Deck 11: Intermolecular Forces and Liquids and Solids
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/149
Play
Full screen (f)
Deck 11: Intermolecular Forces and Liquids and Solids
1
Arrange the following substances in order of increasing boiling point: CH3OH, He, CH3Cl, and N2
A)CH3OH < He < CH3Cl < N2
B)He < N2 < CH3OH < CH3Cl
C)N2 < He < CH3OH < CH3Cl
D)He < N2 < CH3Cl < CH3OH
E)CH3Cl < He < N2 < CH3OH
A)CH3OH < He < CH3Cl < N2
B)He < N2 < CH3OH < CH3Cl
C)N2 < He < CH3OH < CH3Cl
D)He < N2 < CH3Cl < CH3OH
E)CH3Cl < He < N2 < CH3OH
He < N2 < CH3Cl < CH3OH
2
Arrange the following substances in order of increasing boiling point: CH3CH2OH, HOCH2CH2OH, CH3CH2Cl, and ClCH2CH2OH
A)CH3CH2OH < HOCH2CH2OH < CH3CH2Cl < ClCH2CH2OH
B)CH3CH2Cl < CH3CH2OH < ClCH2CH2OH < HOCH2CH2OH
C)CH3CH2OH < CH3CH2Cl < HOCH2CH2OH < ClCH2CH2OH
D)CH3CH2Cl < ClCH2CH2OH < CH3CH2OH < HOCH2CH2OH
E)CH3CH2OH < ClCH2CH2OH < CH3CH2Cl < HOCH2CH2OH `
A)CH3CH2OH < HOCH2CH2OH < CH3CH2Cl < ClCH2CH2OH
B)CH3CH2Cl < CH3CH2OH < ClCH2CH2OH < HOCH2CH2OH
C)CH3CH2OH < CH3CH2Cl < HOCH2CH2OH < ClCH2CH2OH
D)CH3CH2Cl < ClCH2CH2OH < CH3CH2OH < HOCH2CH2OH
E)CH3CH2OH < ClCH2CH2OH < CH3CH2Cl < HOCH2CH2OH `
CH3CH2Cl < CH3CH2OH < ClCH2CH2OH < HOCH2CH2OH
3
Which of the following liquids would have the lowest viscosity at 25°C? 
A)A
B)B
C)C
D)D
E)E

A)A
B)B
C)C
D)D
E)E
A
4
Which one of the following substances is expected to have the lowest melting point?
A)BrI
B)CsI
C)LiI
D)NaI
E)RbI
A)BrI
B)CsI
C)LiI
D)NaI
E)RbI

Unlock Deck
Unlock for access to all 149 flashcards in this deck.
Unlock Deck
k this deck
5
Which one of the following substances is expected to have the highest boiling point?
A)HBr
B)HCl
C)HF
D)HI
A)HBr
B)HCl
C)HF
D)HI

Unlock Deck
Unlock for access to all 149 flashcards in this deck.
Unlock Deck
k this deck
6
For which of the following species are the dispersion forces strongest?
A)C4H10
B)C5H12
C)C6H14
D)C7H16
E)C8H18
A)C4H10
B)C5H12
C)C6H14
D)C7H16
E)C8H18

Unlock Deck
Unlock for access to all 149 flashcards in this deck.
Unlock Deck
k this deck
7
The intermolecular forces present in CO include which of the following? I.dipole-dipole
II.ion-dipole
III.dispersion
IV.hydrogen bonding
A)I, II, III, and IV
B)I and III
C)I, III, and IV
D)I and II
E)II and IV
II.ion-dipole
III.dispersion
IV.hydrogen bonding
A)I, II, III, and IV
B)I and III
C)I, III, and IV
D)I and II
E)II and IV

Unlock Deck
Unlock for access to all 149 flashcards in this deck.
Unlock Deck
k this deck
8
Which of the following would be expected to have the lowest vapor pressure at room temperature?
A)ethanol, bp = 78°C
B)methanol, bp = 65°C
C)water, bp = 100°C
D)acetone, bp = 56°C
A)ethanol, bp = 78°C
B)methanol, bp = 65°C
C)water, bp = 100°C
D)acetone, bp = 56°C

Unlock Deck
Unlock for access to all 149 flashcards in this deck.
Unlock Deck
k this deck
9
Which one of the following substances should exhibit hydrogen bonding in the liquid state?
A)PH3
B)H2
C)H2S
D)CH4
E)NH3
A)PH3
B)H2
C)H2S
D)CH4
E)NH3

Unlock Deck
Unlock for access to all 149 flashcards in this deck.
Unlock Deck
k this deck
10
Which one of the following substances is expected to have the highest melting point?
A)CH4
B)CCl4
C)CO
D)CO2
E)C(diamond)
A)CH4
B)CCl4
C)CO
D)CO2
E)C(diamond)

Unlock Deck
Unlock for access to all 149 flashcards in this deck.
Unlock Deck
k this deck
11
The intermolecular forces present in C6H6 include which of the following? I.dipole-dipole
II.ion-dipole
III.dispersion
IV.hydrogen bonding
A)I, II, III, and IV
B)I and III
C)I, III, and IV
D)I and II
E)III only
II.ion-dipole
III.dispersion
IV.hydrogen bonding
A)I, II, III, and IV
B)I and III
C)I, III, and IV
D)I and II
E)III only

Unlock Deck
Unlock for access to all 149 flashcards in this deck.
Unlock Deck
k this deck
12
Which of the following liquids would have the highest viscosity at 25°C?
A)CH3OCH3
B)CH2Cl2
C)C2H5OH
D)CH3Br
E)HOCH2CH2OH
A)CH3OCH3
B)CH2Cl2
C)C2H5OH
D)CH3Br
E)HOCH2CH2OH

Unlock Deck
Unlock for access to all 149 flashcards in this deck.
Unlock Deck
k this deck
13
Which one of the following substances is expected to have the highest boiling point?
A)Br2
B)Cl2
C)F2
D)I2
A)Br2
B)Cl2
C)F2
D)I2

Unlock Deck
Unlock for access to all 149 flashcards in this deck.
Unlock Deck
k this deck
14
Which of the following characteristics indicates the presence of weak intermolecular forces in a liquid?
A)a low heat of vaporization
B)a high critical temperature
C)a low vapor pressure
D)a high boiling point
E)None of the above.
A)a low heat of vaporization
B)a high critical temperature
C)a low vapor pressure
D)a high boiling point
E)None of the above.

Unlock Deck
Unlock for access to all 149 flashcards in this deck.
Unlock Deck
k this deck
15
Which of the following properties indicates the presence of strong intermolecular forces in a liquid?
A)a low heat of vaporization
B)a low critical temperature
C)a low vapor pressure
D)a low boiling point
E)None of the above.
A)a low heat of vaporization
B)a low critical temperature
C)a low vapor pressure
D)a low boiling point
E)None of the above.

Unlock Deck
Unlock for access to all 149 flashcards in this deck.
Unlock Deck
k this deck
16
The intermolecular forces present in HSCH2CH2SH include which of the following? I.dipole-dipole
II.ion-dipole
III.dispersion
IV.hydrogen bonding
A)I, II, III, and IV
B)I and III
C)I, III, and IV
D)I and II
E)II and IV
II.ion-dipole
III.dispersion
IV.hydrogen bonding
A)I, II, III, and IV
B)I and III
C)I, III, and IV
D)I and II
E)II and IV

Unlock Deck
Unlock for access to all 149 flashcards in this deck.
Unlock Deck
k this deck
17
Each of the following substances is a liquid at -50°C.Place these liquids in order of increasing vapor pressure: dimethyl ether (CH3OCH3), propane (C3H8), and ethanol (CH3CH2OH).
A)ethanol < propane < dimethyl ether
B)ethanol < dimethyl ether < propane
C)propane < dimethyl ether < ethanol
D)dimethyl ether < ethanol < propane
E)propane < ethanol < dimethyl ether
A)ethanol < propane < dimethyl ether
B)ethanol < dimethyl ether < propane
C)propane < dimethyl ether < ethanol
D)dimethyl ether < ethanol < propane
E)propane < ethanol < dimethyl ether

Unlock Deck
Unlock for access to all 149 flashcards in this deck.
Unlock Deck
k this deck
18
Which one of the following substances will have both dispersion forces and dipole-dipole forces?
A)HCl
B)BCl3
C)Br2
D)H2
E)CO2
A)HCl
B)BCl3
C)Br2
D)H2
E)CO2

Unlock Deck
Unlock for access to all 149 flashcards in this deck.
Unlock Deck
k this deck
19
The intermolecular forces present in CH3NH2 include which of the following? I.dipole-dipole
II.ion-dipole
III.dispersion
IV.hydrogen bonding
A)I, II, III, and IV
B)I and III
C)I, III, and IV
D)I and II
E)II and IV
II.ion-dipole
III.dispersion
IV.hydrogen bonding
A)I, II, III, and IV
B)I and III
C)I, III, and IV
D)I and II
E)II and IV

Unlock Deck
Unlock for access to all 149 flashcards in this deck.
Unlock Deck
k this deck
20
Which of the following would be expected to have the highest vapor pressure at room temperature?
A)ethanol, bp = 78°C
B)methanol, bp = 65°C
C)water, bp = 100°C
D)acetone, bp = 56°C
A)ethanol, bp = 78°C
B)methanol, bp = 65°C
C)water, bp = 100°C
D)acetone, bp = 56°C

Unlock Deck
Unlock for access to all 149 flashcards in this deck.
Unlock Deck
k this deck
21
W(s)is classified as which of the following?
A)metallic crystal.
B)covalent solid.
C)molecular crystal.
D)amorphous solid.
E)ionic crystal.
A)metallic crystal.
B)covalent solid.
C)molecular crystal.
D)amorphous solid.
E)ionic crystal.

Unlock Deck
Unlock for access to all 149 flashcards in this deck.
Unlock Deck
k this deck
22
HOCH2CH2OH(s)is classified as which of the following?
A)metallic crystal.
B)covalent solid.
C)molecular crystal.
D)amorphous solid.
E)ionic crystal.
A)metallic crystal.
B)covalent solid.
C)molecular crystal.
D)amorphous solid.
E)ionic crystal.

Unlock Deck
Unlock for access to all 149 flashcards in this deck.
Unlock Deck
k this deck
23
Which of the following properties is not influenced by hydrogen bonding?
A)melting point
B)boiling point
C)vapor pressure
D)viscosity
E)flammability
A)melting point
B)boiling point
C)vapor pressure
D)viscosity
E)flammability

Unlock Deck
Unlock for access to all 149 flashcards in this deck.
Unlock Deck
k this deck
24
Each of the following substances is a gas at 25°C and 1 atmosphere pressure.Which one will liquefy most easily when compressed at a constant temperature?
A)F2
B)H2
C)HF
D)SiH4
E)Ar
A)F2
B)H2
C)HF
D)SiH4
E)Ar

Unlock Deck
Unlock for access to all 149 flashcards in this deck.
Unlock Deck
k this deck
25
Which one of the following substances should exhibit hydrogen bonding in the liquid state?
A)SiH4
B)H2
C)H2S
D)CH4
E)CH3NH2
A)SiH4
B)H2
C)H2S
D)CH4
E)CH3NH2

Unlock Deck
Unlock for access to all 149 flashcards in this deck.
Unlock Deck
k this deck
26
Which property of water allows a razor blade to float on it without sinking?
A)viscosity
B)surface tension
C)density
D)specific heat
E)triple point
A)viscosity
B)surface tension
C)density
D)specific heat
E)triple point

Unlock Deck
Unlock for access to all 149 flashcards in this deck.
Unlock Deck
k this deck
27
The boiling points of propanol (CH3CH2CH2OH)and pentanol (CH3CH2CH2CH2CH2OH)are 97°C and 137°C, respectively.The boiling point of butanol (CH3CH2CH2CH2OH)is predicted to be:
A)< 97°C
B)> 137°C
C)> 97°C and < 137°C
D)97°C
E)137°C
A)< 97°C
B)> 137°C
C)> 97°C and < 137°C
D)97°C
E)137°C

Unlock Deck
Unlock for access to all 149 flashcards in this deck.
Unlock Deck
k this deck
28
The boiling points of chloromethane (CH3Cl)and dichlormethane (CH2Cl2)are - 24 °C and 40.°C respectively.The boiling point of trichloromethane (CHCl3)is predicted to be:
A)< - 24 °C
B)> 40.°C
C)> - 24 °C and < 40.°C
D)- 24 °C
E)40.°C
A)< - 24 °C
B)> 40.°C
C)> - 24 °C and < 40.°C
D)- 24 °C
E)40.°C

Unlock Deck
Unlock for access to all 149 flashcards in this deck.
Unlock Deck
k this deck
29
An example of a covalent network solid is
A)diamond.
B)potassium.
C)iodine.
D)sodium chloride.
E)None of these.
A)diamond.
B)potassium.
C)iodine.
D)sodium chloride.
E)None of these.

Unlock Deck
Unlock for access to all 149 flashcards in this deck.
Unlock Deck
k this deck
30
Which of following can form hydrogen bonds with water molecules? (1)Na+ (2)CH3COOH (3)C2H6 (4)CH3NH2
A)(1)and (2)
B)(1)and (3)
C)(2)and (3)
D)(2)and (4)
E)(3)and (4)
A)(1)and (2)
B)(1)and (3)
C)(2)and (3)
D)(2)and (4)
E)(3)and (4)

Unlock Deck
Unlock for access to all 149 flashcards in this deck.
Unlock Deck
k this deck
31
Arrange the following in order of increasing boiling point: RbCl, CH3Cl, CH3OH, CH4.
A)CH3OH < CH3Cl < RbCl < CH4
B)CH3OH < CH4 < CH3Cl < RbCl
C)RbCl < CH3Cl < CH3OH < CH4
D)CH4 < CH3OH < CH3Cl < RbCl
E)CH4 < CH3Cl < CH3OH < RbCl
A)CH3OH < CH3Cl < RbCl < CH4
B)CH3OH < CH4 < CH3Cl < RbCl
C)RbCl < CH3Cl < CH3OH < CH4
D)CH4 < CH3OH < CH3Cl < RbCl
E)CH4 < CH3Cl < CH3OH < RbCl

Unlock Deck
Unlock for access to all 149 flashcards in this deck.
Unlock Deck
k this deck
32
Glass is classified as which of the following?
A)metallic crystal.
B)covalent solid.
C)molecular crystal.
D)amorphous solid.
E)ionic crystal.
A)metallic crystal.
B)covalent solid.
C)molecular crystal.
D)amorphous solid.
E)ionic crystal.

Unlock Deck
Unlock for access to all 149 flashcards in this deck.
Unlock Deck
k this deck
33
Given the following liquids and their boiling points, which has the highest vapor pressure at its normal boiling point?
A)ethanol, bp = 78°C
B)methanol, bp = 65°C
C)water, bp = 100°C
D)benzene, bp = 80°C
E)The vapor pressure of each of the liquids at its normal boiling point would be the same.
A)ethanol, bp = 78°C
B)methanol, bp = 65°C
C)water, bp = 100°C
D)benzene, bp = 80°C
E)The vapor pressure of each of the liquids at its normal boiling point would be the same.

Unlock Deck
Unlock for access to all 149 flashcards in this deck.
Unlock Deck
k this deck
34
Which one of the following substances should exhibit hydrogen bonding in the liquid state?
A)PH3
B)He
C)H2S
D)CH4
E)CH3OH
A)PH3
B)He
C)H2S
D)CH4
E)CH3OH

Unlock Deck
Unlock for access to all 149 flashcards in this deck.
Unlock Deck
k this deck
35
Which of the following is not true with regard to water?
A)Water has a high heat capacity.
B)Water has an unusually high boiling point.
C)Water can form hydrogen bonds.
D)Ice is more dense than liquid water.
E)Water is a polar molecule.
A)Water has a high heat capacity.
B)Water has an unusually high boiling point.
C)Water can form hydrogen bonds.
D)Ice is more dense than liquid water.
E)Water is a polar molecule.

Unlock Deck
Unlock for access to all 149 flashcards in this deck.
Unlock Deck
k this deck
36
Butter melts over a range of temperatures, rather than with a sharp melting point.Butter is classified as a/an
A)metallic crystal.
B)covalent solid.
C)molecular crystal.
D)amorphous solid.
E)ionic crystal.
A)metallic crystal.
B)covalent solid.
C)molecular crystal.
D)amorphous solid.
E)ionic crystal.

Unlock Deck
Unlock for access to all 149 flashcards in this deck.
Unlock Deck
k this deck
37
Arrange the following in order of increasing melting point: NaCl, H2O, CH4, C6H4(OH)2
A)NaCl < H2O < CH4 < C6H4(OH)2
B)CH4 < H2O < NaCl < C6H4(OH)2
C)CH4 < H2O < C6H4(OH)2 < NaCl
D)CH4 < C6H4(OH)2 < H2O < NaCl
E)CH4 < NaCl < C6H4(OH)2 < H2O
A)NaCl < H2O < CH4 < C6H4(OH)2
B)CH4 < H2O < NaCl < C6H4(OH)2
C)CH4 < H2O < C6H4(OH)2 < NaCl
D)CH4 < C6H4(OH)2 < H2O < NaCl
E)CH4 < NaCl < C6H4(OH)2 < H2O

Unlock Deck
Unlock for access to all 149 flashcards in this deck.
Unlock Deck
k this deck
38
Which one of the following is an example of a covalent network solid?
A)SiO2
B)K
C)I2
D)CaCl2
E)None of these.
A)SiO2
B)K
C)I2
D)CaCl2
E)None of these.

Unlock Deck
Unlock for access to all 149 flashcards in this deck.
Unlock Deck
k this deck
39
Which one of the following substances crystallizes as a molecular solid?
A)KI
B)SiO2
C)Sn
D)CH3OH
E)Al2(SO4)3
A)KI
B)SiO2
C)Sn
D)CH3OH
E)Al2(SO4)3

Unlock Deck
Unlock for access to all 149 flashcards in this deck.
Unlock Deck
k this deck
40
The structural form of the element Ge closely resembles the structure of
A)C (diamond).
B)N (diatomic).
C)As (tetrahedral).
D)S (S8 ring).
E)Kr (monatomic).
A)C (diamond).
B)N (diatomic).
C)As (tetrahedral).
D)S (S8 ring).
E)Kr (monatomic).

Unlock Deck
Unlock for access to all 149 flashcards in this deck.
Unlock Deck
k this deck
41
The number of atoms in a body-centered cubic unit cell is
A)1
B)2
C)3
D)4
E)8
A)1
B)2
C)3
D)4
E)8

Unlock Deck
Unlock for access to all 149 flashcards in this deck.
Unlock Deck
k this deck
42
Platinum has a face-centered cubic crystal structure and a density of 21.5 g/cm3.What is the radius of the platinum atom?
A)69 pm
B)98 pm
C)139 pm
D)196 pm
E)277 pm
A)69 pm
B)98 pm
C)139 pm
D)196 pm
E)277 pm

Unlock Deck
Unlock for access to all 149 flashcards in this deck.
Unlock Deck
k this deck
43
Potassium crystallizes in a body-centered cubic lattice.How many atoms are there per unit cell?
A)1
B)2
C)4
D)6
E)8
A)1
B)2
C)4
D)6
E)8

Unlock Deck
Unlock for access to all 149 flashcards in this deck.
Unlock Deck
k this deck
44
Palladium crystallizes in a face-centered cubic unit cell.Its density is 12.0 g/cm3 at 27°C.Calculate the atomic radius of Pd.
A)154 pm
B)138 pm
C)1.95 × 10-8 nm
D)0.109 nm
E)1.95 × 10-8 cm
A)154 pm
B)138 pm
C)1.95 × 10-8 nm
D)0.109 nm
E)1.95 × 10-8 cm

Unlock Deck
Unlock for access to all 149 flashcards in this deck.
Unlock Deck
k this deck
45
Potassium bromide, KBr, crystallizes like NaCl in a face-centered lattice.The ionic radii of K+ and Br- ions are 133 pm and 195 pm, respectively.Assuming that all Br- ions are positioned in the face and corners of the unit cell, while the K+ ions are positioned along the edge alternating between anions, calculate the length of a unit cell edge.
A)230 pm
B)328 pm
C)523 pm
D)656 pm
E)780 pm
A)230 pm
B)328 pm
C)523 pm
D)656 pm
E)780 pm

Unlock Deck
Unlock for access to all 149 flashcards in this deck.
Unlock Deck
k this deck
46
The number of nearest neighbors (atoms that make contact)around each atom in a face-centered cubic lattice of a metal is
A)2
B)4
C)6
D)8
E)12
A)2
B)4
C)6
D)8
E)12

Unlock Deck
Unlock for access to all 149 flashcards in this deck.
Unlock Deck
k this deck
47
A face-centered cubic unit cell is the repeating unit in which type of crystal packing?
A)hexagonal close-packed
B)cubic close-packed
C)body centered
D)simple
E)all of the above
A)hexagonal close-packed
B)cubic close-packed
C)body centered
D)simple
E)all of the above

Unlock Deck
Unlock for access to all 149 flashcards in this deck.
Unlock Deck
k this deck
48
Vanadium crystallizes in a body-centered cubic lattice, and the length of the edge of a unit cell is 305 pm.What is the density of V?
A)5.96 × 10-30 g/cm3
B)2.98 × 10-6 g/cm3
C)2.98 g/cm3
D)5.96 g/cm3
E)11.9 g/cm3
A)5.96 × 10-30 g/cm3
B)2.98 × 10-6 g/cm3
C)2.98 g/cm3
D)5.96 g/cm3
E)11.9 g/cm3

Unlock Deck
Unlock for access to all 149 flashcards in this deck.
Unlock Deck
k this deck
49
BaCl2 crystallizes such that the Ba2+ ions are in a face-centered cubic arrangement and the Cl- ions are in the holes of the lattice (fluorite structure).How many Cl- ions are present in one unit cell of this crystal?
A)1
B)2
C)4
D)6
E)8
A)1
B)2
C)4
D)6
E)8

Unlock Deck
Unlock for access to all 149 flashcards in this deck.
Unlock Deck
k this deck
50
Which one of the following substances crystallizes as a covalent crystal?
A)CaO
B)SiO2
C)CO2
D)Pb
E)KMnO4
A)CaO
B)SiO2
C)CO2
D)Pb
E)KMnO4

Unlock Deck
Unlock for access to all 149 flashcards in this deck.
Unlock Deck
k this deck
51
The most space efficient arrangement of spheres is found in which type(s)of atom arrangement? I.hexagonal close-packed
II.cubic close-packed
III.simple cubic
IV.body-centered cubic
A)I only
B)II only
C)I and II
D)IV only
E)I, II, and IV
II.cubic close-packed
III.simple cubic
IV.body-centered cubic
A)I only
B)II only
C)I and II
D)IV only
E)I, II, and IV

Unlock Deck
Unlock for access to all 149 flashcards in this deck.
Unlock Deck
k this deck
52
The most space efficient arrangement of spheres is found in which type of unit cell?
A)face-centered cubic
B)body-centered cubic
C)simple cubic
D)face-centered cubic and body-centered cubic
E)body-centered cubic and simple cubic
A)face-centered cubic
B)body-centered cubic
C)simple cubic
D)face-centered cubic and body-centered cubic
E)body-centered cubic and simple cubic

Unlock Deck
Unlock for access to all 149 flashcards in this deck.
Unlock Deck
k this deck
53
The zincblende structure of ZnS has the relatively large sulfide ions arranged at the lattice points of a face-centered cubic structure.The edge length of this cubic unit cell is 540.9 pm.Determine the density of zincblende.
A)1.023 g/cm3
B)2.032 g/cm3
C)2.046 g/cm3
D)3.081 g/cm3
E)4.091 g/cm3
A)1.023 g/cm3
B)2.032 g/cm3
C)2.046 g/cm3
D)3.081 g/cm3
E)4.091 g/cm3

Unlock Deck
Unlock for access to all 149 flashcards in this deck.
Unlock Deck
k this deck
54
MgO has the same crystal structure as NaCl, face-centered cubic.How many oxide ions surround each Mg2+ ion as nearest neighbors?
A)4
B)6
C)8
D)10
E)12
A)4
B)6
C)8
D)10
E)12

Unlock Deck
Unlock for access to all 149 flashcards in this deck.
Unlock Deck
k this deck
55
In the following picture, each arrow represents a molecule or atom.Based on the arrangement in the solid state as shown, which of the following best represents the unit cell? 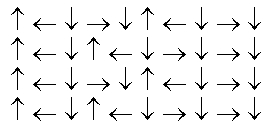
A)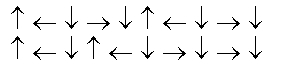
B)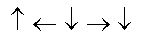
C)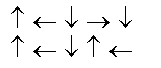
D)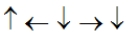
E)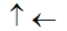

A)

B)

C)

D)
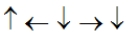
E)
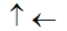

Unlock Deck
Unlock for access to all 149 flashcards in this deck.
Unlock Deck
k this deck
56
SrF2 crystallizes such that the Sr2+ ions are in a face-centered cubic arrangement and the F- ions are in the holes of the lattice (fluorite structure).How many F- ions are present in one unit cell of this crystal?
A)1
B)2
C)4
D)6
E)8
A)1
B)2
C)4
D)6
E)8

Unlock Deck
Unlock for access to all 149 flashcards in this deck.
Unlock Deck
k this deck
57
The mineral manganosite, manganese(II)oxide, crystallizes in the rock salt structure (the face-centered structure adopted by NaCl)with a density of 5.365 g/cm3.Find the unit cell edge length of manganosite.
A)280.0 pm
B)352.8 pm
C)368.2 pm
D)417.9 pm
E)444.5 pm
A)280.0 pm
B)352.8 pm
C)368.2 pm
D)417.9 pm
E)444.5 pm

Unlock Deck
Unlock for access to all 149 flashcards in this deck.
Unlock Deck
k this deck
58
Which one of the following crystallizes in a metallic lattice?
A)C
B)NaMnO4
C)K
D)LiClO4
E)K2Cr2O7
A)C
B)NaMnO4
C)K
D)LiClO4
E)K2Cr2O7

Unlock Deck
Unlock for access to all 149 flashcards in this deck.
Unlock Deck
k this deck
59
In the following picture, each arrow represents a molecule or atom.Based on the arrangement in the solid state as shown, which of the following best represents the unit cell? 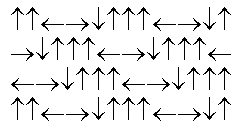
A)
B)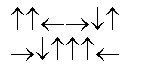
C)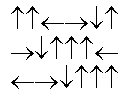
D)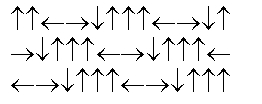
E)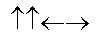

A)

B)

C)

D)

E)
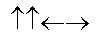

Unlock Deck
Unlock for access to all 149 flashcards in this deck.
Unlock Deck
k this deck
60
The number of atoms in a face-centered cubic unit cell is
A)1
B)2
C)3
D)4
E)8
A)1
B)2
C)3
D)4
E)8

Unlock Deck
Unlock for access to all 149 flashcards in this deck.
Unlock Deck
k this deck
61
The specific heat of liquid ethanol, C2H5OH(l), is 2.46 J/g·°C and the heat of vaporization is 39.3 kJ/mol.The boiling point of ethanol is 78.3 °C.What amount of enthalpy is required to heat 50.0 g of liquid ethanol from 23.0 °C to ethanol vapor at 78.3 °C?
A)42.7 kJ
B)49.5 kJ
C)179 kJ
D)1970kJ
E)6840 kJ
A)42.7 kJ
B)49.5 kJ
C)179 kJ
D)1970kJ
E)6840 kJ

Unlock Deck
Unlock for access to all 149 flashcards in this deck.
Unlock Deck
k this deck
62
What mass of water would need to evaporate from your skin in order to dissipate 1.7 × 105 J of heat from your body? H2O(l) H2O(g) Hvap = 40.7 kJ/mol
A)58.4 g
B)75.2 g
C)418 g
D)7.52 × 104 g
E)6.92 × 106 g
A)58.4 g
B)75.2 g
C)418 g
D)7.52 × 104 g
E)6.92 × 106 g

Unlock Deck
Unlock for access to all 149 flashcards in this deck.
Unlock Deck
k this deck
63
Use the graph of vapor pressure to determine the normal boiling point of O2. 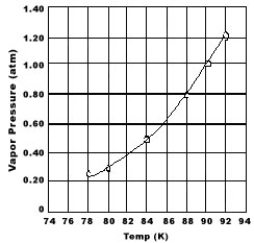
A)84 K
B)88 K
C)90 K
D)92 K
E)O2 doesn't boil because it is always a gas.

A)84 K
B)88 K
C)90 K
D)92 K
E)O2 doesn't boil because it is always a gas.

Unlock Deck
Unlock for access to all 149 flashcards in this deck.
Unlock Deck
k this deck
64
A liquid boils when its
A)vapor pressure is exactly 1 atmosphere.
B)vapor pressure is equal to, or greater than, the external pressure pushing on it.
C)temperature is equal to 273 K (standard temperature).
D)temperature is greater than room temperature.
A)vapor pressure is exactly 1 atmosphere.
B)vapor pressure is equal to, or greater than, the external pressure pushing on it.
C)temperature is equal to 273 K (standard temperature).
D)temperature is greater than room temperature.

Unlock Deck
Unlock for access to all 149 flashcards in this deck.
Unlock Deck
k this deck
65
A phase change from the gas phase directly to the solid phase is called:
A)Sublimation
B)Condensation
C)Freezing
D)Melting
E)Deposition
A)Sublimation
B)Condensation
C)Freezing
D)Melting
E)Deposition

Unlock Deck
Unlock for access to all 149 flashcards in this deck.
Unlock Deck
k this deck
66
Which of the following constants is/are needed to calculate the amount of energy required to heat 30.5g of H2O(s)at -25.0°C to H2O(l)at 55.0°C? Hfus (H2O)
II. Hvap (H2O)
III.specific heat of H2O(s)
IV.specific heat of H2O(l)
V.specific heat of H2O(g)
A)I, II, III, IV, and IV
B)III and IV
C)I, II, III, and IV
D)I, III, and IV
E)I only
II. Hvap (H2O)
III.specific heat of H2O(s)
IV.specific heat of H2O(l)
V.specific heat of H2O(g)
A)I, II, III, IV, and IV
B)III and IV
C)I, II, III, and IV
D)I, III, and IV
E)I only

Unlock Deck
Unlock for access to all 149 flashcards in this deck.
Unlock Deck
k this deck
67
Use the graph of vapor pressure to determine the normal boiling point of CHCl3. 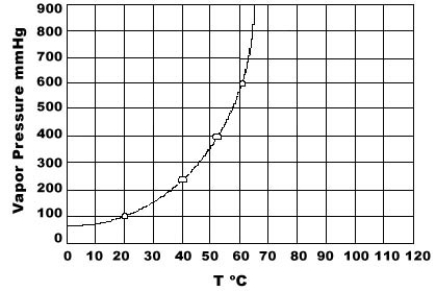
A)19°C
B)52°C
C)60°C
D)64°C
E)70°C

A)19°C
B)52°C
C)60°C
D)64°C
E)70°C

Unlock Deck
Unlock for access to all 149 flashcards in this deck.
Unlock Deck
k this deck
68
How much energy (heat)is required to convert 52.0 g of ice at -10.0°C to steam at 100°C? 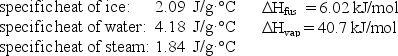
A)40.2 kJ
B)157.8 kJ
C)1,086 kJ
D)2,570 kJ
E)22,957 kJ
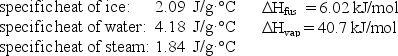
A)40.2 kJ
B)157.8 kJ
C)1,086 kJ
D)2,570 kJ
E)22,957 kJ

Unlock Deck
Unlock for access to all 149 flashcards in this deck.
Unlock Deck
k this deck
69
How much energy (heat)is required to convert 25.5 g of H2O(l)at 35.0°C to H2O(g)at 115.0°C? 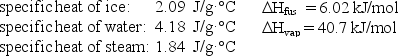
A)207 J
B)7,630 J
C)8,530 J
D)9,130 J
E)65,200 J
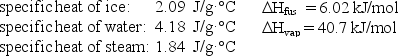
A)207 J
B)7,630 J
C)8,530 J
D)9,130 J
E)65,200 J

Unlock Deck
Unlock for access to all 149 flashcards in this deck.
Unlock Deck
k this deck
70
Which of the following constants is/are needed to calculate the amount of energy required to heat 12.0g of H2O(l)at 30.0°C to H2O(l)at 85.0°C? I. Hfus (H2O)
II. Hvap (H2O)
III.specific heat of H2O(s)
IV.specific heat of H2O(l)
V.specific heat of H2O(g)
A)I and IV
B)II and IV
C)IV only
D)I, II, and IV
E)IV and V
II. Hvap (H2O)
III.specific heat of H2O(s)
IV.specific heat of H2O(l)
V.specific heat of H2O(g)
A)I and IV
B)II and IV
C)IV only
D)I, II, and IV
E)IV and V

Unlock Deck
Unlock for access to all 149 flashcards in this deck.
Unlock Deck
k this deck
71
Which of the following phase changes is endothermic?
A)Sublimation
B)Condensation
C)Freezing
D)Deposition
A)Sublimation
B)Condensation
C)Freezing
D)Deposition

Unlock Deck
Unlock for access to all 149 flashcards in this deck.
Unlock Deck
k this deck
72
The atomic planes in a graphite crystal are separated by 335 pm.At what angle would you find the first-order (n = 1)diffraction of 0.154 nm X-rays from a graphite crystal?
A)0.232°
B)2.63°
C)13.3°
D)27.4°
E)66.8°
A)0.232°
B)2.63°
C)13.3°
D)27.4°
E)66.8°

Unlock Deck
Unlock for access to all 149 flashcards in this deck.
Unlock Deck
k this deck
73
The vapor pressure of a liquid in a closed container depends upon
A)the amount of liquid.
B)the surface area of the liquid.
C)the volume of the container.
D)the temperature.
E)None of the above.
A)the amount of liquid.
B)the surface area of the liquid.
C)the volume of the container.
D)the temperature.
E)None of the above.

Unlock Deck
Unlock for access to all 149 flashcards in this deck.
Unlock Deck
k this deck
74
Which of the following substances is expected to have the highest molar heat of vaporization ( Hvap)?
A)Ar
B)C6H6
C)He
D)NH3
E)H2O
A)Ar
B)C6H6
C)He
D)NH3
E)H2O

Unlock Deck
Unlock for access to all 149 flashcards in this deck.
Unlock Deck
k this deck
75
Calculate the amount of heat that must be absorbed by 10.0 g of ice at -20°C to convert it to liquid water at 60.0°C.Given: specific heat (ice)= 2.1 J/g·°C; specific heat (water)= 4.18 J/g·°C; Hfus = 6.0 kJ/mol.
A)63 kJ
B)7.5 J
C)420 J
D)2,900 J
E)6,300 J
A)63 kJ
B)7.5 J
C)420 J
D)2,900 J
E)6,300 J

Unlock Deck
Unlock for access to all 149 flashcards in this deck.
Unlock Deck
k this deck
76
The heat capacity of liquid water is 4.18 J/g·°C and the heat of vaporization is 40.7 kJ/mol.How many kilojoules of heat must be provided to convert 1.00 g of liquid water at 67°C into 1.00 g of steam at 100°C?
A)40.8 J
B)2.2 kJ
C)2,400 J
D)22.7 kJ
E)40.8 kJ
A)40.8 J
B)2.2 kJ
C)2,400 J
D)22.7 kJ
E)40.8 kJ

Unlock Deck
Unlock for access to all 149 flashcards in this deck.
Unlock Deck
k this deck
77
The triple point of iodine is at 0.12 atm and 115°C.Thus, liquid I2
A)is more dense than I2 (s).
B)cannot exist above 115°C.
C)is liquid at room temperature.
D)cannot have a vapor pressure less than 91 torr.
A)is more dense than I2 (s).
B)cannot exist above 115°C.
C)is liquid at room temperature.
D)cannot have a vapor pressure less than 91 torr.

Unlock Deck
Unlock for access to all 149 flashcards in this deck.
Unlock Deck
k this deck
78
Acetic acid has a heat of fusion of 10.8 kJ/mol and a heat of vaporization of 24.3 kJ/mol.What is the expected value for the heat of sublimation of acetic acid?
A)35.1 kJ/mol
B)-13.5 kJ/mol
C)+13.5 kJ/mol
D)-35.1 kJ/mol
E)Not enough information is given to answer the question.
A)35.1 kJ/mol
B)-13.5 kJ/mol
C)+13.5 kJ/mol
D)-35.1 kJ/mol
E)Not enough information is given to answer the question.

Unlock Deck
Unlock for access to all 149 flashcards in this deck.
Unlock Deck
k this deck
79
Calculate the amount of heat needed to melt 2.00 kg of iron at its melting point (1,809 K), given that Hfus = 13.80 kJ/mol.
A)27,600 J
B)27.6 kJ
C)494 kJ
D)25,000 kJ
E)27,600 kJ
A)27,600 J
B)27.6 kJ
C)494 kJ
D)25,000 kJ
E)27,600 kJ

Unlock Deck
Unlock for access to all 149 flashcards in this deck.
Unlock Deck
k this deck
80
Which of the following phase changes is exothermic?
A)Sublimation
B)Condensation
C)Melting
D)Vaporization
A)Sublimation
B)Condensation
C)Melting
D)Vaporization

Unlock Deck
Unlock for access to all 149 flashcards in this deck.
Unlock Deck
k this deck


