Deck 15: Acids and Bases
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/178
Play
Full screen (f)
Deck 15: Acids and Bases
1
The hydronium ion and the hydroxide ion, in that order, are:
A)H3O+, OH+
B)OH-, H3O-
C)OH-, H+
D)H3O+, OH-
E)H3O-, OH-
A)H3O+, OH+
B)OH-, H3O-
C)OH-, H+
D)H3O+, OH-
E)H3O-, OH-
H3O+, OH-
2
Identify the conjugate base of HSO4 -
A)OH-
B)H2SO4
C)H2O
D)H2SO3
E)SO42-
A)OH-
B)H2SO4
C)H2O
D)H2SO3
E)SO42-
SO42-
3
In the reaction: CH3COOH(aq)+ NH2- (aq) 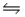 CH3COO- (aq)+ NH3(aq), the conjugate acid-base pairs are:
CH3COO- (aq)+ NH3(aq), the conjugate acid-base pairs are:
A)pair 1: CH3COOH and CH3COO- ; pair 2: NH2- and NH3
B)pair 1: CH3COOH and NH3; pair 2: NH2- and CH3COO-
C)pair 1: CH3COOH and NH2- ; pair 2: NH3 and CH3OO-
D)pair 1: CH3COOH and CH3COO- ; pair 2: NH4+ and NH3
E)pair 1: CH3COOH and CH3COO- ; pair 2: NH2- and NH3+
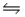 CH3COO- (aq)+ NH3(aq), the conjugate acid-base pairs are:
CH3COO- (aq)+ NH3(aq), the conjugate acid-base pairs are:A)pair 1: CH3COOH and CH3COO- ; pair 2: NH2- and NH3
B)pair 1: CH3COOH and NH3; pair 2: NH2- and CH3COO-
C)pair 1: CH3COOH and NH2- ; pair 2: NH3 and CH3OO-
D)pair 1: CH3COOH and CH3COO- ; pair 2: NH4+ and NH3
E)pair 1: CH3COOH and CH3COO- ; pair 2: NH2- and NH3+
pair 1: CH3COOH and CH3COO- ; pair 2: NH2- and NH3
4
Identify the conjugate base of HCO3-
A)H2CO3
B)CO32-
C)OH-
D)CO2
E)CO
A)H2CO3
B)CO32-
C)OH-
D)CO2
E)CO

Unlock Deck
Unlock for access to all 178 flashcards in this deck.
Unlock Deck
k this deck
5
Identify the conjugate base of HPO42-
A)H2O
B)H2PO4-
C)H3PO4
D)PO43-
E)OH-
A)H2O
B)H2PO4-
C)H3PO4
D)PO43-
E)OH-

Unlock Deck
Unlock for access to all 178 flashcards in this deck.
Unlock Deck
k this deck
6
What is the concentration of H+ in a 2.5 M HCl solution?
A)0
B)1.3 M
C)2.5 M
D)5.0 M
E)10.M
A)0
B)1.3 M
C)2.5 M
D)5.0 M
E)10.M

Unlock Deck
Unlock for access to all 178 flashcards in this deck.
Unlock Deck
k this deck
7
Which of the following is not a conjugate acid-base pair?
A)H3PO4 and H2PO4-
B)H2PO4- and HPO42-
C)H3PO4 and HPO42-
D)HPO42- and PO43-
E)H2O and H3O+
A)H3PO4 and H2PO4-
B)H2PO4- and HPO42-
C)H3PO4 and HPO42-
D)HPO42- and PO43-
E)H2O and H3O+

Unlock Deck
Unlock for access to all 178 flashcards in this deck.
Unlock Deck
k this deck
8
Identify the conjugate acid of CO32-
A)H2CO3
B)HCO3-
C)H2O
D)H3O+
E)CO2
A)H2CO3
B)HCO3-
C)H2O
D)H3O+
E)CO2

Unlock Deck
Unlock for access to all 178 flashcards in this deck.
Unlock Deck
k this deck
9
In the reaction HSO4-(aq)+ OH-(aq) 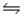 SO42-(aq)+ H2O(l), the conjugate acid-base pairs are
SO42-(aq)+ H2O(l), the conjugate acid-base pairs are 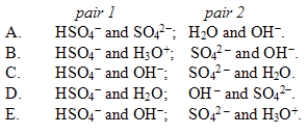
A)A
B)B
C)C
D)D
E)E
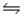 SO42-(aq)+ H2O(l), the conjugate acid-base pairs are
SO42-(aq)+ H2O(l), the conjugate acid-base pairs are 
A)A
B)B
C)C
D)D
E)E

Unlock Deck
Unlock for access to all 178 flashcards in this deck.
Unlock Deck
k this deck
10
In the reaction: 2H2O(l)  H3O+(aq)+ OH- (aq)the conjugate acid-base pairs are
H3O+(aq)+ OH- (aq)the conjugate acid-base pairs are
A)pair 1: H2O and H2O; pair 2: H3O+ and OH-
B)pair 1: H3O+ and OH-; pair 2: H3O+ and H2O
C)pair 1: H3O+ and OH-; pair 2: OH- and H2O
D)pair 1: H2O and OH-; pair 2: H2O and H3O+
E)pair 1: H3O+ and HO-: pair 2: OH- and H3O+
 H3O+(aq)+ OH- (aq)the conjugate acid-base pairs are
H3O+(aq)+ OH- (aq)the conjugate acid-base pairs areA)pair 1: H2O and H2O; pair 2: H3O+ and OH-
B)pair 1: H3O+ and OH-; pair 2: H3O+ and H2O
C)pair 1: H3O+ and OH-; pair 2: OH- and H2O
D)pair 1: H2O and OH-; pair 2: H2O and H3O+
E)pair 1: H3O+ and HO-: pair 2: OH- and H3O+

Unlock Deck
Unlock for access to all 178 flashcards in this deck.
Unlock Deck
k this deck
11
Identify the conjugate acid of SO42-
A)H2SO4
B)HSO4-
C)H2SO3
D)H3O+
E)SO32-
A)H2SO4
B)HSO4-
C)H2SO3
D)H3O+
E)SO32-

Unlock Deck
Unlock for access to all 178 flashcards in this deck.
Unlock Deck
k this deck
12
Which of the following is not a conjugate acid-base pair?
A)H2O and OH-
B)H2O and H3O+
C)H3O+ and OH-
D)HO2- and H2O2
E)O22- and HO2-
A)H2O and OH-
B)H2O and H3O+
C)H3O+ and OH-
D)HO2- and H2O2
E)O22- and HO2-

Unlock Deck
Unlock for access to all 178 flashcards in this deck.
Unlock Deck
k this deck
13
Which of the following does not fit the definition of a Brønsted Base?
A)CO32-
B)NH3
C)H2O
D)NH4+
E)HCO3-
A)CO32-
B)NH3
C)H2O
D)NH4+
E)HCO3-

Unlock Deck
Unlock for access to all 178 flashcards in this deck.
Unlock Deck
k this deck
14
The OH- concentration in a 7.5 × 10-3 M Ca(OH)2 solution is
A)7.5 × 10-3 M.
B)1.5 × 10-2 M.
C)1.3 × 10-12 M.
D)1.0 × 10-7 M.
E)1.0 × 10-14 M.
A)7.5 × 10-3 M.
B)1.5 × 10-2 M.
C)1.3 × 10-12 M.
D)1.0 × 10-7 M.
E)1.0 × 10-14 M.

Unlock Deck
Unlock for access to all 178 flashcards in this deck.
Unlock Deck
k this deck
15
Which one of these statements about strong acids is true?
A)All strong acids have H atoms bonded to electronegative oxygen atoms.
B)Strong acids are 100% ionized in water.
C)The conjugate base of a strong acid is itself a strong base.
D)Strong acids are very concentrated acids.
E)Strong acids produce solutions with a higher pH than weak acids.
A)All strong acids have H atoms bonded to electronegative oxygen atoms.
B)Strong acids are 100% ionized in water.
C)The conjugate base of a strong acid is itself a strong base.
D)Strong acids are very concentrated acids.
E)Strong acids produce solutions with a higher pH than weak acids.

Unlock Deck
Unlock for access to all 178 flashcards in this deck.
Unlock Deck
k this deck
16
The OH- concentration in a 2.5 × 10-3 M Ba(OH)2 solution is
A)4.0 × 10-12 M.
B)2.5 × 10-3 M.
C)5.0 × 10-3 M.
D)1.2 × 10-2 M.
E)0.025 M.
A)4.0 × 10-12 M.
B)2.5 × 10-3 M.
C)5.0 × 10-3 M.
D)1.2 × 10-2 M.
E)0.025 M.

Unlock Deck
Unlock for access to all 178 flashcards in this deck.
Unlock Deck
k this deck
17
What is the H+ ion concentration in a 4.8 × 10-2 M KOH solution?
A)4.8 × 10-2 M
B)1.0 × 10-7 M
C)4.8 × 10-11 M
D)4.8 × 10-12 M
E)2.1 × 10-13 M
A)4.8 × 10-2 M
B)1.0 × 10-7 M
C)4.8 × 10-11 M
D)4.8 × 10-12 M
E)2.1 × 10-13 M

Unlock Deck
Unlock for access to all 178 flashcards in this deck.
Unlock Deck
k this deck
18
Which of the following does not fit the definition of a Brønsted Acid?
A)H3PO4
B)H2PO4-
C)H2O
D)NH4+
E)CO2
A)H3PO4
B)H2PO4-
C)H2O
D)NH4+
E)CO2

Unlock Deck
Unlock for access to all 178 flashcards in this deck.
Unlock Deck
k this deck
19
The OH- concentration in a 1.0 × 10-3 M Ba(OH)2 solution is
A)0.50 × 10-3 M.
B)1.0 × 10-3 M.
C)2.0 × 10-3 M.
D)1.0 × 10-2 M.
E)0.020 M.
A)0.50 × 10-3 M.
B)1.0 × 10-3 M.
C)2.0 × 10-3 M.
D)1.0 × 10-2 M.
E)0.020 M.

Unlock Deck
Unlock for access to all 178 flashcards in this deck.
Unlock Deck
k this deck
20
Identify the conjugate acid of HCO3-
A)H2O
B)CO32-
C)H2CO3
D)CO2
E)H3O+
A)H2O
B)CO32-
C)H2CO3
D)CO2
E)H3O+

Unlock Deck
Unlock for access to all 178 flashcards in this deck.
Unlock Deck
k this deck
21
A 0.10 M HF solution is 8.4% ionized.Calculate the H+ ion concentration.
A)0.84 M
B)0.12 M
C)0.10 M
D)0.084 M
E)8.4 × 10-3 M
A)0.84 M
B)0.12 M
C)0.10 M
D)0.084 M
E)8.4 × 10-3 M

Unlock Deck
Unlock for access to all 178 flashcards in this deck.
Unlock Deck
k this deck
22
Calculate the pH of 2.6 × 10-2 M KOH.
A)12.41
B)15.59
C)2.06
D)7.00
E)1.59
A)12.41
B)15.59
C)2.06
D)7.00
E)1.59

Unlock Deck
Unlock for access to all 178 flashcards in this deck.
Unlock Deck
k this deck
23
What is the pH of 10.0 mL of 0.0020 M HCl?
A)0.70
B)2.70
C)3.70
D)5.70
E)10.0
A)0.70
B)2.70
C)3.70
D)5.70
E)10.0

Unlock Deck
Unlock for access to all 178 flashcards in this deck.
Unlock Deck
k this deck
24
Calculate the pH of a 6.7 × 10-2 M NaOH solution.
A)12.83
B)2.17
C)11.82
D)6.71
E)1.17
A)12.83
B)2.17
C)11.82
D)6.71
E)1.17

Unlock Deck
Unlock for access to all 178 flashcards in this deck.
Unlock Deck
k this deck
25
Determine the pH of a KOH solution made by mixing 0.251 g KOH with enough water to make 1.0 × 102 mL of solution.
A)1.35
B)2.35
C)7.00
D)11.65
E)12.65
A)1.35
B)2.35
C)7.00
D)11.65
E)12.65

Unlock Deck
Unlock for access to all 178 flashcards in this deck.
Unlock Deck
k this deck
26
Consider the weak acid CH3COOH (acetic acid).If a 0.048 M CH3COOH solution is 5.2% ionized, determine the [H3O+] concentration at equilibrium.
A)0.25 M
B)9.2 × 10-3 M
C)0.048 M
D)0.052 M
E)2.5 × 10-3 M
A)0.25 M
B)9.2 × 10-3 M
C)0.048 M
D)0.052 M
E)2.5 × 10-3 M

Unlock Deck
Unlock for access to all 178 flashcards in this deck.
Unlock Deck
k this deck
27
Which of the following solutions is basic?
A)[OH-] = 1.0 x 10-14 M
B)[OH-] = 1.0 x 10-7 M
C)[H3O+] = 1.0 x 10-14 M
D)[H3O+] > 1.0 x 10-7 M
E)[OH-] < 1.0 x 10-7 M
A)[OH-] = 1.0 x 10-14 M
B)[OH-] = 1.0 x 10-7 M
C)[H3O+] = 1.0 x 10-14 M
D)[H3O+] > 1.0 x 10-7 M
E)[OH-] < 1.0 x 10-7 M

Unlock Deck
Unlock for access to all 178 flashcards in this deck.
Unlock Deck
k this deck
28
Calculate the pH of a 3.5 × 10-3 M HNO3 solution.
A)-2.46
B)0.54
C)2.46
D)3.00
E)3.46
A)-2.46
B)0.54
C)2.46
D)3.00
E)3.46

Unlock Deck
Unlock for access to all 178 flashcards in this deck.
Unlock Deck
k this deck
29
Calculate the pH of a 0.14 M HNO2 solution that is 5.7% ionized.
A)0.85
B)1.70
C)2.10
D)11.90
E)13.10
A)0.85
B)1.70
C)2.10
D)11.90
E)13.10

Unlock Deck
Unlock for access to all 178 flashcards in this deck.
Unlock Deck
k this deck
30
Calculate the H+ ion concentration in a 8.8 × 10-4 M Ca(OH)2 solution.
A)8.8 × 10-4 M
B)1.8 × 10-3 M
C)2.2 × 10-11 M
D)1.1 × 10-11 M
E)5.7 × 10-12 M
A)8.8 × 10-4 M
B)1.8 × 10-3 M
C)2.2 × 10-11 M
D)1.1 × 10-11 M
E)5.7 × 10-12 M

Unlock Deck
Unlock for access to all 178 flashcards in this deck.
Unlock Deck
k this deck
31
A 0.14 M HNO2 solution is 5.7% ionized.Calculate the H+ ion concentration.
A)8.0 × 10-3 M
B)0.057 M
C)0.13 M
D)0.14 M
E)0.80 M
A)8.0 × 10-3 M
B)0.057 M
C)0.13 M
D)0.14 M
E)0.80 M

Unlock Deck
Unlock for access to all 178 flashcards in this deck.
Unlock Deck
k this deck
32
Which of the following solutions is acidic?
A)[H3O+] = 7.0 x 10-7 M
B)[H3O+] = 1.5 x 10-10 M
C)[H3O+] < 7.0 x 10-7 M
D)[H3O+] > 7.0 x 10-7 M
E)[H3O+] = 1.0 x 10-14 M
A)[H3O+] = 7.0 x 10-7 M
B)[H3O+] = 1.5 x 10-10 M
C)[H3O+] < 7.0 x 10-7 M
D)[H3O+] > 7.0 x 10-7 M
E)[H3O+] = 1.0 x 10-14 M

Unlock Deck
Unlock for access to all 178 flashcards in this deck.
Unlock Deck
k this deck
33
What is the OH- ion concentration in a 5.2 × 10-4 M HNO3 solution?
A)1.9 × 10-11 M
B)1.0 × 10-7 M
C)5.2 × 10-4 M
D)0
E)1.0 × 10-4 M
A)1.9 × 10-11 M
B)1.0 × 10-7 M
C)5.2 × 10-4 M
D)0
E)1.0 × 10-4 M

Unlock Deck
Unlock for access to all 178 flashcards in this deck.
Unlock Deck
k this deck
34
Calculate the pH of a carbonated beverage in which the hydrogen ion concentration is 3.4 × 10-4 M.
A)2.34
B)3.47
C)6.01
D)7.99
E)10.53
A)2.34
B)3.47
C)6.01
D)7.99
E)10.53

Unlock Deck
Unlock for access to all 178 flashcards in this deck.
Unlock Deck
k this deck
35
Calculate the H+ ion concentration in lemon juice having a pH of 2.40.
A)4.0 × 10-2 M
B)250 M
C)0.38 M
D)4.0 × 10-3 M
E)12 M
A)4.0 × 10-2 M
B)250 M
C)0.38 M
D)4.0 × 10-3 M
E)12 M

Unlock Deck
Unlock for access to all 178 flashcards in this deck.
Unlock Deck
k this deck
36
Calculate the pH of a 0.10 M HCN solution that is 0.0070% ionized.
A)1.00
B)0.00070
C)3.15
D)5.15
E)7.00
A)1.00
B)0.00070
C)3.15
D)5.15
E)7.00

Unlock Deck
Unlock for access to all 178 flashcards in this deck.
Unlock Deck
k this deck
37
What is the pH of a 0.0055 M HA (weak acid)solution that is 8.2% ionized?
A)2.26
B)3.35
C)4.52
D)8.21
E)10.65
A)2.26
B)3.35
C)4.52
D)8.21
E)10.65

Unlock Deck
Unlock for access to all 178 flashcards in this deck.
Unlock Deck
k this deck
38
Which of the following solutions is acidic?
A)[OH-] = 1.0 x 10-7 M
B)[OH-] > 1.0 x 10-7 M
C)[OH-] = 1.0 x 10-10 M
D)[H3O+] = 1.0 x 10-10 M
E)[H3O+] < 1.0 x 10-7 M
A)[OH-] = 1.0 x 10-7 M
B)[OH-] > 1.0 x 10-7 M
C)[OH-] = 1.0 x 10-10 M
D)[H3O+] = 1.0 x 10-10 M
E)[H3O+] < 1.0 x 10-7 M

Unlock Deck
Unlock for access to all 178 flashcards in this deck.
Unlock Deck
k this deck
39
Which of the following solutions is basic?
A)[H3O+] = 1.0 x 10-10 M
B)[OH-] = 1.0 x 10-10 M
C)[H3O+] > 1.0 x 10-7 M
D)[OH-] < 1.0 x 10-10 M
E)[OH-] = 1.0 x 10-7 M
A)[H3O+] = 1.0 x 10-10 M
B)[OH-] = 1.0 x 10-10 M
C)[H3O+] > 1.0 x 10-7 M
D)[OH-] < 1.0 x 10-10 M
E)[OH-] = 1.0 x 10-7 M

Unlock Deck
Unlock for access to all 178 flashcards in this deck.
Unlock Deck
k this deck
40
A 0.10 M NH3 solution is 1.3% ionized.Calculate the H+ ion concentration. NH3 + H2O 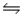
NH4+ + OH-
A)1.3 × 10-3 M
B)7.7 × 10-2 M
C)7.7 × 10-12 M
D)0.13 M
E)0.10 M
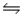
NH4+ + OH-
A)1.3 × 10-3 M
B)7.7 × 10-2 M
C)7.7 × 10-12 M
D)0.13 M
E)0.10 M

Unlock Deck
Unlock for access to all 178 flashcards in this deck.
Unlock Deck
k this deck
41
If the pH of an acid rain storm is approximately 3.0, how many times greater is the [H+] in the rain than in a cup of coffee having a pH of 5.0?
A)1000
B)100
C)20
D)1.7
E)0.60
A)1000
B)100
C)20
D)1.7
E)0.60

Unlock Deck
Unlock for access to all 178 flashcards in this deck.
Unlock Deck
k this deck
42
A 1.5 L sample of a 0.44 M HBr solution is mixed with 2.2 L of a 0.080 M HClO4 solution.What is the pH of the mixture?
A)0.28
B)0.36
C)1.45
D)0.73
E)0.65
A)0.28
B)0.36
C)1.45
D)0.73
E)0.65

Unlock Deck
Unlock for access to all 178 flashcards in this deck.
Unlock Deck
k this deck
43
The pOH of a solution is 10.40.Calculate the hydrogen ion concentration in the solution.
A)4.0 × 10-11 M
B)3.6 M
C)4.0 × 10-10 M
D)2.5 × 10-4 M
E)1.8 × 10-4 M
A)4.0 × 10-11 M
B)3.6 M
C)4.0 × 10-10 M
D)2.5 × 10-4 M
E)1.8 × 10-4 M

Unlock Deck
Unlock for access to all 178 flashcards in this deck.
Unlock Deck
k this deck
44
Calculate the pOH for a solution with [H3O+] = 2.5 x 10-5 M
A)4.60
B)9.40
C)4.0 x 10-10
D)2.50
E)11.50
A)4.60
B)9.40
C)4.0 x 10-10
D)2.50
E)11.50

Unlock Deck
Unlock for access to all 178 flashcards in this deck.
Unlock Deck
k this deck
45
What is the pH of a solution prepared by mixing 10.0 mL of a strong acid solution with pH = 2.0 and 10.0 mL of a strong acid solution with pH = 6.0?
A)2.0
B)2.3
C)4.0
D)6.0
E)8.0
A)2.0
B)2.3
C)4.0
D)6.0
E)8.0

Unlock Deck
Unlock for access to all 178 flashcards in this deck.
Unlock Deck
k this deck
46
What is the pH of a 0.001 M Ca(OH)2 solution?
A)3.0
B)11.0
C)2.7
D)17.0
E)11.3
A)3.0
B)11.0
C)2.7
D)17.0
E)11.3

Unlock Deck
Unlock for access to all 178 flashcards in this deck.
Unlock Deck
k this deck
47
A 5.5 L sample of a 0.25 M HNO3 solution is mixed with 1.2 L of a 0.34 M HCl solution.What is the pH of the mixture?
A)0.23
B)0.57
C)1.07
D)0.50
E)0.84
A)0.23
B)0.57
C)1.07
D)0.50
E)0.84

Unlock Deck
Unlock for access to all 178 flashcards in this deck.
Unlock Deck
k this deck
48
Calculate the hydrogen ion concentration in a solution having a pH of 4.60.
A)4.0 × 10-3 M
B)4.0 × 10-9 M
C)4.0 × 10-10 M
D)2.5 × 10-5 M
E)2.5 × 10-4 M
A)4.0 × 10-3 M
B)4.0 × 10-9 M
C)4.0 × 10-10 M
D)2.5 × 10-5 M
E)2.5 × 10-4 M

Unlock Deck
Unlock for access to all 178 flashcards in this deck.
Unlock Deck
k this deck
49
A 2.1 L sample of a 0.23 M NaOH solution is mixed with 1.9 L of a 0.021 M KOH solution.What is the pH of the mixture?
A)13.40
B)13.12
C)11.68
D)12.84
E)13.04
A)13.40
B)13.12
C)11.68
D)12.84
E)13.04

Unlock Deck
Unlock for access to all 178 flashcards in this deck.
Unlock Deck
k this deck
50
The pH of tomato juice is about 4.5.Calculate the concentration of hydrogen ions in this juice.
A)3 × 10-10 M
B)3 × 10-5 M
C)5 × 10-4 M
D)4 M
E)3 × 1010 M
A)3 × 10-10 M
B)3 × 10-5 M
C)5 × 10-4 M
D)4 M
E)3 × 1010 M

Unlock Deck
Unlock for access to all 178 flashcards in this deck.
Unlock Deck
k this deck
51
What is the pH of a 0.014 M Ca(OH)2 solution?
A)1.85
B)1.55
C)12.15
D)12.45
E)15.85
A)1.85
B)1.55
C)12.15
D)12.45
E)15.85

Unlock Deck
Unlock for access to all 178 flashcards in this deck.
Unlock Deck
k this deck
52
Diet cola drinks have a pH of about 3.0, while milk has a pH of about 7.0.How many times greater is the H3O+ concentration in diet cola than in milk?
A)2.3 times higher in diet cola than in milk
B)400 times higher in diet cola than in milk
C)0.43 times higher in diet cola than in milk
D)1,000 times higher in diet cola than in milk
E)10,000 times higher in diet cola than in milk
A)2.3 times higher in diet cola than in milk
B)400 times higher in diet cola than in milk
C)0.43 times higher in diet cola than in milk
D)1,000 times higher in diet cola than in milk
E)10,000 times higher in diet cola than in milk

Unlock Deck
Unlock for access to all 178 flashcards in this deck.
Unlock Deck
k this deck
53
Calculate the hydrogen ion concentration in a solution of iced tea with lemon having a pH of 2.87.
A)2.9 × 10-2 M
B)5.7 × 10-2 M
C)1.3 × 10-3 M
D)2.9 × 10-3 M
E)5.7 × 10-4 M
A)2.9 × 10-2 M
B)5.7 × 10-2 M
C)1.3 × 10-3 M
D)2.9 × 10-3 M
E)5.7 × 10-4 M

Unlock Deck
Unlock for access to all 178 flashcards in this deck.
Unlock Deck
k this deck
54
A 5.2 L sample of a 1.1 M KOH solution is mixed with 2.3 L of a 0.20 M Sc(OH)3 solution.What is the pH of the mixture?
A)13.67
B)13.89
C)14.11
D)14.23
E)13.98
A)13.67
B)13.89
C)14.11
D)14.23
E)13.98

Unlock Deck
Unlock for access to all 178 flashcards in this deck.
Unlock Deck
k this deck
55
The pH of a Ba(OH)2 solution is 10.00.What is the H+ ion concentration of this solution?
A)4.0 × 10-11 M
B)1.6 × 10-10 M
C)1.3 × 10-5 M
D)1.0 × 10-10 M
E)10.M
A)4.0 × 10-11 M
B)1.6 × 10-10 M
C)1.3 × 10-5 M
D)1.0 × 10-10 M
E)10.M

Unlock Deck
Unlock for access to all 178 flashcards in this deck.
Unlock Deck
k this deck
56
The pH of coffee is approximately 5.0.How many times greater is the [H3O+] in coffee than in tap water having a pH of 8.0?
A)0.62
B)1.6
C)30
D)1,000
E)1.0 × 104
A)0.62
B)1.6
C)30
D)1,000
E)1.0 × 104

Unlock Deck
Unlock for access to all 178 flashcards in this deck.
Unlock Deck
k this deck
57
Calculate the pH of a 1.6 M KOH solution.
A)1.60
B)-0.20
C)0.20
D)14.20
E)13.80
A)1.60
B)-0.20
C)0.20
D)14.20
E)13.80

Unlock Deck
Unlock for access to all 178 flashcards in this deck.
Unlock Deck
k this deck
58
The pH of coffee is approximately 5.0.How many times greater is the [H+] in coffee than in neutral water?
A)200
B)100
C)5.0
D)1.4
E)0.01
A)200
B)100
C)5.0
D)1.4
E)0.01

Unlock Deck
Unlock for access to all 178 flashcards in this deck.
Unlock Deck
k this deck
59
Calculate the hydrogen ion concentration in a solution of fruit juice having a pH of 4.25.
A)1.0 × 10-14 M
B)5.6 × 10-5 M
C)4.0 × 10-25 M
D)2.5 × 10-4 M
E)5.6 × 10-4 M
A)1.0 × 10-14 M
B)5.6 × 10-5 M
C)4.0 × 10-25 M
D)2.5 × 10-4 M
E)5.6 × 10-4 M

Unlock Deck
Unlock for access to all 178 flashcards in this deck.
Unlock Deck
k this deck
60
The pOH of a solution is 9.60.Calculate the hydrogen ion concentration in this solution.
A)2.5 × 10-10 M
B)6.0 × 10-9 M
C)4.0 × 10-5 M
D)2.4 × 10-4 M
E)1.0 × 10-14 M
A)2.5 × 10-10 M
B)6.0 × 10-9 M
C)4.0 × 10-5 M
D)2.4 × 10-4 M
E)1.0 × 10-14 M

Unlock Deck
Unlock for access to all 178 flashcards in this deck.
Unlock Deck
k this deck
61
Which one of the following equations represents the ionization of a weak monoprotic acid in water?
A)HNO2(aq)+ OH- (aq)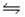 H2O(l)+ NO2-(aq)
H2O(l)+ NO2-(aq)
B)HNO2(aq)+ NH3(aq)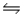 NH4NO2(aq)
NH4NO2(aq)
C)HNO2(aq)+ H2O(l)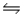 H3O+(aq)+ NO2-(aq)
H3O+(aq)+ NO2-(aq)
D)HNO3(aq)+ OH-(aq)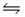 H2O(l)+ NO3-(aq)
H2O(l)+ NO3-(aq)
E)NO2- (aq)+ H2O(aq)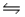 HNO2(aq)+ OH-(aq)
HNO2(aq)+ OH-(aq)
A)HNO2(aq)+ OH- (aq)
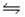 H2O(l)+ NO2-(aq)
H2O(l)+ NO2-(aq)B)HNO2(aq)+ NH3(aq)
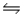 NH4NO2(aq)
NH4NO2(aq)C)HNO2(aq)+ H2O(l)
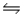 H3O+(aq)+ NO2-(aq)
H3O+(aq)+ NO2-(aq)D)HNO3(aq)+ OH-(aq)
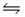 H2O(l)+ NO3-(aq)
H2O(l)+ NO3-(aq)E)NO2- (aq)+ H2O(aq)
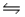 HNO2(aq)+ OH-(aq)
HNO2(aq)+ OH-(aq)
Unlock Deck
Unlock for access to all 178 flashcards in this deck.
Unlock Deck
k this deck
62
Given the following Ka values, which anion is the strongest base? 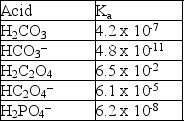
A)HCO3-
B)CO32-
C)HC2O4-
D)C2O42-
E)HPO42-

A)HCO3-
B)CO32-
C)HC2O4-
D)C2O42-
E)HPO42-

Unlock Deck
Unlock for access to all 178 flashcards in this deck.
Unlock Deck
k this deck
63
Given the following Kb values, which cation is the strongest acid? ![<strong>Given the following K<sub>b</sub> values, which cation is the strongest acid? </strong> A)NH<sub>4</sub><sup>+</sup> B)C<sub>2</sub>H<sub>5</sub>NH<sub>3</sub><sup>+</sup> C)C<sub>5</sub>H<sub>6</sub>N<sup>+</sup> D)[C<sub>8</sub>H<sub>11</sub>N<sub>4</sub>O<sub>2</sub>]<sup>+</sup> E)[NH<sub>2</sub>NH<sub>3</sub>CO]<sup>+</sup>](https://storage.examlex.com/TB3246/11ea7cbf_8f25_d3ea_a2ab_bd115a8af7d1_TB3246_00.jpg)
A)NH4+
B)C2H5NH3+
C)C5H6N+
D)[C8H11N4O2]+
E)[NH2NH3CO]+
![<strong>Given the following K<sub>b</sub> values, which cation is the strongest acid? </strong> A)NH<sub>4</sub><sup>+</sup> B)C<sub>2</sub>H<sub>5</sub>NH<sub>3</sub><sup>+</sup> C)C<sub>5</sub>H<sub>6</sub>N<sup>+</sup> D)[C<sub>8</sub>H<sub>11</sub>N<sub>4</sub>O<sub>2</sub>]<sup>+</sup> E)[NH<sub>2</sub>NH<sub>3</sub>CO]<sup>+</sup>](https://storage.examlex.com/TB3246/11ea7cbf_8f25_d3ea_a2ab_bd115a8af7d1_TB3246_00.jpg)
A)NH4+
B)C2H5NH3+
C)C5H6N+
D)[C8H11N4O2]+
E)[NH2NH3CO]+

Unlock Deck
Unlock for access to all 178 flashcards in this deck.
Unlock Deck
k this deck
64
When comparing acid strength of binary acids HX, as X varies within a particular group of the periodic table, which one of these factors dominates in affecting the acid strength?
A)bond strength
B)electron withdrawing effects
C)percent ionic character of the H-X bond
D)solubility
E)Le Châtelier's principle
A)bond strength
B)electron withdrawing effects
C)percent ionic character of the H-X bond
D)solubility
E)Le Châtelier's principle

Unlock Deck
Unlock for access to all 178 flashcards in this deck.
Unlock Deck
k this deck
65
The equilibrium expression for the ionization of a weak monoprotic acid, HA, in water is:
A)Ka = [HA]/[OH-][H3O+]
B)Ka = [OH-][H3O+]/[HA]
C)Ka = [H3O+][HA]/[A-]
D)Ka = [H3O+][A-]/[HA]
E)Ka = [HA]/[H3O+][A-]
A)Ka = [HA]/[OH-][H3O+]
B)Ka = [OH-][H3O+]/[HA]
C)Ka = [H3O+][HA]/[A-]
D)Ka = [H3O+][A-]/[HA]
E)Ka = [HA]/[H3O+][A-]

Unlock Deck
Unlock for access to all 178 flashcards in this deck.
Unlock Deck
k this deck
66
Which one of these equations represents the reaction of a weak acid with a weak base?
A)H+(aq)+ OH-(aq) H2O(aq)
B)H+(aq)+ CH3NH2(aq) CH3NH3+(aq)
C)OH-(aq)+ HCN(aq) H2O(aq)+ CN-(aq)
D)HCN(aq)+ CH3NH2(aq) CH3NH3+(aq)+ CN-(aq)
A)H+(aq)+ OH-(aq) H2O(aq)
B)H+(aq)+ CH3NH2(aq) CH3NH3+(aq)
C)OH-(aq)+ HCN(aq) H2O(aq)+ CN-(aq)
D)HCN(aq)+ CH3NH2(aq) CH3NH3+(aq)+ CN-(aq)

Unlock Deck
Unlock for access to all 178 flashcards in this deck.
Unlock Deck
k this deck
67
Which one of the following equations represents the ionization of a weak base in water?
A)NH3(aq)+ H2O(l)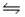 NH2+(aq)+ H3O+(aq)
NH2+(aq)+ H3O+(aq)
B)NH3(aq)+ H2O(l)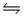 NH4+(aq)+ OH-(aq)
NH4+(aq)+ OH-(aq)
C)NH3(aq)+ H2O(l)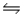 NH4- (aq)+ OH+(aq)
NH4- (aq)+ OH+(aq)
D)NH3(aq)+ OH-(aq)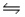 NH2- (aq)+ H2O(l)
NH2- (aq)+ H2O(l)
E)NH3(aq)+ H3O+(aq)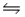 NH4+(aq)+ H2O(l)
NH4+(aq)+ H2O(l)
A)NH3(aq)+ H2O(l)
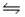 NH2+(aq)+ H3O+(aq)
NH2+(aq)+ H3O+(aq)B)NH3(aq)+ H2O(l)
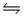 NH4+(aq)+ OH-(aq)
NH4+(aq)+ OH-(aq)C)NH3(aq)+ H2O(l)
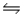 NH4- (aq)+ OH+(aq)
NH4- (aq)+ OH+(aq)D)NH3(aq)+ OH-(aq)
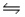 NH2- (aq)+ H2O(l)
NH2- (aq)+ H2O(l)E)NH3(aq)+ H3O+(aq)
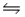 NH4+(aq)+ H2O(l)
NH4+(aq)+ H2O(l)
Unlock Deck
Unlock for access to all 178 flashcards in this deck.
Unlock Deck
k this deck
68
Which solution will have the lowest pH?
A)0.10 M HCN
B)0.10 M HNO3
C)0.10 M NaCl
D)0.10 M H2CO3
E)0.10 M NaOH
A)0.10 M HCN
B)0.10 M HNO3
C)0.10 M NaCl
D)0.10 M H2CO3
E)0.10 M NaOH

Unlock Deck
Unlock for access to all 178 flashcards in this deck.
Unlock Deck
k this deck
69
Which one of the following equations represents the ionization of a weak monoprotic acid in water?
A)HCN(aq)+ H2O(l)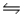 H3O+(aq)+ CN-(aq)
H3O+(aq)+ CN-(aq)
B)HCN(aq)+ OH-(aq) H2O(l)+ CN+(aq)
H2O(l)+ CN+(aq)
C)CN- (aq)+ H2O(aq) HCN(aq)+ OH-(aq)
HCN(aq)+ OH-(aq)
D)HCN(aq)+ OH- (aq) H2O(l)+ CN-(aq)
H2O(l)+ CN-(aq)
E)HCN(aq)+ NH3(aq) NH4CN(aq)
NH4CN(aq)
A)HCN(aq)+ H2O(l)
 H3O+(aq)+ CN-(aq)
H3O+(aq)+ CN-(aq)B)HCN(aq)+ OH-(aq)
 H2O(l)+ CN+(aq)
H2O(l)+ CN+(aq)C)CN- (aq)+ H2O(aq)
 HCN(aq)+ OH-(aq)
HCN(aq)+ OH-(aq)D)HCN(aq)+ OH- (aq)
 H2O(l)+ CN-(aq)
H2O(l)+ CN-(aq)E)HCN(aq)+ NH3(aq)
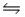 NH4CN(aq)
NH4CN(aq)
Unlock Deck
Unlock for access to all 178 flashcards in this deck.
Unlock Deck
k this deck
70
Arrange the acids HOBr, HBrO3, and HBrO2 in order of increasing acid strength.
A)HOBr < HBrO3 < HBrO2
B)HOBr < HBrO2 < HBrO3
C)HBrO2 < HOBr < HBrO3
D)HBrO3 < HOBr < HBrO2
E)HBrO3 < HBrO2 < HOBr
A)HOBr < HBrO3 < HBrO2
B)HOBr < HBrO2 < HBrO3
C)HBrO2 < HOBr < HBrO3
D)HBrO3 < HOBr < HBrO2
E)HBrO3 < HBrO2 < HOBr

Unlock Deck
Unlock for access to all 178 flashcards in this deck.
Unlock Deck
k this deck
71
Which one of these net ionic equations represents the reaction of a strong acid with a weak base?
A)H+(aq)+ OH-(aq) H2O(aq)
B)H+(aq)+ CH3NH2(aq) CH3NH3+(aq)
C)OH-(aq)+ HCN(aq) H2O(aq)+ CN-(aq)
D)HCN(aq)+ CH3NH2(aq) CH3NH3+ (aq)+ CN-(aq)
A)H+(aq)+ OH-(aq) H2O(aq)
B)H+(aq)+ CH3NH2(aq) CH3NH3+(aq)
C)OH-(aq)+ HCN(aq) H2O(aq)+ CN-(aq)
D)HCN(aq)+ CH3NH2(aq) CH3NH3+ (aq)+ CN-(aq)

Unlock Deck
Unlock for access to all 178 flashcards in this deck.
Unlock Deck
k this deck
72
Given the following Ka values, which anion is the strongest base? 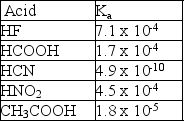
A)F-
B)HCOO-
C)CN-
D)NO2-
E)CH3COO-

A)F-
B)HCOO-
C)CN-
D)NO2-
E)CH3COO-

Unlock Deck
Unlock for access to all 178 flashcards in this deck.
Unlock Deck
k this deck
73
Calculate the pOH for a solution with [H3O+] = 3.1 x 10-9 M
A)3.10
B)10.90
C)8.51
D)5.49
E)3.2 x 10-6
A)3.10
B)10.90
C)8.51
D)5.49
E)3.2 x 10-6

Unlock Deck
Unlock for access to all 178 flashcards in this deck.
Unlock Deck
k this deck
74
Which solution will have the lowest pH?
A)0.25 M HClO
B)0.25 M HClO2
C)0.25 M HClO3
D)0.25 M HClO4
E)0.25 M NaClO4
A)0.25 M HClO
B)0.25 M HClO2
C)0.25 M HClO3
D)0.25 M HClO4
E)0.25 M NaClO4

Unlock Deck
Unlock for access to all 178 flashcards in this deck.
Unlock Deck
k this deck
75
Arrange the acids HOCl, HClO3, and HClO2 in order of increasing acid strength.
A)HOCl < HClO3 < HClO2
B)HOCl < HClO2 < HClO3
C)HClO2 < HOCl < HClO3
D)HClO3 < HOCl < HClO2
E)HClO3 < HClO2 < HOCl
A)HOCl < HClO3 < HClO2
B)HOCl < HClO2 < HClO3
C)HClO2 < HOCl < HClO3
D)HClO3 < HOCl < HClO2
E)HClO3 < HClO2 < HOCl

Unlock Deck
Unlock for access to all 178 flashcards in this deck.
Unlock Deck
k this deck
76
Which one of these net ionic equations represents the reaction of a strong acid with a strong base?
A)H+(aq)+ OH-(aq) H2O(aq)
B)H+(aq)+ CH3NH2(aq) CH3NH3+(aq)
C)OH-(aq)+ HCN(aq) H2O(aq)+ CN-(aq)
D)HCN(aq)+ CH3NH2(aq) CH3NH3+(aq)+ CN-(aq)
A)H+(aq)+ OH-(aq) H2O(aq)
B)H+(aq)+ CH3NH2(aq) CH3NH3+(aq)
C)OH-(aq)+ HCN(aq) H2O(aq)+ CN-(aq)
D)HCN(aq)+ CH3NH2(aq) CH3NH3+(aq)+ CN-(aq)

Unlock Deck
Unlock for access to all 178 flashcards in this deck.
Unlock Deck
k this deck
77
Which one of the following statements is true for a 0.1 M solution of a weak acid HA?
A)The concentration of H+ is slightly greater than the concentration of A-.
B)The pH equals 1.0.
C)The concentration of H+ is exactly equal to the concentration of A-.
D)The pH is less than 1.0.
E)The concentration of H+ is slightly less than the concentration of A-.
A)The concentration of H+ is slightly greater than the concentration of A-.
B)The pH equals 1.0.
C)The concentration of H+ is exactly equal to the concentration of A-.
D)The pH is less than 1.0.
E)The concentration of H+ is slightly less than the concentration of A-.

Unlock Deck
Unlock for access to all 178 flashcards in this deck.
Unlock Deck
k this deck
78
Which one of these equations represents the reaction of a weak acid with a strong base?
A)H+(aq)+ OH-(aq) H2O(aq)
B)H+(aq)+ CH3NH2(aq) CH3NH3+(aq)
C)OH-(aq)+ HCN(aq) H2O(aq)+ CN-(aq)
D)HCN(aq)+ CH3NH2(aq) CH3NH3+(aq)+ CN-(aq)
A)H+(aq)+ OH-(aq) H2O(aq)
B)H+(aq)+ CH3NH2(aq) CH3NH3+(aq)
C)OH-(aq)+ HCN(aq) H2O(aq)+ CN-(aq)
D)HCN(aq)+ CH3NH2(aq) CH3NH3+(aq)+ CN-(aq)

Unlock Deck
Unlock for access to all 178 flashcards in this deck.
Unlock Deck
k this deck
79
Arrange the acids H2Se, H2Te, and H2S in order of increasing acid strength.
A)H2S < H2Se < H2Te
B)H2S < H2Te < H2Se
C)H2Te < H2S < H2Se
D)H2Se < H2S < H2Te
E)H2Se < H2Te < H2S
A)H2S < H2Se < H2Te
B)H2S < H2Te < H2Se
C)H2Te < H2S < H2Se
D)H2Se < H2S < H2Te
E)H2Se < H2Te < H2S

Unlock Deck
Unlock for access to all 178 flashcards in this deck.
Unlock Deck
k this deck
80
Arrange the acids HBr, H2Se, and H3As in order of increasing acid strength.
A)HBr < H2Se < H3As
B)HBr < H3As < H2Se
C)H2Se < H3As < HBr
D)H3As < H2Se < HBr
E)H3As < HBr < H2Se
A)HBr < H2Se < H3As
B)HBr < H3As < H2Se
C)H2Se < H3As < HBr
D)H3As < H2Se < HBr
E)H3As < HBr < H2Se

Unlock Deck
Unlock for access to all 178 flashcards in this deck.
Unlock Deck
k this deck


