Deck 14: Aldehydes and Ketones: Nucleophilic Addition Reactions
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/30
Play
Full screen (f)
Deck 14: Aldehydes and Ketones: Nucleophilic Addition Reactions
1
Instructions: Consider the data below to answer the following question(s).
Cyanohydrins are important intermediates in the synthesis of a-hydroxycarboxylic acids from ketones and aldehydes.The nitrile functional group can be hydrolyzed by aqueous acid to yield a carboxylic acid.Nitriles can also be hydrolyzed to carboxylic acids using aqueous base.When a cyanohydrin is treated with aqueous base,however,the original carbonyl compound is isolated.
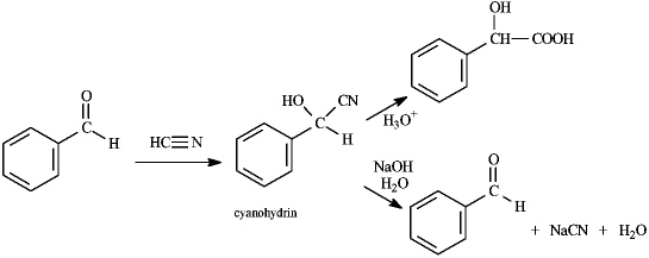
Refer to instructions.Identify the electrophile and nucloephile in the reaction of benzaldehyde with hydrogen cyanide.
Cyanohydrins are important intermediates in the synthesis of a-hydroxycarboxylic acids from ketones and aldehydes.The nitrile functional group can be hydrolyzed by aqueous acid to yield a carboxylic acid.Nitriles can also be hydrolyzed to carboxylic acids using aqueous base.When a cyanohydrin is treated with aqueous base,however,the original carbonyl compound is isolated.

Refer to instructions.Identify the electrophile and nucloephile in the reaction of benzaldehyde with hydrogen cyanide.
Benzaldehyde is the electrophile and HCN is the nucleophile in this reaction.
2
Instructions: Consider the data below to answer the following question(s).
C7H14O

Refer to instructions.Interpret the 1H NMR data.
C7H14O

Refer to instructions.Interpret the 1H NMR data.
The 1H NMR indicates that there are only three different kinds of hydrogen in the molecule.The 6 H triplet is due to two equivalent CH3 groups next to two equivalent CH2 groups,the 4 H triplet is due to two equivalent CH2 groups next to two other equivalent CH2 groups,shifted by a C=O,and the 4 H multiplet is two equivalent CH2 groups between a CH2 and a CH3.
3
The nucleophillic addition of water to an aldehyde or ketone
A)is irreversible.
B)dependent on the structure of the carbonyl.
C)favored by neutral conditions.
D)produces a stable tetrahedral product.
E)all of these.
A)is irreversible.
B)dependent on the structure of the carbonyl.
C)favored by neutral conditions.
D)produces a stable tetrahedral product.
E)all of these.
dependent on the structure of the carbonyl.
4
Many nucleophilic addition reactions of aldehydes and ketones are catalyzed by acid or base.Bases catalyze hydration by:
A)making the carbonyl group more electrophilic
B)shifting the equilibrium of the reaction
C)making the carbonyl group less electrophilic
D)converting the water to hydroxide ion,a much better nucleophile
A)making the carbonyl group more electrophilic
B)shifting the equilibrium of the reaction
C)making the carbonyl group less electrophilic
D)converting the water to hydroxide ion,a much better nucleophile

Unlock Deck
Unlock for access to all 30 flashcards in this deck.
Unlock Deck
k this deck
5
Instructions: Consider the reaction below to answer the following question.
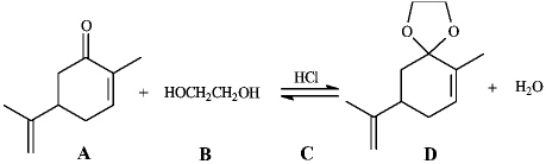
Refer to instructions.The electrophile,the nucleophile and the catalyst in this reaction are indicated by letters _____,_____,and _____,respectively.
A)A
B)B
C)C
D)D

Refer to instructions.The electrophile,the nucleophile and the catalyst in this reaction are indicated by letters _____,_____,and _____,respectively.
A)A
B)B
C)C
D)D

Unlock Deck
Unlock for access to all 30 flashcards in this deck.
Unlock Deck
k this deck
6
Predict the products of the following reactions.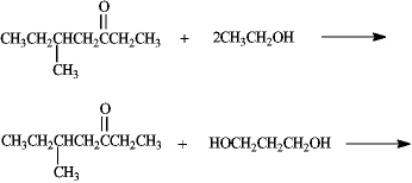


Unlock Deck
Unlock for access to all 30 flashcards in this deck.
Unlock Deck
k this deck
7
Instructions: Consider the data below to answer the following question(s).
Cyanohydrins are important intermediates in the synthesis of a-hydroxycarboxylic acids from ketones and aldehydes.The nitrile functional group can be hydrolyzed by aqueous acid to yield a carboxylic acid.Nitriles can also be hydrolyzed to carboxylic acids using aqueous base.When a cyanohydrin is treated with aqueous base,however,the original carbonyl compound is isolated.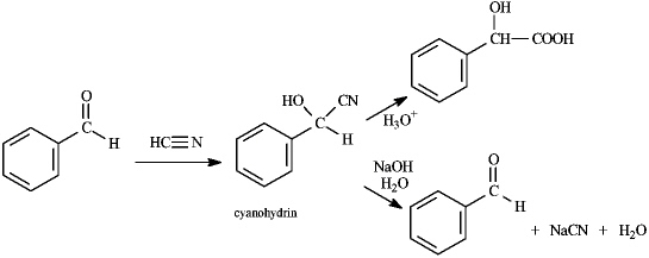
Refer to instructions.The reaction of an aldehyde with hydrogen cyanide is an example of _____ reaction.
A)a nucleophilic substitution
B)an electrophilic addition
C)an electrophilic substitution
D)a nucleophilic addition
Cyanohydrins are important intermediates in the synthesis of a-hydroxycarboxylic acids from ketones and aldehydes.The nitrile functional group can be hydrolyzed by aqueous acid to yield a carboxylic acid.Nitriles can also be hydrolyzed to carboxylic acids using aqueous base.When a cyanohydrin is treated with aqueous base,however,the original carbonyl compound is isolated.

Refer to instructions.The reaction of an aldehyde with hydrogen cyanide is an example of _____ reaction.
A)a nucleophilic substitution
B)an electrophilic addition
C)an electrophilic substitution
D)a nucleophilic addition

Unlock Deck
Unlock for access to all 30 flashcards in this deck.
Unlock Deck
k this deck
8
The substance formed in the following reaction is called: 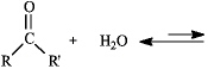
A)vicinal diol
B)geminal diol
C)acetal
D)hydrate
E)b or d
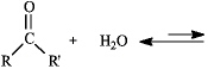
A)vicinal diol
B)geminal diol
C)acetal
D)hydrate
E)b or d

Unlock Deck
Unlock for access to all 30 flashcards in this deck.
Unlock Deck
k this deck
9
Instructions: Consider the data below to answer the following question(s).
C7H14O

Refer to instructions.What functional group is indicated by the IR data?
C7H14O

Refer to instructions.What functional group is indicated by the IR data?

Unlock Deck
Unlock for access to all 30 flashcards in this deck.
Unlock Deck
k this deck
10
Instructions: Predict the products from the information given for the following question(s).
Predict: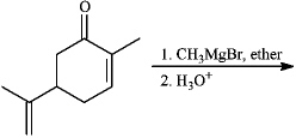
Predict:


Unlock Deck
Unlock for access to all 30 flashcards in this deck.
Unlock Deck
k this deck
11
What is the IUPAC name of the following compound? 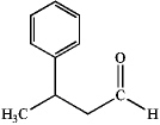
A)3-methyl-3-phenylpropanol
B)3-phenylbutanal
C)3-phenyl-1-butanone
D)3-phenylbutanoic acid

A)3-methyl-3-phenylpropanol
B)3-phenylbutanal
C)3-phenyl-1-butanone
D)3-phenylbutanoic acid

Unlock Deck
Unlock for access to all 30 flashcards in this deck.
Unlock Deck
k this deck
12
Instructions: Consider the data below to answer the following question(s).
C7H14O

Refer to instructions.Calculate the degree of unsaturation for this compound.
C7H14O

Refer to instructions.Calculate the degree of unsaturation for this compound.

Unlock Deck
Unlock for access to all 30 flashcards in this deck.
Unlock Deck
k this deck
13
Instructions: Consider the data below to answer the following question(s).
C7H14O

Refer to instructions.Interpret the mass spectral data.
C7H14O

Refer to instructions.Interpret the mass spectral data.

Unlock Deck
Unlock for access to all 30 flashcards in this deck.
Unlock Deck
k this deck
14
Instructions: Predict the products from the information given for the following question(s).
Predict: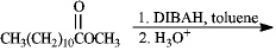
Predict:
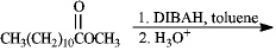

Unlock Deck
Unlock for access to all 30 flashcards in this deck.
Unlock Deck
k this deck
15
Instructions: Consider the reaction below to answer the following question. 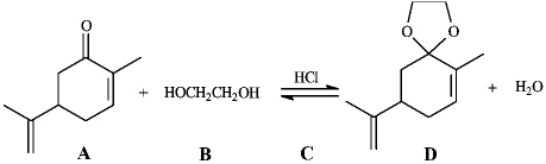
Refer to instructions.The product of this reaction is called:
A)an ylide
B)an acetal
C)a gem diol
D)a hydrate

Refer to instructions.The product of this reaction is called:
A)an ylide
B)an acetal
C)a gem diol
D)a hydrate

Unlock Deck
Unlock for access to all 30 flashcards in this deck.
Unlock Deck
k this deck
16
What hemiacetal would form from the internal nucleophillic addition reaction of the following compound?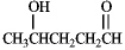
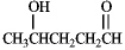

Unlock Deck
Unlock for access to all 30 flashcards in this deck.
Unlock Deck
k this deck
17
Instructions: a,b-Unsaturated aldehydes and ketones can undergo reaction with nucleophiles at the b carbon,as shown below.Use this information to answer the following question(s). 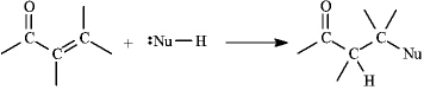
Refer to instructions.This reaction is called a(n)_____ reaction.
A)conjugate addition.
B)electrophilic addition.
C)direct addition
D)1,2-addition.
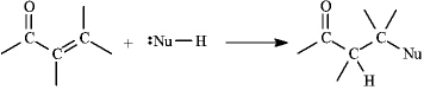
Refer to instructions.This reaction is called a(n)_____ reaction.
A)conjugate addition.
B)electrophilic addition.
C)direct addition
D)1,2-addition.

Unlock Deck
Unlock for access to all 30 flashcards in this deck.
Unlock Deck
k this deck
18
Instructions: a,b-Unsaturated aldehydes and ketones can undergo reaction with nucleophiles at the b carbon,as shown below.Use this information to answer the following question(s).
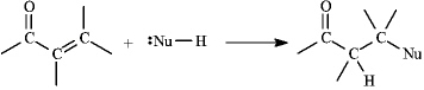
Refer to instructions.Draw a resonance form for the unsaturated carbonyl that accounts for this reactivity.
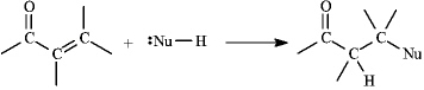
Refer to instructions.Draw a resonance form for the unsaturated carbonyl that accounts for this reactivity.

Unlock Deck
Unlock for access to all 30 flashcards in this deck.
Unlock Deck
k this deck
19
Instructions: Consider the data below to answer the following question(s).
C7H14O

Refer to instructions.Propose a structure consistent with the spectral data presented above.
C7H14O

Refer to instructions.Propose a structure consistent with the spectral data presented above.

Unlock Deck
Unlock for access to all 30 flashcards in this deck.
Unlock Deck
k this deck
20
Predict the products of the following reactions.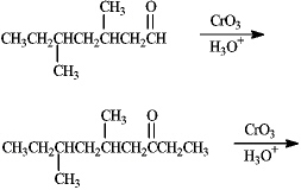


Unlock Deck
Unlock for access to all 30 flashcards in this deck.
Unlock Deck
k this deck
21
What is the IUPAC name of the following compound? 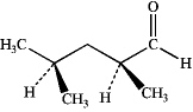
A)(2S,4R)-dimethylpentanal
B)(2S,4S)-dimethylpentanal
C)(R)-2,4-dimethylpentanal
D)(S)-2,4-dimethylpentanal

A)(2S,4R)-dimethylpentanal
B)(2S,4S)-dimethylpentanal
C)(R)-2,4-dimethylpentanal
D)(S)-2,4-dimethylpentanal

Unlock Deck
Unlock for access to all 30 flashcards in this deck.
Unlock Deck
k this deck
22
Based on the following IR spectrum, 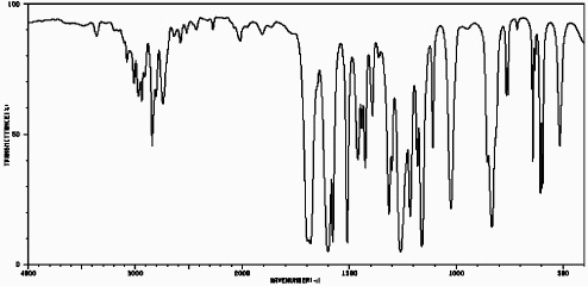
Spectrum obtained from: SDBSWeb : http://riodb01.ibase.aist.go.jp/sdbs/ (National Institute of Advanced Industrial Science and Technology,11-06-08)
The compound represented would be classified as an:
A)aromatic ketone
B)aromatic aldehyde
C)aliphatic ketone
D)aliphatic aldehyde

Spectrum obtained from: SDBSWeb : http://riodb01.ibase.aist.go.jp/sdbs/ (National Institute of Advanced Industrial Science and Technology,11-06-08)
The compound represented would be classified as an:
A)aromatic ketone
B)aromatic aldehyde
C)aliphatic ketone
D)aliphatic aldehyde

Unlock Deck
Unlock for access to all 30 flashcards in this deck.
Unlock Deck
k this deck
23
Draw the product(s)of the following reaction.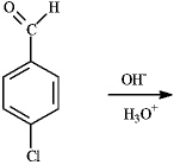


Unlock Deck
Unlock for access to all 30 flashcards in this deck.
Unlock Deck
k this deck
24
What is the name of the major organic product obtained from the following reaction? 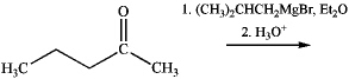
A)2,3-dimethylheptan-3-ol
B)2,4-dimethylheptan-4-ol
C)3,5-dimethylheptan-4-ol
D)3,5-dimethylheptan-3-ol

A)2,3-dimethylheptan-3-ol
B)2,4-dimethylheptan-4-ol
C)3,5-dimethylheptan-4-ol
D)3,5-dimethylheptan-3-ol

Unlock Deck
Unlock for access to all 30 flashcards in this deck.
Unlock Deck
k this deck
25
Predict whether the following reactions of the depicted carbonyl containing compounds and amines will result in the formation of an enamine or an imine.
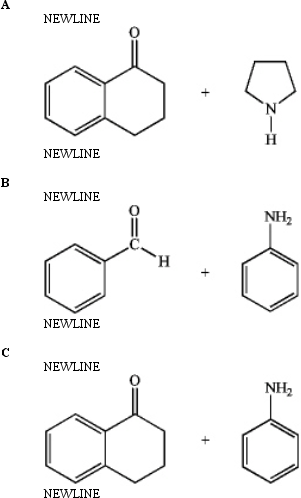


Unlock Deck
Unlock for access to all 30 flashcards in this deck.
Unlock Deck
k this deck
26
Which of the following would correctly describe the respective 13C NMR and 1H NMR spectra for the indicated atoms for the compound shown below? 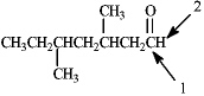
A)Atom 1 would produce a peak at 205 and atom 2 would appear as a singlet.
B)Atom 1 would produce a peak at 195 and atom 2 would appear as a singlet.
C)Atom 1 would produce a peak at 205 and atom 2 would appear as a triplet.
D)Atom 1 would produce a peak at 195 and atom 2 would appear as a triplet.
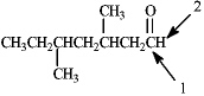
A)Atom 1 would produce a peak at 205 and atom 2 would appear as a singlet.
B)Atom 1 would produce a peak at 195 and atom 2 would appear as a singlet.
C)Atom 1 would produce a peak at 205 and atom 2 would appear as a triplet.
D)Atom 1 would produce a peak at 195 and atom 2 would appear as a triplet.

Unlock Deck
Unlock for access to all 30 flashcards in this deck.
Unlock Deck
k this deck
27
What is the correct assignment of the functional groups in each of the following compounds? 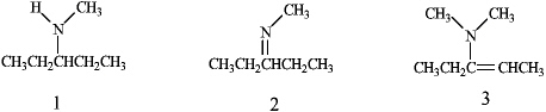
A)1 = amine;2 = imine;3 = enamine
B)1 = enamine;2 = imine;3 = amine
C)1 = imine;2 = enamine;3 = amine
D)1 = amine;2 =enamine;3 = imine

A)1 = amine;2 = imine;3 = enamine
B)1 = enamine;2 = imine;3 = amine
C)1 = imine;2 = enamine;3 = amine
D)1 = amine;2 =enamine;3 = imine

Unlock Deck
Unlock for access to all 30 flashcards in this deck.
Unlock Deck
k this deck
28
Draw the structure of the product obtained from the following reaction.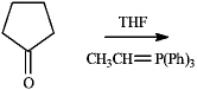
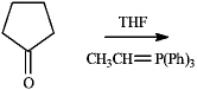

Unlock Deck
Unlock for access to all 30 flashcards in this deck.
Unlock Deck
k this deck
29
In the box,what is the name of the reactant used in the following reaction? 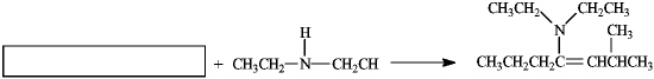
A)2-methylhept-4-ene
B)heptan-4-one
C)2-methylheptan-4-one
D)2-methylhep-3-ene-4-one

A)2-methylhept-4-ene
B)heptan-4-one
C)2-methylheptan-4-one
D)2-methylhep-3-ene-4-one

Unlock Deck
Unlock for access to all 30 flashcards in this deck.
Unlock Deck
k this deck
30
Synthesize the following alkene through the Wittig reaction of a carbonyl compound and a phosphorus ylide.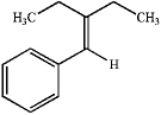


Unlock Deck
Unlock for access to all 30 flashcards in this deck.
Unlock Deck
k this deck


