Deck 12: Organic Acids and Bases
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/62
Play
Full screen (f)
Deck 12: Organic Acids and Bases
1
The salt of propanoic acid and the sodium ion would be named as sodium propanoate.
True
2
Serotonin is classified as a tryptamine.
True
3
In decarboxylation reactions, the carboxylic acid group produces carbon dioxide.
True
4
The following represents the general structure of a thioester. 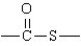

Unlock Deck
Unlock for access to all 62 flashcards in this deck.
Unlock Deck
k this deck
5
When phenol dissolves in water, it functions as
A)a weak base
B)a weak acid
C)a neutral compound
A)a weak base
B)a weak acid
C)a neutral compound

Unlock Deck
Unlock for access to all 62 flashcards in this deck.
Unlock Deck
k this deck
6
The structure of isoleucine is 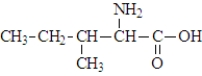 The zwitterion form of the amino acid isoleucine is
The zwitterion form of the amino acid isoleucine is 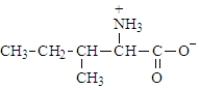
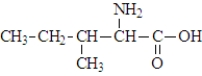 The zwitterion form of the amino acid isoleucine is
The zwitterion form of the amino acid isoleucine is 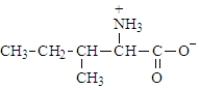

Unlock Deck
Unlock for access to all 62 flashcards in this deck.
Unlock Deck
k this deck
7
The following compound contains a thioester bond. 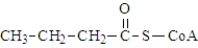
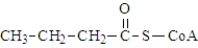

Unlock Deck
Unlock for access to all 62 flashcards in this deck.
Unlock Deck
k this deck
8
The following illustrates an amine acting as hydrogen bond donor with water. 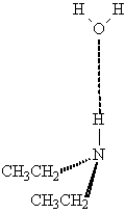


Unlock Deck
Unlock for access to all 62 flashcards in this deck.
Unlock Deck
k this deck
9
Only amino acids can form zwitterions.

Unlock Deck
Unlock for access to all 62 flashcards in this deck.
Unlock Deck
k this deck
10
One of the necessary components for fatty acid synthesis is a complex of malonic acid (shown below) and CoA. 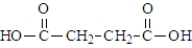 The structure of this complex at physiological pH would be:
The structure of this complex at physiological pH would be: 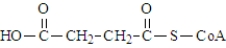
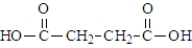 The structure of this complex at physiological pH would be:
The structure of this complex at physiological pH would be: 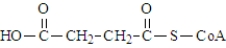

Unlock Deck
Unlock for access to all 62 flashcards in this deck.
Unlock Deck
k this deck
11
The ketone carbonyl group in the following compound is on the α carbon atom. 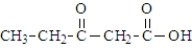
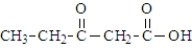

Unlock Deck
Unlock for access to all 62 flashcards in this deck.
Unlock Deck
k this deck
12
The water solubility of heptanoic acid is less than that of potassium heptanoate.

Unlock Deck
Unlock for access to all 62 flashcards in this deck.
Unlock Deck
k this deck
13
The hexanoate ion has the formula: 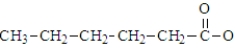
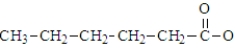

Unlock Deck
Unlock for access to all 62 flashcards in this deck.
Unlock Deck
k this deck
14
Which of the following is the molecular formula for the salt of the magnesium ion and the propanoate anion?
A)Mg(C3H5O2)2
B)Mg2C3H5O2
C)Mg(C3H6O2)2
D)MgC3H5O2
A)Mg(C3H5O2)2
B)Mg2C3H5O2
C)Mg(C3H6O2)2
D)MgC3H5O2

Unlock Deck
Unlock for access to all 62 flashcards in this deck.
Unlock Deck
k this deck
15
Certain amines called tryptamines, have an effect on the central nervous system and share a common structural feature.

Unlock Deck
Unlock for access to all 62 flashcards in this deck.
Unlock Deck
k this deck
16
The amine shown below is a tertiary amine. 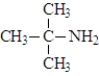
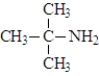

Unlock Deck
Unlock for access to all 62 flashcards in this deck.
Unlock Deck
k this deck
17
Which of the following would not be soluble in water?
A)potassium nonanoate
B)sodium nonanoate
C)nonanoic acid
D)All would exhibit about the same solubility in water.
A)potassium nonanoate
B)sodium nonanoate
C)nonanoic acid
D)All would exhibit about the same solubility in water.

Unlock Deck
Unlock for access to all 62 flashcards in this deck.
Unlock Deck
k this deck
18
The formula for cyclopentylamine is: 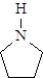


Unlock Deck
Unlock for access to all 62 flashcards in this deck.
Unlock Deck
k this deck
19
When diethylamine ionizes in water, a product of the reaction is: 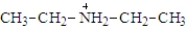
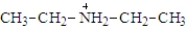

Unlock Deck
Unlock for access to all 62 flashcards in this deck.
Unlock Deck
k this deck
20
Compounds such as morphine (an amine) are often administered to a patient as a salt because the salt is more soluble in body fluids.

Unlock Deck
Unlock for access to all 62 flashcards in this deck.
Unlock Deck
k this deck
21
The following compounds are constitutional isomers. Which has the lowest boiling point?
A)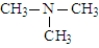
B)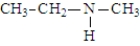
C)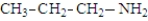
D)All boiling points are about the same.
A)
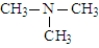
B)
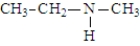
C)
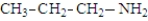
D)All boiling points are about the same.

Unlock Deck
Unlock for access to all 62 flashcards in this deck.
Unlock Deck
k this deck
22
What is the name of the following substance?
CH3-NH-CH2−CH2-CH3
A)methylpropylamine
B)propylmethylamine
C)aminobutane
D)butanamine
CH3-NH-CH2−CH2-CH3
A)methylpropylamine
B)propylmethylamine
C)aminobutane
D)butanamine

Unlock Deck
Unlock for access to all 62 flashcards in this deck.
Unlock Deck
k this deck
23
Which of the following is true of both a decarboxylation reaction and an oxidative decarboxylation reaction?
A)thiol reactant
B)production of CO2
C)presence of coenzyme
D)molecular location of the ketone group
A)thiol reactant
B)production of CO2
C)presence of coenzyme
D)molecular location of the ketone group

Unlock Deck
Unlock for access to all 62 flashcards in this deck.
Unlock Deck
k this deck
24
Which of the following solutions is most likely to have the lowest pH?
A)0.010 M propanol
B)0.010 M propanoic acid
C)0.010 M propyl amine
D)0.010 M phenol
E)0.010 M propanethiol
A)0.010 M propanol
B)0.010 M propanoic acid
C)0.010 M propyl amine
D)0.010 M phenol
E)0.010 M propanethiol

Unlock Deck
Unlock for access to all 62 flashcards in this deck.
Unlock Deck
k this deck
25
Imitrex was developed in the late 1980's and is used in the treatment of migraine. The active ingredient is shown below. 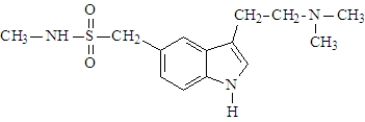 The substance would be classified as a(n):
The substance would be classified as a(n):
A)phenethylamine
B)tryptamine
C)alkaloid
D)a and c
E)b and c
 The substance would be classified as a(n):
The substance would be classified as a(n):A)phenethylamine
B)tryptamine
C)alkaloid
D)a and c
E)b and c

Unlock Deck
Unlock for access to all 62 flashcards in this deck.
Unlock Deck
k this deck
26
The following compounds are constitutional isomers. Which can form hydrogen bonds with water?
A)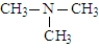
B)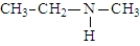
C)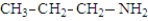
D)All can form hydrogen bonds with water.
A)
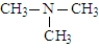
B)
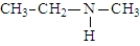
C)
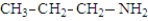
D)All can form hydrogen bonds with water.

Unlock Deck
Unlock for access to all 62 flashcards in this deck.
Unlock Deck
k this deck
27
Which of the following classes of compounds cannot form hydrogen bonds with like molecules?
A)alcohol
B)phenol
C)thiol
A)alcohol
B)phenol
C)thiol

Unlock Deck
Unlock for access to all 62 flashcards in this deck.
Unlock Deck
k this deck
28
The name of the following alkylammonium salt is: 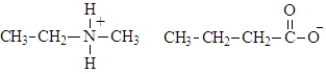
A)propylammonium butanoate
B)ethylmethylammonium butanoate
C)ethylmethylbutanoate
D)methylethylammonium propanoate
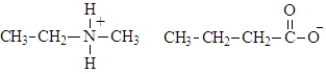
A)propylammonium butanoate
B)ethylmethylammonium butanoate
C)ethylmethylbutanoate
D)methylethylammonium propanoate

Unlock Deck
Unlock for access to all 62 flashcards in this deck.
Unlock Deck
k this deck
29
When heated, what is the product of the decarboxylation of the following substance? 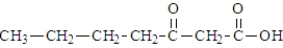
A)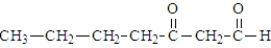
B)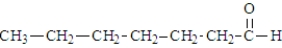
C)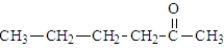
D)This compound does not decarboxylate when heated.
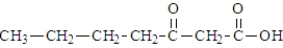
A)
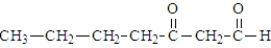
B)
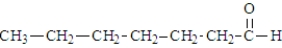
C)
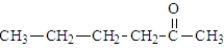
D)This compound does not decarboxylate when heated.

Unlock Deck
Unlock for access to all 62 flashcards in this deck.
Unlock Deck
k this deck
30
The following compounds are constitutional isomers. Which can function as only a hydrogen bond acceptor?
A)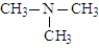
B)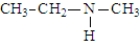
C)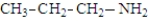
D)All can only be hydrogen bond acceptors.
A)
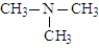
B)
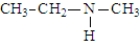
C)
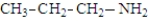
D)All can only be hydrogen bond acceptors.

Unlock Deck
Unlock for access to all 62 flashcards in this deck.
Unlock Deck
k this deck
31
The following amine is classified as: 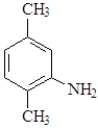
A)primary.
B)secondary.
C)tertiary.

A)primary.
B)secondary.
C)tertiary.

Unlock Deck
Unlock for access to all 62 flashcards in this deck.
Unlock Deck
k this deck
32
What is the basis for classifying amines as primary, secondary, or tertiary?
A)the number of carbon atoms bonded directly to the nitrogen atom
B)the number of carbon atoms bonded to a carbon bearing nitrogen
C)the number of nitrogen atoms present
D)the number of hydrogen atoms present
A)the number of carbon atoms bonded directly to the nitrogen atom
B)the number of carbon atoms bonded to a carbon bearing nitrogen
C)the number of nitrogen atoms present
D)the number of hydrogen atoms present

Unlock Deck
Unlock for access to all 62 flashcards in this deck.
Unlock Deck
k this deck
33
When propanthiol (structure shown below) ionizes in water, a product of the reaction is:
CH3-CH2-CH2-SH
A)CH3-CH2-CH2-SH2+
B)CH3-CH2-CH2-OH
C)CH3-CH2-CH2-S-
D)CH3-CH2-CH2-SOH
CH3-CH2-CH2-SH
A)CH3-CH2-CH2-SH2+
B)CH3-CH2-CH2-OH
C)CH3-CH2-CH2-S-
D)CH3-CH2-CH2-SOH

Unlock Deck
Unlock for access to all 62 flashcards in this deck.
Unlock Deck
k this deck
34
The active ingredient in Prozac is shown below. 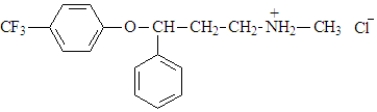 This compound:
This compound:
A)is a hydrochloride salt.
B)contains the conjugate acid of an amine.
C)was formed by the reaction of an amine and HCl.
D)all of the above
 This compound:
This compound:A)is a hydrochloride salt.
B)contains the conjugate acid of an amine.
C)was formed by the reaction of an amine and HCl.
D)all of the above

Unlock Deck
Unlock for access to all 62 flashcards in this deck.
Unlock Deck
k this deck
35
Which of the following solutions is most likely to have the highest pH?
A)0.010 M propanol
B)0.010 M propanoic acid
C)0.010 M propyl amine
D)0.010 M phenol
E)0.010 M propanethiol
A)0.010 M propanol
B)0.010 M propanoic acid
C)0.010 M propyl amine
D)0.010 M phenol
E)0.010 M propanethiol

Unlock Deck
Unlock for access to all 62 flashcards in this deck.
Unlock Deck
k this deck
36
The formula for the organic product of the following reaction is: 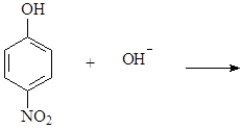
A)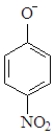
B)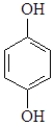
C)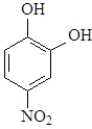
D)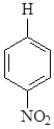
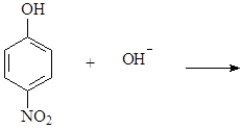
A)

B)

C)

D)


Unlock Deck
Unlock for access to all 62 flashcards in this deck.
Unlock Deck
k this deck
37
Which of the following classes of amines can act as both a hydrogen bond acceptor and a hydrogen bond donor when dissolved in water?
A)primary
B)secondary
C)tertiary
D)both primary and secondary
E)primary, secondary and tertiary
A)primary
B)secondary
C)tertiary
D)both primary and secondary
E)primary, secondary and tertiary

Unlock Deck
Unlock for access to all 62 flashcards in this deck.
Unlock Deck
k this deck
38
Which of the following compounds would be the most likely to undergo decarboxylation when simply heated?
A)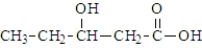
B)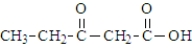
C)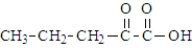
D)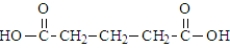
A)
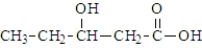
B)
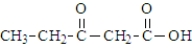
C)
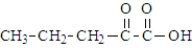
D)
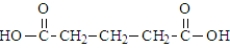

Unlock Deck
Unlock for access to all 62 flashcards in this deck.
Unlock Deck
k this deck
39
The following compound undergoes oxidative decarboxylation using coenzyme A (CoA) as the thiol. 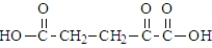 Which of the following is a product of this reaction?
Which of the following is a product of this reaction?
A)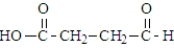
B)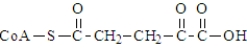
C)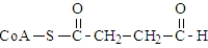
D)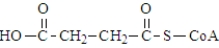
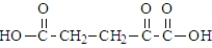 Which of the following is a product of this reaction?
Which of the following is a product of this reaction?A)
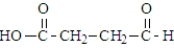
B)
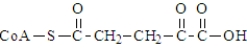
C)
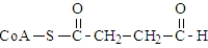
D)
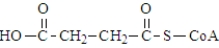

Unlock Deck
Unlock for access to all 62 flashcards in this deck.
Unlock Deck
k this deck
40
In the formation of a thioester bond, the involved hydrogen atoms:
A)are transferred to FAD.
B)are transferred to NAD+.
C)remain with the thiol.
D)remain with the aldehyde.
A)are transferred to FAD.
B)are transferred to NAD+.
C)remain with the thiol.
D)remain with the aldehyde.

Unlock Deck
Unlock for access to all 62 flashcards in this deck.
Unlock Deck
k this deck
41
Which of the following types of compounds is a good candidate for decarboxylation?
A)carboxylic acid
B)ketone
C)b-keto carboxylic acid
D)a-keto carboxylic acid
A)carboxylic acid
B)ketone
C)b-keto carboxylic acid
D)a-keto carboxylic acid

Unlock Deck
Unlock for access to all 62 flashcards in this deck.
Unlock Deck
k this deck
42
Consider the following nitrogen containing compounds. 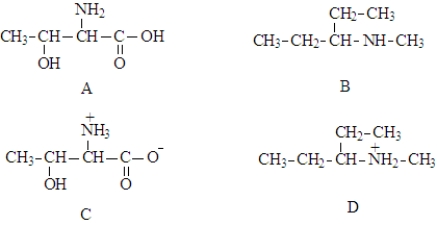 Fill in the blanks with the appropriate letter (A,B,C,D).
Fill in the blanks with the appropriate letter (A,B,C,D).
Structure___________________ contains an amino group.
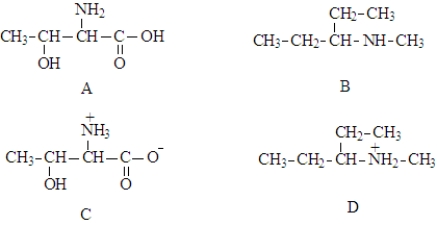 Fill in the blanks with the appropriate letter (A,B,C,D).
Fill in the blanks with the appropriate letter (A,B,C,D).Structure___________________ contains an amino group.

Unlock Deck
Unlock for access to all 62 flashcards in this deck.
Unlock Deck
k this deck
43
Consider the structures given below. 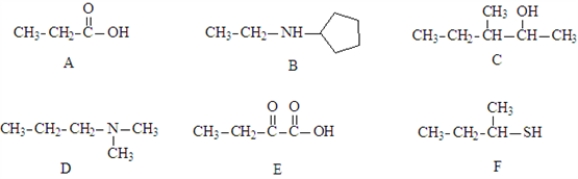 Fill in the blanks with appropriate letter (A,B,C,D,E,F).
Fill in the blanks with appropriate letter (A,B,C,D,E,F).
Structure ____________________ could be a hydrogen bond donor or acceptor in water.
 Fill in the blanks with appropriate letter (A,B,C,D,E,F).
Fill in the blanks with appropriate letter (A,B,C,D,E,F).Structure ____________________ could be a hydrogen bond donor or acceptor in water.

Unlock Deck
Unlock for access to all 62 flashcards in this deck.
Unlock Deck
k this deck
44
Which of the following is characteristic of the structure of tryptamines?
A)aromatic
B)alkene
C)secondary amine
D)all of the above
A)aromatic
B)alkene
C)secondary amine
D)all of the above

Unlock Deck
Unlock for access to all 62 flashcards in this deck.
Unlock Deck
k this deck
45
Consider the structures given below.  Fill in the blanks with appropriate letter (A,B,C,D,E,F).
Fill in the blanks with appropriate letter (A,B,C,D,E,F).
Structure__________________ and structure _______________ could react to form ethyldimethylammonium propanoate.
 Fill in the blanks with appropriate letter (A,B,C,D,E,F).
Fill in the blanks with appropriate letter (A,B,C,D,E,F).Structure__________________ and structure _______________ could react to form ethyldimethylammonium propanoate.

Unlock Deck
Unlock for access to all 62 flashcards in this deck.
Unlock Deck
k this deck
46
Consider the structures given below.  Fill in the blanks with appropriate letter (A,B,C,D,E,F).
Fill in the blanks with appropriate letter (A,B,C,D,E,F).
Structure ___________________ would exist as a negative ion at pH 9.
 Fill in the blanks with appropriate letter (A,B,C,D,E,F).
Fill in the blanks with appropriate letter (A,B,C,D,E,F).Structure ___________________ would exist as a negative ion at pH 9.

Unlock Deck
Unlock for access to all 62 flashcards in this deck.
Unlock Deck
k this deck
47
Consider the following nitrogen containing compounds. 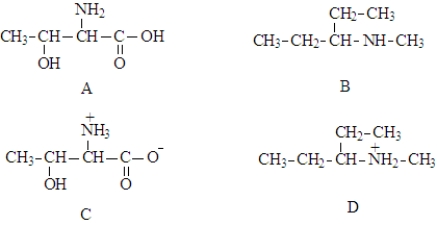 Fill in the blanks with the appropriate letter (A,B,C,D).
Fill in the blanks with the appropriate letter (A,B,C,D).
Structure________________ would be the form present in a pH 2 solution.
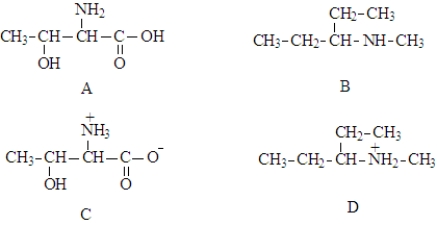 Fill in the blanks with the appropriate letter (A,B,C,D).
Fill in the blanks with the appropriate letter (A,B,C,D).Structure________________ would be the form present in a pH 2 solution.

Unlock Deck
Unlock for access to all 62 flashcards in this deck.
Unlock Deck
k this deck
48
Consider the structures given below.  Fill in the blanks with appropriate letter (A,B,C,D,E,F).
Fill in the blanks with appropriate letter (A,B,C,D,E,F).
Structure__________________ would produce a solution near pH 7.
 Fill in the blanks with appropriate letter (A,B,C,D,E,F).
Fill in the blanks with appropriate letter (A,B,C,D,E,F).Structure__________________ would produce a solution near pH 7.

Unlock Deck
Unlock for access to all 62 flashcards in this deck.
Unlock Deck
k this deck
49
Draw the structure of sodium benzoate.

Unlock Deck
Unlock for access to all 62 flashcards in this deck.
Unlock Deck
k this deck
50
Using structural formulas, write the equation for the ionization of p-cresol (show below) in water. 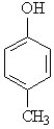


Unlock Deck
Unlock for access to all 62 flashcards in this deck.
Unlock Deck
k this deck
51
Consider the structures given below.  Fill in the blanks with appropriate letter (A,B,C,D,E,F).
Fill in the blanks with appropriate letter (A,B,C,D,E,F).
Structure____________________ would not be neutralized by the buffers found in the body.
 Fill in the blanks with appropriate letter (A,B,C,D,E,F).
Fill in the blanks with appropriate letter (A,B,C,D,E,F).Structure____________________ would not be neutralized by the buffers found in the body.

Unlock Deck
Unlock for access to all 62 flashcards in this deck.
Unlock Deck
k this deck
52
Consider the structures given below.  Fill in the blanks with appropriate letter (A,B,C,D,E,F).
Fill in the blanks with appropriate letter (A,B,C,D,E,F).
Structure ___________________ would exist as a positive ion at pH 4.
 Fill in the blanks with appropriate letter (A,B,C,D,E,F).
Fill in the blanks with appropriate letter (A,B,C,D,E,F).Structure ___________________ would exist as a positive ion at pH 4.

Unlock Deck
Unlock for access to all 62 flashcards in this deck.
Unlock Deck
k this deck
53
Consider the structures given below.  Fill in the blanks with appropriate letter (A,B,C,D,E,F).
Fill in the blanks with appropriate letter (A,B,C,D,E,F).
Structure _____________________ would from a thioester bond with CoA.
 Fill in the blanks with appropriate letter (A,B,C,D,E,F).
Fill in the blanks with appropriate letter (A,B,C,D,E,F).Structure _____________________ would from a thioester bond with CoA.

Unlock Deck
Unlock for access to all 62 flashcards in this deck.
Unlock Deck
k this deck
54
When a amine dissolves in water, the pH is
A)greater than 7.
B)less than 7.
C)neutral at about 7.
A)greater than 7.
B)less than 7.
C)neutral at about 7.

Unlock Deck
Unlock for access to all 62 flashcards in this deck.
Unlock Deck
k this deck
55
Consider the structures given below.  Fill in the blanks with appropriate letter (A,B,C,D,E,F).
Fill in the blanks with appropriate letter (A,B,C,D,E,F).
Structure __________________ is a secondary amine.
 Fill in the blanks with appropriate letter (A,B,C,D,E,F).
Fill in the blanks with appropriate letter (A,B,C,D,E,F).Structure __________________ is a secondary amine.

Unlock Deck
Unlock for access to all 62 flashcards in this deck.
Unlock Deck
k this deck
56
Consider the following nitrogen containing compounds. 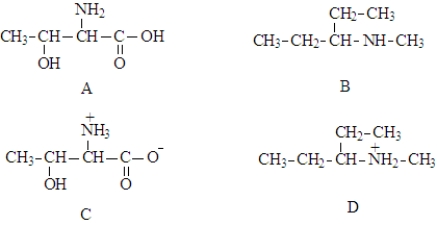 Fill in the blanks with the appropriate letter (A,B,C,D).
Fill in the blanks with the appropriate letter (A,B,C,D).
Structure __________________ represents a zwitterion.
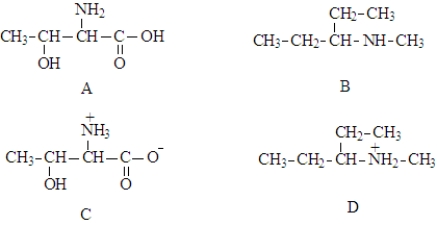 Fill in the blanks with the appropriate letter (A,B,C,D).
Fill in the blanks with the appropriate letter (A,B,C,D).Structure __________________ represents a zwitterion.

Unlock Deck
Unlock for access to all 62 flashcards in this deck.
Unlock Deck
k this deck
57
The structure of procaine, whose hydrochloride salt is the local anesthetic known as Novocain, is shown below. 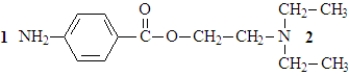 At physiological pH,
At physiological pH,
A)only nitrogen 1 will be protonated.
B)only nitrogen 2 will be protonated.
C)both nitrogen 1 and 2 will be protonated.
D)neither nitrogen 1 nor 2 will be protonated.
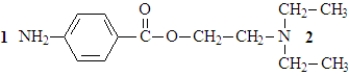 At physiological pH,
At physiological pH,A)only nitrogen 1 will be protonated.
B)only nitrogen 2 will be protonated.
C)both nitrogen 1 and 2 will be protonated.
D)neither nitrogen 1 nor 2 will be protonated.

Unlock Deck
Unlock for access to all 62 flashcards in this deck.
Unlock Deck
k this deck
58
Consider the structures given below.  Fill in the blanks with appropriate letter (A,B,C,D,E,F).
Fill in the blanks with appropriate letter (A,B,C,D,E,F).
Structure ____________________ would ionize in water and produce OH- ions.
 Fill in the blanks with appropriate letter (A,B,C,D,E,F).
Fill in the blanks with appropriate letter (A,B,C,D,E,F).Structure ____________________ would ionize in water and produce OH- ions.

Unlock Deck
Unlock for access to all 62 flashcards in this deck.
Unlock Deck
k this deck
59
Consider the structures given below.  Fill in the blanks with appropriate letter (A,B,C,D,E,F).
Fill in the blanks with appropriate letter (A,B,C,D,E,F).
Structure _________________________ would undergo oxidative decarboxylation.
 Fill in the blanks with appropriate letter (A,B,C,D,E,F).
Fill in the blanks with appropriate letter (A,B,C,D,E,F).Structure _________________________ would undergo oxidative decarboxylation.

Unlock Deck
Unlock for access to all 62 flashcards in this deck.
Unlock Deck
k this deck
60
Which of the following statements about decarboxylation and oxidative decarboxylation reactions is(are) correct?
A)Decarboxylation reactions and oxidative decarboxylation reactions both require the presence of NAD+.
B)Decarboxylation reactions and oxidative decarboxylation reactions require the use of any type of carboxylic acid.
C)Thiols are require for decarboxylation but not for oxidative decarboxylation.
D)Decarboxylation reactions and oxidative decarboxylation reactions produce a product containing a carbonyl group.
E)All of these characterize these two types of reactions.
A)Decarboxylation reactions and oxidative decarboxylation reactions both require the presence of NAD+.
B)Decarboxylation reactions and oxidative decarboxylation reactions require the use of any type of carboxylic acid.
C)Thiols are require for decarboxylation but not for oxidative decarboxylation.
D)Decarboxylation reactions and oxidative decarboxylation reactions produce a product containing a carbonyl group.
E)All of these characterize these two types of reactions.

Unlock Deck
Unlock for access to all 62 flashcards in this deck.
Unlock Deck
k this deck
61
Draw the structure of the products of both steps of the oxidative decarboxylation of the following substance in the presence of coenzyme A. Use CoA to represent the portion of the molecule that does not change in the reaction. 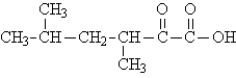
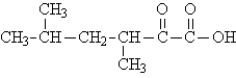

Unlock Deck
Unlock for access to all 62 flashcards in this deck.
Unlock Deck
k this deck
62
Write the structural formula for the organic product of the decarboxylation of the following substance. 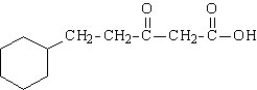
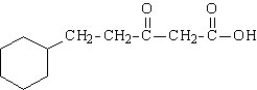

Unlock Deck
Unlock for access to all 62 flashcards in this deck.
Unlock Deck
k this deck


