimes in the gas phase.
D)Both a and b are true.
 H2O(g)
H2O(g)Has reached equilibrium, what can we say about any specific water molecule?
A)If it was present in the liquid it will always remain in the liquid.
B)If it was present in the gas it will always remain in the gas.
C)It will sometimes be in the liquid phase and sometimes in the gas phase.
D)Both a and b are true.

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
51
When the following equation is balanced using integers, 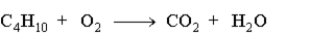 the number of atoms of carbon represented in reactants or products is:
the number of atoms of carbon represented in reactants or products is:
A)8 atoms.
B)4 atoms
C)6 atoms.
D)10 atoms.
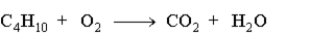 the number of atoms of carbon represented in reactants or products is:
the number of atoms of carbon represented in reactants or products is:A)8 atoms.
B)4 atoms
C)6 atoms.
D)10 atoms.

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
52
If the 'Nutrition Facts' on a food label contains Total Fat 5.0 g, Total Carbohydrate 35 g, and Total Protein 7.0 g, how many Total kcal would be needed for the average healthy person to burn this food item?
A)1.8 × 102 kcal
B)2.1 × 102 kcal
C)4.2 × 102 kcal
D)4.0 × 102 kcal
A)1.8 × 102 kcal
B)2.1 × 102 kcal
C)4.2 × 102 kcal
D)4.0 × 102 kcal

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
53
Complete the following sentence using the terms provided below.
nutritive value
carbohydrate
protein
fat
Calorie
kilocalorie
energy content
_______________________refers to the characteristic amount of heat produced when a nutrient is burned.
nutritive value
carbohydrate
protein
fat
Calorie
kilocalorie
energy content
_______________________refers to the characteristic amount of heat produced when a nutrient is burned.

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
54
Copper reacts with nitric acid as given in the following reaction. 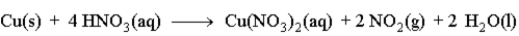 Predict the effect of the given change on the rate of the reaction by filling in the blank with one of the following terms.
Predict the effect of the given change on the rate of the reaction by filling in the blank with one of the following terms.
increase
decrease
no effect
A catalyst is added to the reaction mixture. ____________________
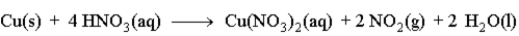 Predict the effect of the given change on the rate of the reaction by filling in the blank with one of the following terms.
Predict the effect of the given change on the rate of the reaction by filling in the blank with one of the following terms.increase
decrease
no effect
A catalyst is added to the reaction mixture. ____________________

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
55
Consider the following energy diagram showing a reaction with and without a catalyst.. 
Fill in the blank with the appropriate letter (A,B,C,etc.).
The lowering of the activation energy by the catalyst is represented by__________________.

Fill in the blank with the appropriate letter (A,B,C,etc.).
The lowering of the activation energy by the catalyst is represented by__________________.

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
56
Which of the following the appropriate ending for the name of many aromatic compounds?
A)benzene
B)ane
C)yne
D)ene
A)benzene
B)ane
C)yne
D)ene

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
57
Copper reacts with nitric acid as given in the following reaction. 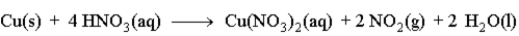 Predict the effect of the given change on the rate of the reaction by filling in the blank with one of the following terms.
Predict the effect of the given change on the rate of the reaction by filling in the blank with one of the following terms.
increase
decrease
no effect
The copper metal is ground into a very fine powder. ______________________
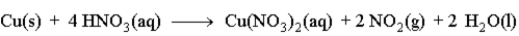 Predict the effect of the given change on the rate of the reaction by filling in the blank with one of the following terms.
Predict the effect of the given change on the rate of the reaction by filling in the blank with one of the following terms.increase
decrease
no effect
The copper metal is ground into a very fine powder. ______________________

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
58
Per gram ________________________ produces the largest amount of energy when burned.

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
59
Copper reacts with nitric acid as given in the following reaction. 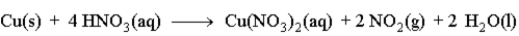 Predict the effect of the given change on the rate of the reaction by filling in the blank with one of the following terms.
Predict the effect of the given change on the rate of the reaction by filling in the blank with one of the following terms.
increase
decrease
no effect
The temperature of the reaction is decreased. _____________________
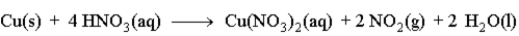 Predict the effect of the given change on the rate of the reaction by filling in the blank with one of the following terms.
Predict the effect of the given change on the rate of the reaction by filling in the blank with one of the following terms.increase
decrease
no effect
The temperature of the reaction is decreased. _____________________

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
60
Many molecular collisions do not result in chemical reaction. Which of the following explains this observation?
A)The colliding molecules may not be the correct chemicals.
B)The colliding molecules do not have sufficient energy.
C)The colliding molecules do not have the correct orientations.
D)All of the above are potential factors.
A)The colliding molecules may not be the correct chemicals.
B)The colliding molecules do not have sufficient energy.
C)The colliding molecules do not have the correct orientations.
D)All of the above are potential factors.

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
61
Complete each of the following sentences using one of the terms listed below.
physical change
chemical change
reactant
product
coefficient
physical state
Consider the image below. In the balanced chemical equation hydrogen would be written as H2(g) where the parenthetical designation represents its________________.
physical change
chemical change
reactant
product
coefficient
physical state
Consider the image below. In the balanced chemical equation hydrogen would be written as H2(g) where the parenthetical designation represents its________________.


Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
62
Complete each of the following sentences using one of the terms listed below.
physical change
chemical change
reactant
product
coefficient
physical state
The image below represents a________________.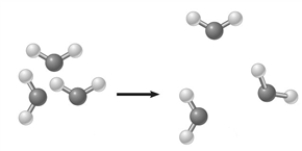
physical change
chemical change
reactant
product
coefficient
physical state
The image below represents a________________.


Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
63
When 15.0 g of sucrose is burned during metabolism in the body according to the following equation, what mass in grams of oxygen is needed? 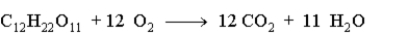
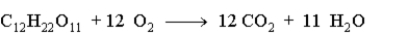

Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
64
Complete each of the following sentences using one of the terms listed below.
physical change
chemical change
reactant
product
coefficient
physical state
Consider the image below. If the balanced equation for this reaction were written oxygen would have a ___________ of 1.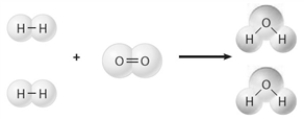
physical change
chemical change
reactant
product
coefficient
physical state
Consider the image below. If the balanced equation for this reaction were written oxygen would have a ___________ of 1.


Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
65
When a nickel-cadmium battery is recharged, the following reaction occurs. What mass in grams of nickel(II) hydroxide is consumed if 2.40 g of Cd is produced? 


Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
66
Draw the energy diagram for the following reaction which represents the burning of the amino acid alanine. 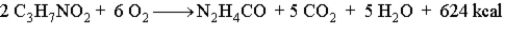 Indicate on the diagram the activation energy and the heat of reaction.
Indicate on the diagram the activation energy and the heat of reaction.
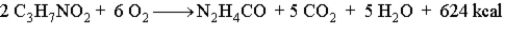 Indicate on the diagram the activation energy and the heat of reaction.
Indicate on the diagram the activation energy and the heat of reaction.
Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
67
Complete each of the following sentences using one of the terms listed below.
physical change
chemical change
reactant
product
coefficient
physical state
The image below represents a________________.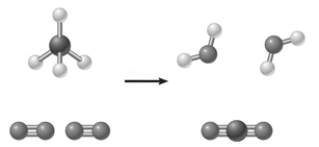
physical change
chemical change
reactant
product
coefficient
physical state
The image below represents a________________.


Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck
68
Complete each of the following sentences using one of the terms listed below.
physical change
chemical change
reactant
product
coefficient
physical state
In the image below hydrogen sulfide represents the________________.
physical change
chemical change
reactant
product
coefficient
physical state
In the image below hydrogen sulfide represents the________________.


Unlock Deck
Unlock for access to all 68 flashcards in this deck.
Unlock Deck
k this deck


