Deck 8: Nuclear Chemistry
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/65
Play
Full screen (f)
Deck 8: Nuclear Chemistry
1
Which of the following reactions represents the nuclear equation for the alpha decay of Kr-76?
A)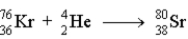
B)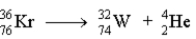
C)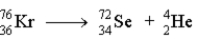
D)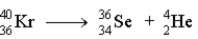
A)
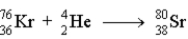
B)
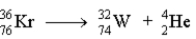
C)
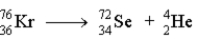
D)
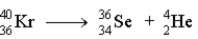
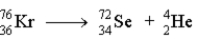
2
A scintillation counter is more sensitive in measuring radiation than a Geiger counter.
True
3
Ultraviolet radiation is an example of ionizing radiation.
False
4
The half-life of a sample depends on the identity of the isotope and not the amount present while activity does depend on the amount of the sample.

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
5
Background radiation is ionizing radiation emitted from natural sources and essentially cannot be avoided.

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
6
What is the nuclear composition of 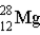 ?
?
A)12 neutrons and 28 protons
B)12 neutrons and 16 protons
C)12 protons and 28 neutrons
D)12 protons and 16 neutrons
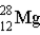 ?
?A)12 neutrons and 28 protons
B)12 neutrons and 16 protons
C)12 protons and 28 neutrons
D)12 protons and 16 neutrons

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
7
What is the nuclear symbol for atom that contains 30 protons and 35 neutrons?
A)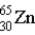
B)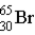
C)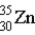
D)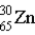
E)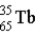
A)
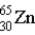
B)
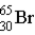
C)
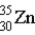
D)
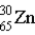
E)
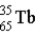

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
8
The decay of 1 kg of uranium produces more energy than the combustion of an identical mass of uranium.

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
9
The following image could illustrate the process of nuclear fusion. 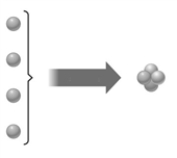


Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
10
The fusion of deuterium ( 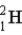 ) and tritium (
) and tritium ( 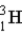 ) holds some promise as a source of nuclear energy. If a neutron is produced, the other product of the reaction is
) holds some promise as a source of nuclear energy. If a neutron is produced, the other product of the reaction is 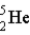 .
.
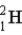 ) and tritium (
) and tritium ( 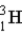 ) holds some promise as a source of nuclear energy. If a neutron is produced, the other product of the reaction is
) holds some promise as a source of nuclear energy. If a neutron is produced, the other product of the reaction is 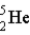 .
.
Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
11
Consider the graph of activity versus time for a naturally occurring isotope. 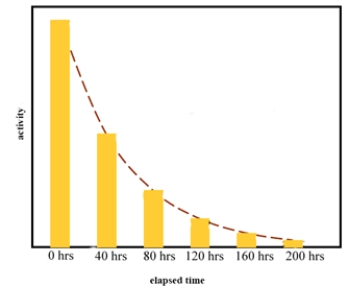 The half-life of this isotope is between 80 and 120 hours.
The half-life of this isotope is between 80 and 120 hours.
 The half-life of this isotope is between 80 and 120 hours.
The half-life of this isotope is between 80 and 120 hours.
Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
12
The chemical symbol for a positron is: 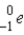
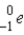

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
13
The following correctly applies to balancing nuclear equations.
"The sums of the mass numbers of the products and reactants must be equal."
"The sums of the mass numbers of the products and reactants must be equal."

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
14
A reaction produces 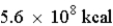 when one mole of reactant is converted to product. This reaction is likely to be a chemical reaction.
when one mole of reactant is converted to product. This reaction is likely to be a chemical reaction.
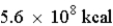 when one mole of reactant is converted to product. This reaction is likely to be a chemical reaction.
when one mole of reactant is converted to product. This reaction is likely to be a chemical reaction.
Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
15
The following represents a nuclear reaction. 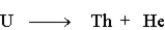
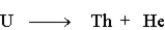

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
16
In medicine, an equivalent dose commonly has units of μCi while activity has units of mrem.

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
17
The following image depicts beta decay. 


Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
18
The following reaction would be classified alpha decay. 


Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
19
In the following reaction, 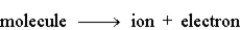 another name for the ion in the product would be a radical.
another name for the ion in the product would be a radical.
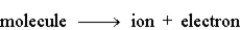 another name for the ion in the product would be a radical.
another name for the ion in the product would be a radical.
Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
20
The nuclear symbol for an americium atom with a mass number of 243 is:
A)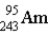
B)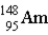
C)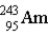
D)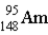
A)
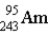
B)
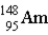
C)
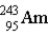
D)
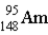

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
21
Which of the following is true of nuclear fission but not nuclear fusion?
A)Large amounts of energy are produced during the reaction.
B)The mass of the products is less than the mass of the reactants.
C)The mass number of the product nuclei is larger than those of reactants.
D)Type of reaction used to generate electrical energy.
E)By products of the reaction do not pose serious health or storage threats.
A)Large amounts of energy are produced during the reaction.
B)The mass of the products is less than the mass of the reactants.
C)The mass number of the product nuclei is larger than those of reactants.
D)Type of reaction used to generate electrical energy.
E)By products of the reaction do not pose serious health or storage threats.

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
22
Which of the following would be classified as a particle?
A)X-rays
B)radio waves
C)ultraviolet light
D)beta radiation
A)X-rays
B)radio waves
C)ultraviolet light
D)beta radiation

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
23
One person standing 10 m from a radiation source absorbs 1000 particles. A second person is standing 30 m from the same source, how many particles does the second person absorb? Assume that there is no difference in shielding between the two people.
A)1000 particles
B)333 particles
C)111 particles
D)33 particles
E)50 particles
A)1000 particles
B)333 particles
C)111 particles
D)33 particles
E)50 particles

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
24
If you absorb 0.0056 rad of energy from neutrons that have a WR of 8, what is the equivalent dose?
A)0.70 mrem
B)0.045 mrem
C)45 mrem
D)5.6 mrem
E)1.4 mrem
A)0.70 mrem
B)0.045 mrem
C)45 mrem
D)5.6 mrem
E)1.4 mrem

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
25
Iodine-131 is used in the treatment of thyroid disorders. 1.0 μg of this isotope produces 3.5 mCi. If a patient is given a dose of 165 mCi, what mass of iodine-131 was in the sample?
A)0.021 μg
B)47 μg
C)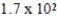 μg
μg
D)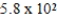 μg
μg
A)0.021 μg
B)47 μg
C)
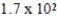 μg
μgD)
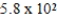 μg
μg
Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
26
When uranium-235 fissions the energy is emitted
A)as the kinetic energy of high speed particles.
B)as gamma radiation.
C)as X-rays.
D)as a combination kinetic energy of high speed particles and gamma radiation.
E)as a combination of kinetic energy of high speed particles, X-rays, and gamma radiation.
A)as the kinetic energy of high speed particles.
B)as gamma radiation.
C)as X-rays.
D)as a combination kinetic energy of high speed particles and gamma radiation.
E)as a combination of kinetic energy of high speed particles, X-rays, and gamma radiation.

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
27
What is the element produced by the alpha decay of 243Am ?
A)Bk
B)Np
C)Pa
D)Es
A)Bk
B)Np
C)Pa
D)Es

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
28
Which of the following correctly ranks the amount of energy produced in the reactions given from smallest to largest?
A)burn 1 g carbon < fission 1 g uranium < fusion 1 g hydrogen
B)fission 1 g uranium < burn 1 g carbon < fusion 1 g hydrogen
C)burn 1 g carbon < fusion 1 g hydrogen < fission 1 g uranium
D)burn 1 g carbon < fission 1 g uranium = fusion 1 g hydrogen
A)burn 1 g carbon < fission 1 g uranium < fusion 1 g hydrogen
B)fission 1 g uranium < burn 1 g carbon < fusion 1 g hydrogen
C)burn 1 g carbon < fusion 1 g hydrogen < fission 1 g uranium
D)burn 1 g carbon < fission 1 g uranium = fusion 1 g hydrogen

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
29
Consider the process illustrated in the image below. 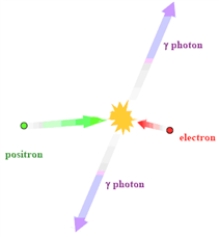 Which of the following is true of this process?
Which of the following is true of this process?
A)The process illustrated is annihilation.
B)This process is used in radiation therapy.
C)Positrons have fairly long half-lives making for easy detection.
D)All of the statements are true of this process.
 Which of the following is true of this process?
Which of the following is true of this process?A)The process illustrated is annihilation.
B)This process is used in radiation therapy.
C)Positrons have fairly long half-lives making for easy detection.
D)All of the statements are true of this process.

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
30
Consider the following reaction. 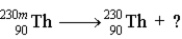 Which of the following is not true of this reaction?
Which of the following is not true of this reaction?
A)The question mark represents γ.
B)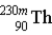 is a metastable isotope.
is a metastable isotope.
C)Ionizing radiation is a product of the reaction.
D)a and b
E)a, b, and c
 Which of the following is not true of this reaction?
Which of the following is not true of this reaction?A)The question mark represents γ.
B)
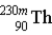 is a metastable isotope.
is a metastable isotope.C)Ionizing radiation is a product of the reaction.
D)a and b
E)a, b, and c

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
31
The following decay 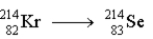 occurs through the emission of
occurs through the emission of
A)an alpha particle
B)a beta particle
C)a positron
D)a proton
E)neutron
 occurs through the emission of
occurs through the emission ofA)an alpha particle
B)a beta particle
C)a positron
D)a proton
E)neutron

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
32
A patient received 67 mrem of radiation during a treatment. What is this equivalent dose in Sv?
A)67 Sv
B)0.67 Sv
C)0.00067 Sv
D)6.7 Sv
A)67 Sv
B)0.67 Sv
C)0.00067 Sv
D)6.7 Sv

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
33
Which of the following is not a form of ionizing radiation?
A)beta particles
B)gamma radiation
C)microwaves
D)X-rays
E)All are forms of ionizing radiation.
A)beta particles
B)gamma radiation
C)microwaves
D)X-rays
E)All are forms of ionizing radiation.

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
34
The half-life of 14C is 5,700 years. In a living organism the 14C content is a constant of 0.22 Bq/g. If an unknown sample contains a 14C activity of about 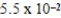 Bq/g , approximately how old is the sample?
Bq/g , approximately how old is the sample?
A)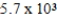 yr
yr
B)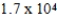 yr
yr
C)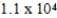 yr
yr
D)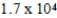 yr
yr
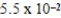 Bq/g , approximately how old is the sample?
Bq/g , approximately how old is the sample?A)
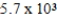 yr
yrB)
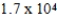 yr
yrC)
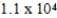 yr
yrD)
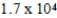 yr
yr
Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
35
Which of the following radiation units takes into consideration the effect on biological systems?
A)Bq
B)Sv
C)Ci
D)rad
A)Bq
B)Sv
C)Ci
D)rad

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
36
Which of the measurements of activity given below is equivalent to 23 disintegrations per second?
A)23 Ci
B)23 Bq
C)23 Sv
D)23 rem
A)23 Ci
B)23 Bq
C)23 Sv
D)23 rem

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
37
Emission of which of the following does not produce a change in the identity of the element?
A)alpha
B)beta
C)gamma
D)positron
E)All produce a change in the identity of the element.
A)alpha
B)beta
C)gamma
D)positron
E)All produce a change in the identity of the element.

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
38
Which of the following types of diagnostic tools does not use ionizing radiation?
A)MRI
B)CT
C)PET
D)X-rays
A)MRI
B)CT
C)PET
D)X-rays

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
39
The activity of 1.0 μg of fluorine-18 is 89 Ci. Which of the following is not equivalent to this activity?
A)8.9 × 104 mCi
B)8.9 × 107 μCi
C)3.3 × 1012 Bq
D)3.3 × 103 GBq
E)All of these quantities are equivalent.
A)8.9 × 104 mCi
B)8.9 × 107 μCi
C)3.3 × 1012 Bq
D)3.3 × 103 GBq
E)All of these quantities are equivalent.

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
40
The half-life of is bromine-80 is 17.6 min. Approximately how long will it take for the activity of this isotope to drop from to 320 mCi to 10 mCi?
A)70 min
B)106 min
C)53 min
D)88 min
E)123 mi
A)70 min
B)106 min
C)53 min
D)88 min
E)123 mi

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
41
The image below is a representation of the formation of radical (X)by different types of radiation (1,2,and 3). 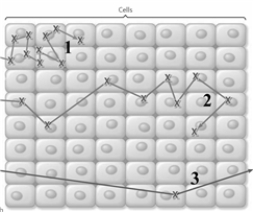 Based on this diagram answer the following questions.
Based on this diagram answer the following questions.
This type of radiation is useful for diagnostic imaging:
_____
 Based on this diagram answer the following questions.
Based on this diagram answer the following questions.This type of radiation is useful for diagnostic imaging:
_____

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
42
Write the nuclear equation for the beta decay of iodine-131.

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
43
Write the nuclear equation for the fusion of carbon-11 and helium-4.

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
44
Fill in the blanks with the appropriate term from the list below.
alpha
beta
gamma
___________________ radiation poses the smallest hazard if taken internally.
alpha
beta
gamma
___________________ radiation poses the smallest hazard if taken internally.

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
45
A common questions asked of every patient admitted to a hospital is 'Have you experienced an unexpected/unintentional weight loss of 15 or more pounds in the last three months'. Why do think this is an important question to ask? What could it be alluding to?

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
46
As the strength of a radioactive source increases, the likelihood of radiation damage
A)decreases.
B)increases.
C)remains constant.
A)decreases.
B)increases.
C)remains constant.

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
47
Write the nuclear equation for the positron emission of carbon-11.

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
48
If there has been no change in a patient's diet, yet they've experienced weight loss, and are found to have a cancer-explain why having a cancer could cause weight loss.

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
49
Cobalt-60 used in cancer radiation therapy can be produced by the following reaction in a nuclear reactor.  Supply the identity of the missing particle represented by the ?.
Supply the identity of the missing particle represented by the ?.
 Supply the identity of the missing particle represented by the ?.
Supply the identity of the missing particle represented by the ?.
Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
50
The image below is a representation of the formation of radical (X)by different types of radiation (1,2,and 3).  Based on this diagram answer the following questions.
Based on this diagram answer the following questions.
Radiation that can be represented as shown below:
_____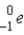
 Based on this diagram answer the following questions.
Based on this diagram answer the following questions.Radiation that can be represented as shown below:
_____

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
51
Write the nuclear equation for the alpha decay of radium-226.

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
52
Fill in the blanks with the appropriate term from the list below.
alpha
beta
gamma
___________________produce the largest number of radicals upon tissue exposure.
alpha
beta
gamma
___________________produce the largest number of radicals upon tissue exposure.

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
53
X-rays have radiation properties (effects) most similar to ____________________radiation.

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
54
A sample of a radioacitive isotope has an activity of 80 μCi when produced. 60 min later when it is being used the activity has dropped to 10 μCi. What is the half-life of this isotope?
A)60 min
B)30 min
C)20 min
D)15 min
A)60 min
B)30 min
C)20 min
D)15 min

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
55
In addition to a paper barrier, Plexiglas® is would be needed for shielding against _____________________ radiation.

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
56
The image below is a representation of the formation of radical (X)by different types of radiation (1,2,and 3).  Based on this diagram answer the following questions.
Based on this diagram answer the following questions.
This type of radiation can produce "secondary" X-ray photons. _____
 Based on this diagram answer the following questions.
Based on this diagram answer the following questions.This type of radiation can produce "secondary" X-ray photons. _____

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
57
Fill in the blanks with the appropriate term from the list below.
alpha
beta
gamma
______________________ poses the largest hazard when exposure is from an external source.
alpha
beta
gamma
______________________ poses the largest hazard when exposure is from an external source.

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
58
Write the nuclear symbol for sulfur-32.

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
59
131I has a half life of 8 days. Approximately how long will it take a sample with an activity of 60 mCi to drop to about 3.75 mCi?
A)8 days
B)16 days
C)32 days
D)64 days
A)8 days
B)16 days
C)32 days
D)64 days

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
60
Fill in the blanks with the appropriate term from the list below.
alpha
beta
gamma
__________________ particles have the largest WR factor.
alpha
beta
gamma
__________________ particles have the largest WR factor.

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
61
Strontium-90 has a half-life of 28 years and is formed during nuclear explosions. If a water sample had an activity of 84 pCi in June of 2010, approximately what will be the activity in pCi at the same time in June of 2094?

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
62
Two workers absorbed equal amounts of energy from nuclear radiation. Worker A was exposed to neutrons with a WR of 14 and received a dose of 85 mrem. Worker B was exposed to beta particles. What dose did Worker B receive?

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
63
An exposure to equal amounts of energy from nuclear radiation occurred at two different sites. At the first site an employee was exposed to neutrons with a WR of 16 and received a dose of 33 mrem. At the second site a worker was exposed to alpha particles. What dose did this second empolyee receive?

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
64
Which type of radiation is the least damaging to tissue if exposure occurs from an external source? Briefly explain your reasoning.

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck
65
Which type of radiation is the most damaging to tissue if exposure occurs internally? Briefly explain your reasoning.

Unlock Deck
Unlock for access to all 65 flashcards in this deck.
Unlock Deck
k this deck


