Deck 5: Gases
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/109
Play
Full screen (f)
Deck 5: Gases
1
At what temperature will a fixed amount of gas with a volume of 175 L at 15°C and 760 mmHg occupy a volume of 198 L at a pressure of 640 mm Hg?
A)274°C
B)214°C
C)114°C
D)1°C
E)-59°C
A)274°C
B)214°C
C)114°C
D)1°C
E)-59°C
1°C
2
The pressure of a gas sample was measured to be 489 mmHg.Which of the following is not an equivalent statement of that pressure? (1 atm = 1.01325 * 105 Pa)
A)65.2 kPa
B)6.52 * 104 Pa
C)489 torr
D)0.811 atm
A)65.2 kPa
B)6.52 * 104 Pa
C)489 torr
D)0.811 atm
0.811 atm
3
What will happen to the height (h)of the mercury column in the manometer shown below if the stopcock is opened, given that the atmospheric pressure is 755 mmHg? 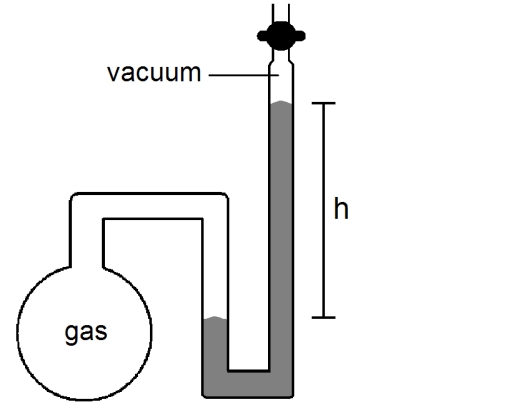
A)h will decrease
B)h will not change
C)h will increase
D)not enough information given to answer the question

A)h will decrease
B)h will not change
C)h will increase
D)not enough information given to answer the question
h will decrease
4
The gas pressure in an aerosol can is 1.8 atm at 25°C. If the gas is an ideal gas, what pressure would develop in the can if it were heated to 475°C?
A)0.095 atm
B)0.717 atm
C)3.26 atm
D)4.52 atm
E)34.2 atm
A)0.095 atm
B)0.717 atm
C)3.26 atm
D)4.52 atm
E)34.2 atm

Unlock Deck
Unlock for access to all 109 flashcards in this deck.
Unlock Deck
k this deck
5
The pressure of a gas sample was measured to be 654 mmHg.What is the pressure in kPa? (1 atm = 1.01325 * 105 Pa)
A)87.2 kPa
B)118 kPa
C)6.63 * 104 kPa
D)8.72 * 104 kPa
E)8.72 * 107 kPa
A)87.2 kPa
B)118 kPa
C)6.63 * 104 kPa
D)8.72 * 104 kPa
E)8.72 * 107 kPa

Unlock Deck
Unlock for access to all 109 flashcards in this deck.
Unlock Deck
k this deck
6
The temperature of an ideal gas in a 5.00 L container originally at 1 atm pressure and 25°C is lowered to 220 K. Calculate the new pressure of the gas.
A)1.0 atm
B)1.35 atm
C)8.8 atm
D)0.738 atm
E)0.114 atm
A)1.0 atm
B)1.35 atm
C)8.8 atm
D)0.738 atm
E)0.114 atm

Unlock Deck
Unlock for access to all 109 flashcards in this deck.
Unlock Deck
k this deck
7
Which of these properties is/are characteristic(s)of gases?
A)High compressibility
B)Relatively large distances between molecules
C)Formation of homogeneous mixtures regardless of the nature of gases
D)A and B.
E)A, B, and C.
A)High compressibility
B)Relatively large distances between molecules
C)Formation of homogeneous mixtures regardless of the nature of gases
D)A and B.
E)A, B, and C.

Unlock Deck
Unlock for access to all 109 flashcards in this deck.
Unlock Deck
k this deck
8
If 30.0 L of oxygen are cooled from 200ºC to 1°C at constant pressure, what is the new volume of oxygen?
A)0.150 L
B)17.4 L
C)23.0 L
D)51.8 L
E)6.00 * 103 L
A)0.150 L
B)17.4 L
C)23.0 L
D)51.8 L
E)6.00 * 103 L

Unlock Deck
Unlock for access to all 109 flashcards in this deck.
Unlock Deck
k this deck
9
A sample of nitrogen gas has a volume of 32.4 L at 20°C. The gas is heated to 220ºC at constant pressure. What is the final volume of nitrogen?
A)2.94 L
B)19.3 L
C)31.4 L
D)54.5 L
E)356 L
A)2.94 L
B)19.3 L
C)31.4 L
D)54.5 L
E)356 L

Unlock Deck
Unlock for access to all 109 flashcards in this deck.
Unlock Deck
k this deck
10
A sample of N2 gas occupies 2.40 L at 20°C. If the gas is in a container that can contract or expand at constant pressure, at what temperature will the N2 occupy 4.80 L?
A)10°C
B)40°C
C)146°C
D)313°C
E)685°C
A)10°C
B)40°C
C)146°C
D)313°C
E)685°C

Unlock Deck
Unlock for access to all 109 flashcards in this deck.
Unlock Deck
k this deck
11
At what temperature will a fixed mass of gas with a volume of 125 L at 15°C and 750 mmHg occupy a volume of 101 L at a pressure of 645 mm Hg?
A)-73°C
B)10.4°C
C)2°C
D)34°C
E)200°C
A)-73°C
B)10.4°C
C)2°C
D)34°C
E)200°C

Unlock Deck
Unlock for access to all 109 flashcards in this deck.
Unlock Deck
k this deck
12
If the pressure of a gas sample is quadrupled and the absolute temperature is doubled, by what factor does the volume of the sample change?
A)8
B)2
C)1/2
D)1/4
E)1/8
A)8
B)2
C)1/2
D)1/4
E)1/8

Unlock Deck
Unlock for access to all 109 flashcards in this deck.
Unlock Deck
k this deck
13
If the pressure on a gas sample is tripled and the absolute temperature is quadrupled, by what factor will the volume of the sample change?
A)12
B)4/3
C)3/4
D)1/3
E)4
A)12
B)4/3
C)3/4
D)1/3
E)4

Unlock Deck
Unlock for access to all 109 flashcards in this deck.
Unlock Deck
k this deck
14
A small bubble rises from the bottom of a lake, where the temperature and pressure are 4°C and 3.0 atm, to the water's surface, where the temperature is 25°C and the pressure is 0.95 atm.Calculate the final volume of the bubble if its initial volume was 2.1 mL.
A)0.72 mL
B)6.2 mL
C)41.4 mL
D)22.4 mL
E)7.1 mL
A)0.72 mL
B)6.2 mL
C)41.4 mL
D)22.4 mL
E)7.1 mL

Unlock Deck
Unlock for access to all 109 flashcards in this deck.
Unlock Deck
k this deck
15
A sample of a gas occupies 1.40 *103 mL at 25°C and 760 mmHg. What volume will it occupy at the same temperature and 380 mmHg?
A)2,800 mL
B)2,100 mL
C)1,400 mL
D)1,050 mL
E)700 mL
A)2,800 mL
B)2,100 mL
C)1,400 mL
D)1,050 mL
E)700 mL

Unlock Deck
Unlock for access to all 109 flashcards in this deck.
Unlock Deck
k this deck
16
What will happen to the height (h)of the column of mercury in the manometer shown below if the stopcock is opened? 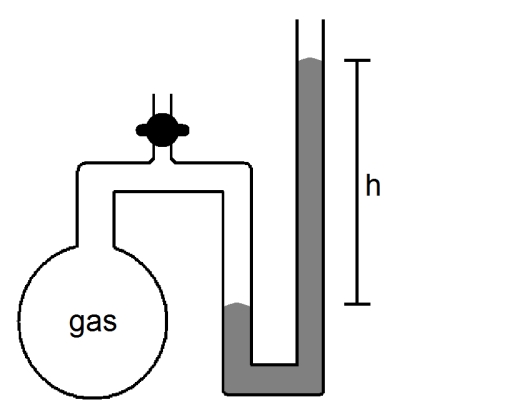
A)h will decrease
B)h will not change
C)h will increase
D)not enough information given to answer the question

A)h will decrease
B)h will not change
C)h will increase
D)not enough information given to answer the question

Unlock Deck
Unlock for access to all 109 flashcards in this deck.
Unlock Deck
k this deck
17
Calculate the volume occupied by 35.2 g of methane gas (CH4)at 25°C and 1.0 atm.R = 0.08206 L.atm/K.mol.
A)0.0186 L
B)4.5 L
C)11.2 L
D)49.2 L
E)53.7 L
A)0.0186 L
B)4.5 L
C)11.2 L
D)49.2 L
E)53.7 L

Unlock Deck
Unlock for access to all 109 flashcards in this deck.
Unlock Deck
k this deck
18
Calculate the number of moles of gas contained in a 10.0 L tank at 22°C and 105 atm.(R = 0.08206 L.atm/K.mol)
A)1.71 * 10-3 mol
B)0.0231 mol
C)1.03 mol
D)43.4 mol
E)582 mol
A)1.71 * 10-3 mol
B)0.0231 mol
C)1.03 mol
D)43.4 mol
E)582 mol

Unlock Deck
Unlock for access to all 109 flashcards in this deck.
Unlock Deck
k this deck
19
What is the pressure of the sample of gas trapped in the open-tube mercury manometer shown below if atmospheric pressure is 736 mmHg and h = 9.2 cm? 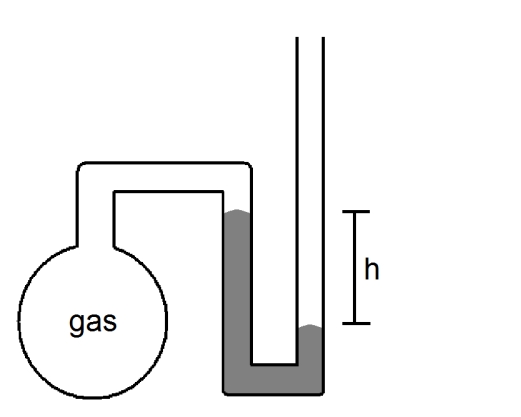
A)92 mmHg
B)644 mmHg
C)736 mmHg
D)828 mmHg

A)92 mmHg
B)644 mmHg
C)736 mmHg
D)828 mmHg

Unlock Deck
Unlock for access to all 109 flashcards in this deck.
Unlock Deck
k this deck
20
A pressure that will support a column of Hg to a height of 256 mm would support a column of water to what height? The density of mercury is 13.6 g/cm3; the density of water is 1.00 g/cm3.
A)1.00 * 102 ft
B)18.8 mm
C)33.8 ft
D)76.0 cm
E)348 cm
A)1.00 * 102 ft
B)18.8 mm
C)33.8 ft
D)76.0 cm
E)348 cm

Unlock Deck
Unlock for access to all 109 flashcards in this deck.
Unlock Deck
k this deck
21
Determine the molar mass of chloroform gas if a sample weighing 0.389 g is collected in a flask with a volume of 102 cm3 at 97°C. The pressure of the chloroform is 728 mmHg.
A)187 g/mol
B)121 g/mol
C)112 g/mol
D)31.6 g/mol
E)8.28 * 10-3 g/mol
A)187 g/mol
B)121 g/mol
C)112 g/mol
D)31.6 g/mol
E)8.28 * 10-3 g/mol

Unlock Deck
Unlock for access to all 109 flashcards in this deck.
Unlock Deck
k this deck
22
Calculate the mass, in grams, of 2.74 L of CO gas measured at 33°C and 945 mmHg.
A)0.263 g
B)2.46 g
C)3.80 g
D)35.2 g
E)206 g
A)0.263 g
B)2.46 g
C)3.80 g
D)35.2 g
E)206 g

Unlock Deck
Unlock for access to all 109 flashcards in this deck.
Unlock Deck
k this deck
23
Calculate the density of Br2(g)at 59.0°C and 1.00 atm pressure.
A)27.2 g/L
B)5.83 g/L
C)769 g/L
D)22.4 g/L
E)3.45 g/L
A)27.2 g/L
B)5.83 g/L
C)769 g/L
D)22.4 g/L
E)3.45 g/L

Unlock Deck
Unlock for access to all 109 flashcards in this deck.
Unlock Deck
k this deck
24
Two moles of chlorine gas at 20.0°C are heated to 350°C while the volume is kept constant.The density of the gas
A)increases.
B)decreases.
C)remains the same.
D)Not enough information is given to correctly answer the question.
A)increases.
B)decreases.
C)remains the same.
D)Not enough information is given to correctly answer the question.

Unlock Deck
Unlock for access to all 109 flashcards in this deck.
Unlock Deck
k this deck
25
What is the molar mass of Freon-11 gas if its density is 6.13 g/L at STP?
A)0.274 g/mol
B)3.64 g/mol
C)78.2 g/mol
D)137 g/mol
E)365 g/mol
A)0.274 g/mol
B)3.64 g/mol
C)78.2 g/mol
D)137 g/mol
E)365 g/mol

Unlock Deck
Unlock for access to all 109 flashcards in this deck.
Unlock Deck
k this deck
26
Calculate the density of CO2(g)at 100°C and 10.0 atm pressure.
A)1.44 g/L
B)134 g/L
C)44.0 g/L
D)53.6 g/L
E)14.4 g/L
A)1.44 g/L
B)134 g/L
C)44.0 g/L
D)53.6 g/L
E)14.4 g/L

Unlock Deck
Unlock for access to all 109 flashcards in this deck.
Unlock Deck
k this deck
27
A 1.2 L flask contains 0.500 mole of ammonia (NH3)at 150°C. Calculate the pressure of the ammonia inside the flask.
A)6.91 * 10-2 atm
B)5.13 atm
C)12.2 atm
D)14.5 atm
E)22.4 atm
A)6.91 * 10-2 atm
B)5.13 atm
C)12.2 atm
D)14.5 atm
E)22.4 atm

Unlock Deck
Unlock for access to all 109 flashcards in this deck.
Unlock Deck
k this deck
28
A 0.271 g sample of an unknown vapor occupies 294 mL at 140°C and 847 mmHg.The empirical formula of the compound is CH2. What is the molecular formula of the compound?
A)CH2
B)C2H4
C)C3H6
D)C4H8
E)C6H12
A)CH2
B)C2H4
C)C3H6
D)C4H8
E)C6H12

Unlock Deck
Unlock for access to all 109 flashcards in this deck.
Unlock Deck
k this deck
29
Calculate the volume occupied by 25.2 g of CO2 at 0.84 atm and 25°C.R = 0.08206 L.atm/K.mol.
A)0.060 L
B)1.34 L
C)16.9 L
D)24.2 L
E)734 L
A)0.060 L
B)1.34 L
C)16.9 L
D)24.2 L
E)734 L

Unlock Deck
Unlock for access to all 109 flashcards in this deck.
Unlock Deck
k this deck
30
Gases are sold in large cylinders for laboratory use. What pressure, in atmospheres, will be exerted by 2,500 g of oxygen gas (O2)when stored at 22°C in a 40.0 L cylinder?
A)3.55 atm
B)1,510 atm
C)47.3 atm
D)7.56 * 104 atm
E)10.2 atm
A)3.55 atm
B)1,510 atm
C)47.3 atm
D)7.56 * 104 atm
E)10.2 atm

Unlock Deck
Unlock for access to all 109 flashcards in this deck.
Unlock Deck
k this deck
31
A 1.17 g sample of an alkane hydrocarbon gas occupies a volume of 674 mL at 28°C and 741 mmHg.Alkanes are known to have the general formula CnH2n+2.What is the molecular formula of the gas in this sample? (R = 0.08206 L.atm/K.mol)
A)CH4
B)C2H6
C)C3H8
D)C4H10
E)C5H12
A)CH4
B)C2H6
C)C3H8
D)C4H10
E)C5H12

Unlock Deck
Unlock for access to all 109 flashcards in this deck.
Unlock Deck
k this deck
32
Calculate the density, in g/L, of CO2 gas at 27°C and 0.50 atm pressure.
A)0.89 g/L
B)1.12 g/L
C)9.93 g/L
D)46.0 g/L
E)2.17 kg/L
A)0.89 g/L
B)1.12 g/L
C)9.93 g/L
D)46.0 g/L
E)2.17 kg/L

Unlock Deck
Unlock for access to all 109 flashcards in this deck.
Unlock Deck
k this deck
33
Determine the molar mass of Freon-11 gas if a sample weighing 0.597 g occupies 100.cm3 at 95°C, and 1,000.mmHg.
A)0.19 g/mol
B)35.3 g/mol
C)70.9 g/mol
D)137 g/mol
E)384 g/mol
A)0.19 g/mol
B)35.3 g/mol
C)70.9 g/mol
D)137 g/mol
E)384 g/mol

Unlock Deck
Unlock for access to all 109 flashcards in this deck.
Unlock Deck
k this deck
34
Calculate the density of Ar(g)at -11°C and 675 mmHg.
A)1.52 g/L
B)1.65 g/L
C)-39.3 g/L
D)39.95 g/L
E)1254 g/L
A)1.52 g/L
B)1.65 g/L
C)-39.3 g/L
D)39.95 g/L
E)1254 g/L

Unlock Deck
Unlock for access to all 109 flashcards in this deck.
Unlock Deck
k this deck
35
Which of these gases will have the greatest density at the same specified temperature and pressure?
A)H2
B)CClF3
C)CO2
D)C2H6
E)CF4
A)H2
B)CClF3
C)CO2
D)C2H6
E)CF4

Unlock Deck
Unlock for access to all 109 flashcards in this deck.
Unlock Deck
k this deck
36
How many molecules of N2 gas can be present in a 2.5 L flask at 50°C and 650 mmHg?
A)2.1 * 10-23 molecules
B)4.9 * 1022 molecules
C)3.1 * 1023 molecules
D)3.6 * 1025 molecules
E)0.081 molecules
A)2.1 * 10-23 molecules
B)4.9 * 1022 molecules
C)3.1 * 1023 molecules
D)3.6 * 1025 molecules
E)0.081 molecules

Unlock Deck
Unlock for access to all 109 flashcards in this deck.
Unlock Deck
k this deck
37
Calculate the density, in g/L, of SF6 gas at 27°C and 0.500 atm pressure.
A)3.38 * 10-3 g/L
B)2.96 g/L
C)22.4 g/L
D)32.9 g/L
E)3.38 kg/L
A)3.38 * 10-3 g/L
B)2.96 g/L
C)22.4 g/L
D)32.9 g/L
E)3.38 kg/L

Unlock Deck
Unlock for access to all 109 flashcards in this deck.
Unlock Deck
k this deck
38
Calculate the density, in g/L, of chlorine (Cl2)gas at STP.
A)2.13 * 10-2 g/L
B)46.9 g/L
C)1.58 g/L
D)3.16 g/L
E)0.316 kg/L
A)2.13 * 10-2 g/L
B)46.9 g/L
C)1.58 g/L
D)3.16 g/L
E)0.316 kg/L

Unlock Deck
Unlock for access to all 109 flashcards in this deck.
Unlock Deck
k this deck
39
Which one of these gases is "lighter-than-air"?
A)Cl2
B)SO2
C)PH3
D)NO2
E)Ne
A)Cl2
B)SO2
C)PH3
D)NO2
E)Ne

Unlock Deck
Unlock for access to all 109 flashcards in this deck.
Unlock Deck
k this deck
40
A gas evolved during the fermentation of sugar was collected.After purification its volume was found to be 25.0 L at 22.5°C and 702 mmHg.How many moles of gas were collected?
A)0.95 mol
B)1.05 mol
C)12.5 mol
D)22.4 mol
E)724 mol
A)0.95 mol
B)1.05 mol
C)12.5 mol
D)22.4 mol
E)724 mol

Unlock Deck
Unlock for access to all 109 flashcards in this deck.
Unlock Deck
k this deck
41
How many liters of oxygen gas at 153°C and 0.820 atm can be produced by the decomposition of 22.4 g of solid KClO3? (The other decomposition product is solid potassium chloride.)
A)3.0 L
B)0.085 L
C)4.20 L
D)7.79 L
E)11.7 L
A)3.0 L
B)0.085 L
C)4.20 L
D)7.79 L
E)11.7 L

Unlock Deck
Unlock for access to all 109 flashcards in this deck.
Unlock Deck
k this deck
42
A gaseous compound is 30.4% nitrogen and 69.6% oxygen by mass. A 5.25-g sample of the gas occupies a volume of 1.00 L and exerts a pressure of 1.26 atm at -4.0°C. Which of these choices is its molecular formula?
A)NO
B)NO2
C)N3O6
D)N2O4
E)N2O5
A)NO
B)NO2
C)N3O6
D)N2O4
E)N2O5

Unlock Deck
Unlock for access to all 109 flashcards in this deck.
Unlock Deck
k this deck
43
How many liters of chlorine gas at 25°C and 0.950 atm can be produced by the reaction of 12.0 g of MnO2 with excess HCl(aq)according to the following chemical equation? MnO2(s)+ 4HCl(aq) MnCl2(aq)+ 2H2O(l)+ Cl2(g)
A)5.36 * 10-3 L
B)0.138 L
C)0.282 L
D)3.09 L
E)3.55 L
A)5.36 * 10-3 L
B)0.138 L
C)0.282 L
D)3.09 L
E)3.55 L

Unlock Deck
Unlock for access to all 109 flashcards in this deck.
Unlock Deck
k this deck
44
Which statement is false?
A)The average kinetic energies of molecules from samples of different "ideal" gases is the same at the same temperature.
B)The molecules of an ideal gas are relatively far apart.
C)All molecules of an ideal gas have the same kinetic energy at constant temperature.
D)Molecules of a gas undergo many collisions with each other and the container walls.
E)Molecules of greater mass have a lower average speed than those of less mass at the same temperature.
A)The average kinetic energies of molecules from samples of different "ideal" gases is the same at the same temperature.
B)The molecules of an ideal gas are relatively far apart.
C)All molecules of an ideal gas have the same kinetic energy at constant temperature.
D)Molecules of a gas undergo many collisions with each other and the container walls.
E)Molecules of greater mass have a lower average speed than those of less mass at the same temperature.

Unlock Deck
Unlock for access to all 109 flashcards in this deck.
Unlock Deck
k this deck
45
What volume of CO2 gas at 645 torr and 800 K could be produced by the reaction of 45 g of CaCO3 according to the equation? CaCO3(s) CaO(s)+ CO2(g)
A)0.449 L
B)22.4 L
C)25.0 L
D)34.8 L
E)45.7 mL
A)0.449 L
B)22.4 L
C)25.0 L
D)34.8 L
E)45.7 mL

Unlock Deck
Unlock for access to all 109 flashcards in this deck.
Unlock Deck
k this deck
46
What mass of KClO3 must be decomposed to produce 126 L of oxygen gas at 133°C and 0.880 atm? (The other reaction product is solid KCl.)
A)24.6 g
B)70.8 g
C)272 g
D)408 g
E)612 g
A)24.6 g
B)70.8 g
C)272 g
D)408 g
E)612 g

Unlock Deck
Unlock for access to all 109 flashcards in this deck.
Unlock Deck
k this deck
47
A sample of hydrogen gas was collected over water at 21°C and 685 mmHg. The volume of the container was 7.80 L. Calculate the mass of H2(g)collected. (Vapor pressure of water = 18.6 mmHg at 21°C.)
A)0.283 g
B)0.572 g
C)0.589 g
D)7.14 g
E)435 g
A)0.283 g
B)0.572 g
C)0.589 g
D)7.14 g
E)435 g

Unlock Deck
Unlock for access to all 109 flashcards in this deck.
Unlock Deck
k this deck
48
Calculate the volume of H2(g)at 273 K and 2.00 atm that will be formed when 275 mL of 0.725 M HCl solution reacts with excess Mg to give hydrogen gas and aqueous magnesium chloride.
A)0.56 L
B)1.12 L
C)2.23 L
D)4.47 L
E)3.54 L
A)0.56 L
B)1.12 L
C)2.23 L
D)4.47 L
E)3.54 L

Unlock Deck
Unlock for access to all 109 flashcards in this deck.
Unlock Deck
k this deck
49
What volume of oxygen gas at 320 K and 680 torr will react completely with 2.50 L of NO gas at the same temperature and pressure? 2NO(g)+ O2(g) 2NO2(g)
A)1.25 L
B)2.50 L
C)3.00 L
D)1.00 L
E)5.00 L
A)1.25 L
B)2.50 L
C)3.00 L
D)1.00 L
E)5.00 L

Unlock Deck
Unlock for access to all 109 flashcards in this deck.
Unlock Deck
k this deck
50
A sample of carbon monoxide gas was collected in a 2.0 L flask by displacing water at 28°C and 810 mmHg. Calculate the number of CO molecules in the flask. The vapor pressure of water at 28°C is 28.3 mmHg.
A)5.0 * 1022
B)5.2 * 1022
C)3.8 * 1023
D)5.4 * 1023
E)3.8 * 1025
A)5.0 * 1022
B)5.2 * 1022
C)3.8 * 1023
D)5.4 * 1023
E)3.8 * 1025

Unlock Deck
Unlock for access to all 109 flashcards in this deck.
Unlock Deck
k this deck
51
Which gas has molecules with the greatest average molecular speed at 25°C?
A)CH4
B)Kr
C)N2
D)CO2
E)Ar
A)CH4
B)Kr
C)N2
D)CO2
E)Ar

Unlock Deck
Unlock for access to all 109 flashcards in this deck.
Unlock Deck
k this deck
52
Which of these gas molecules have the highest average kinetic energy at 25°C?
A)H2
B)O2
C)N2
D)Cl2
E)All the gases have the same average kinetic energy.
A)H2
B)O2
C)N2
D)Cl2
E)All the gases have the same average kinetic energy.

Unlock Deck
Unlock for access to all 109 flashcards in this deck.
Unlock Deck
k this deck
53
The molecules of different samples of an ideal gas have the same average kinetic energies, at the same
A)pressure.
B)temperature.
C)volume.
D)density.
A)pressure.
B)temperature.
C)volume.
D)density.

Unlock Deck
Unlock for access to all 109 flashcards in this deck.
Unlock Deck
k this deck
54
Gas A and gas B are combined in a flask at initial pressures of 1.0 atm each.The flask is sealed and over time they react to completion to give gas C according to the following chemical equation: 2A(g)+ B(g) C(g)
Assuming the temperature stays constant, what will be the total pressure in the flask after the reaction goes to completion?
A)0.33 atm
B)0.50 atm
C)0.67 atm
D)0.75 atm
E)1.0 atm
Assuming the temperature stays constant, what will be the total pressure in the flask after the reaction goes to completion?
A)0.33 atm
B)0.50 atm
C)0.67 atm
D)0.75 atm
E)1.0 atm

Unlock Deck
Unlock for access to all 109 flashcards in this deck.
Unlock Deck
k this deck
55
If equal masses of O2(g)and HBr(g)are in separate containers of equal volume and temperature, which one of these statements is true?
A)The pressure in the O2 container is greater than that in the HBr container.
B)There are more HBr molecules than O2 molecules.
C)The average velocity of the O2 molecules is less than that of the HBr molecules.
D)The average kinetic energy of HBr molecules is greater than that of O2 molecules.
E)The pressures of both gases are the same.
A)The pressure in the O2 container is greater than that in the HBr container.
B)There are more HBr molecules than O2 molecules.
C)The average velocity of the O2 molecules is less than that of the HBr molecules.
D)The average kinetic energy of HBr molecules is greater than that of O2 molecules.
E)The pressures of both gases are the same.

Unlock Deck
Unlock for access to all 109 flashcards in this deck.
Unlock Deck
k this deck
56
A mixture of three gases has a total pressure of 1,380 mmHg at 298 K. The mixture is analyzed and is found to contain 1.27 mol CO2, 3.04 mol CO, and 1.50 mol Ar. What is the partial pressure of Ar?
A)0.258 atm
B)301 mmHg
C)356 mmHg
D)5,345 mmHg
E)8,020 mmHg
A)0.258 atm
B)301 mmHg
C)356 mmHg
D)5,345 mmHg
E)8,020 mmHg

Unlock Deck
Unlock for access to all 109 flashcards in this deck.
Unlock Deck
k this deck
57
Liquid nitrogen has a density of 0.807 g/mL at -195.8 °C.If 1.00 L of N2(l)is allowed to warm to 25°C at a pressure of 1.0 atm, what volume will the gas occupy? (R = 0.08206 L.atm/K.mol)
A)59.1 L
B)182 L
C)705 L
D)1.41 * 103 L
E)1.97 * 104 L
A)59.1 L
B)182 L
C)705 L
D)1.41 * 103 L
E)1.97 * 104 L

Unlock Deck
Unlock for access to all 109 flashcards in this deck.
Unlock Deck
k this deck
58
When active metals such as magnesium are immersed in acid solution, hydrogen gas is evolved.Calculate the volume of H2(g)at 30.1°C and 0.85 atm that can be formed when 275 mL of 0.725 M HCl solution reacts with excess Mg to give hydrogen gas and aqueous magnesium chloride.
A)3.4 * 10-3 L
B)2.2 L
C)2.9 L
D)5.8 L
E)11.7 L
A)3.4 * 10-3 L
B)2.2 L
C)2.9 L
D)5.8 L
E)11.7 L

Unlock Deck
Unlock for access to all 109 flashcards in this deck.
Unlock Deck
k this deck
59
A 1.07 g sample of a Noble gas occupies a volume of 363 mL at 35°C and 678 mmHg.Identify the Noble gas in this sample? (R = 0.08206 L.atm/K.mol)
A)He
B)Ne
C)Ar
D)Kr
E)Xe
A)He
B)Ne
C)Ar
D)Kr
E)Xe

Unlock Deck
Unlock for access to all 109 flashcards in this deck.
Unlock Deck
k this deck
60
Air contains 78% N2, 21% O2, and 1% Ar, by volume. What is the density of air at 1,000.torr and -10°C?
A)1.0 g/L
B)6.1 g/L
C)1.3 g/L
D)1.8 g/L
E)0.56 g/L
A)1.0 g/L
B)6.1 g/L
C)1.3 g/L
D)1.8 g/L
E)0.56 g/L

Unlock Deck
Unlock for access to all 109 flashcards in this deck.
Unlock Deck
k this deck
61
A method of removing CO2 from a spacecraft is to allow the CO2 to react with sodium hydroxide. (The products of the reaction are sodium carbonate and water.)What volume of carbon dioxide at 25°C and 749 mmHg can be removed per kilogram of sodium hydroxide that reacts?
A)301 L
B)284 L
C)276 L
D)310 L
E)620 L
A)301 L
B)284 L
C)276 L
D)310 L
E)620 L

Unlock Deck
Unlock for access to all 109 flashcards in this deck.
Unlock Deck
k this deck
62
What is standard temperature and standard pressure?

Unlock Deck
Unlock for access to all 109 flashcards in this deck.
Unlock Deck
k this deck
63
What is the pressure (in atmospheres)of the sample of gas trapped in the open-tube mercury manometer shown below if atmospheric pressure is 735 mmHg and h = 8.3 cm? 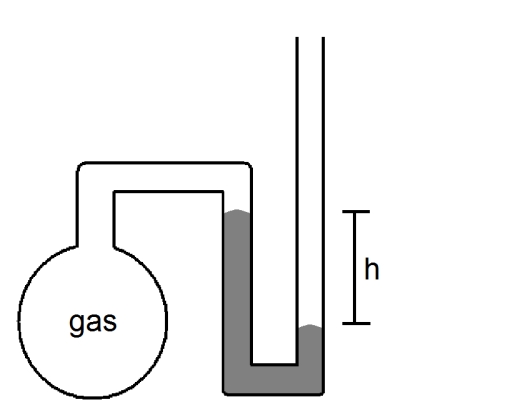


Unlock Deck
Unlock for access to all 109 flashcards in this deck.
Unlock Deck
k this deck
64
What is the pressure of the gas trapped in the apparatus shown below when the atmospheric pressure is 720 mmHg? 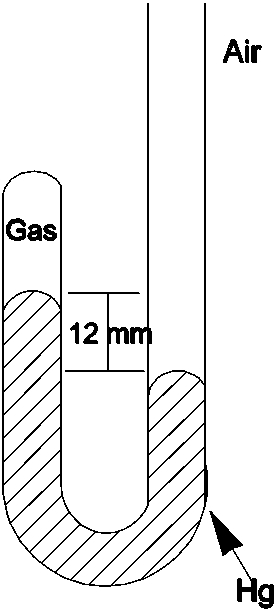
A)12 mmHg
B)708 mmHg
C)720 mmHg
D)732 mmHg
E)760 mmHg

A)12 mmHg
B)708 mmHg
C)720 mmHg
D)732 mmHg
E)760 mmHg

Unlock Deck
Unlock for access to all 109 flashcards in this deck.
Unlock Deck
k this deck
65
A spacecraft is filled with 0.500 atm of N2 and 0.500 atm of O2. Suppose a micrometeor strikes this spacecraft and puts a very small hole in it's side. Under these circumstances,
A)O2 is lost from the craft 6.9% faster than N2 is lost.
B)O2 is lost from the craft 14% faster than N2 is lost.
C)N2 is lost from the craft 6.9% faster than O2 is lost.
D)N2 is lost from the craft 14% faster than O2 is lost.
E)N2 and O2 are lost from the craft at the same rate.
A)O2 is lost from the craft 6.9% faster than N2 is lost.
B)O2 is lost from the craft 14% faster than N2 is lost.
C)N2 is lost from the craft 6.9% faster than O2 is lost.
D)N2 is lost from the craft 14% faster than O2 is lost.
E)N2 and O2 are lost from the craft at the same rate.

Unlock Deck
Unlock for access to all 109 flashcards in this deck.
Unlock Deck
k this deck
66
A 2.50-L flask contains a mixture of methane (CH4)and propane (C3H8)at a pressure of 1.45 atm and 20°C. When this gas mixture is then burned in excess oxygen, 8.60 g of carbon dioxide is formed. (The other product is water.)What is the mole fraction of methane in the original gas mixture?
A)0.341
B)1.00
C)0.659
D)0.855
E)0.145
A)0.341
B)1.00
C)0.659
D)0.855
E)0.145

Unlock Deck
Unlock for access to all 109 flashcards in this deck.
Unlock Deck
k this deck
67
Samples of the following volatile liquids are opened simultaneously at one end of a room. If you are standing at the opposite end of this room, which species would you smell first? (Assume that your nose is equally sensitive to all these species.)
A)ethyl acetate (CH3COOC2H5)
B)camphor (C10H16O)
C)diethyl ether (C2H5OC2H5)
D)naphthalene (C10H8)
E)pentanethiol (C5H11SH)
A)ethyl acetate (CH3COOC2H5)
B)camphor (C10H16O)
C)diethyl ether (C2H5OC2H5)
D)naphthalene (C10H8)
E)pentanethiol (C5H11SH)

Unlock Deck
Unlock for access to all 109 flashcards in this deck.
Unlock Deck
k this deck
68
A spacecraft is filled with 0.500 atm of O2 and 0.500 atm of He. If there is a very small hole in the side of this craft such that gas is lost slowly into outer space,
A)He is lost 2.8 times faster than O2 is lost.
B)He is lost 8 times faster than O2 is lost.
C)He is lost twice as fast as O2 is lost.
D)O2 is lost 2.8 times faster than He is lost.
E)O2 is lost 8 times faster than He is lost.
A)He is lost 2.8 times faster than O2 is lost.
B)He is lost 8 times faster than O2 is lost.
C)He is lost twice as fast as O2 is lost.
D)O2 is lost 2.8 times faster than He is lost.
E)O2 is lost 8 times faster than He is lost.

Unlock Deck
Unlock for access to all 109 flashcards in this deck.
Unlock Deck
k this deck
69
What is the pressure of the sample of gas trapped in the closed-tube mercury manometer shown below if atmospheric pressure is 751 mmHg and h = 17.3 cm? 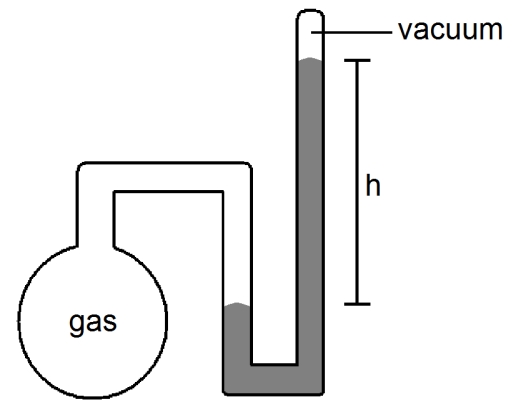


Unlock Deck
Unlock for access to all 109 flashcards in this deck.
Unlock Deck
k this deck
70
A sample of mercury(II)oxide is placed in a 5.00 L evacuated container and heated until it decomposes entirely to mercury metal and oxygen gas. The container is then cooled to 25°C. One now finds that the gas pressure inside the container is 1.73 atm. What mass of mercury(II)oxide was originally placed into the container?
A)913 g
B)76.6 g
C)1.51 g
D)45.6 g
E)153 g
A)913 g
B)76.6 g
C)1.51 g
D)45.6 g
E)153 g

Unlock Deck
Unlock for access to all 109 flashcards in this deck.
Unlock Deck
k this deck
71
What is the significance of the magnitude of the van der Waals "a" constant?

Unlock Deck
Unlock for access to all 109 flashcards in this deck.
Unlock Deck
k this deck
72
For a substance that remains a gas under the conditions listed, deviation from the ideal gas law would be most pronounced at
A)100°C and 2.0 atm.
B)0°C and 2.0 atm.
C)-100°C and 2.0 atm.
D)-100°C and 4.0 atm.
E)100°C and 4.0 atm.
A)100°C and 2.0 atm.
B)0°C and 2.0 atm.
C)-100°C and 2.0 atm.
D)-100°C and 4.0 atm.
E)100°C and 4.0 atm.

Unlock Deck
Unlock for access to all 109 flashcards in this deck.
Unlock Deck
k this deck
73
Deviations from the ideal gas law are greater at
A)low temperatures and low pressures.
B)low temperatures and high pressures.
C)high temperatures and high pressures.
D)high temperatures and low pressures.
A)low temperatures and low pressures.
B)low temperatures and high pressures.
C)high temperatures and high pressures.
D)high temperatures and low pressures.

Unlock Deck
Unlock for access to all 109 flashcards in this deck.
Unlock Deck
k this deck
74
What is the definition of a "gas"?

Unlock Deck
Unlock for access to all 109 flashcards in this deck.
Unlock Deck
k this deck
75
A block of dry ice (solid CO2, density = 1.56 g/mL)of dimensions 25.0 cm * 25.0 cm * 25.0 cm is left to sublime (i.e.to pass from the solid phase to the gas phase)in a closed chamber of dimensions 4.00 m * 5.00 m *3.00 m. The partial pressure of carbon dioxide in this chamber at 25°C will be
A)171 mmHg
B)107 mmHg
C)0.225 mmHg
D)0.171 mmHg
E)14.4 mmHg
A)171 mmHg
B)107 mmHg
C)0.225 mmHg
D)0.171 mmHg
E)14.4 mmHg

Unlock Deck
Unlock for access to all 109 flashcards in this deck.
Unlock Deck
k this deck
76
What is the pressure of the sample of gas trapped in the open-tube mercury manometer shown below if atmospheric pressure is 742 mmHg and h = 16.7 cm? 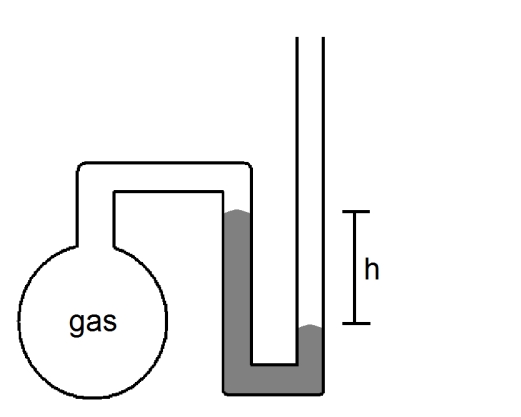


Unlock Deck
Unlock for access to all 109 flashcards in this deck.
Unlock Deck
k this deck
77
Determine the pressure of the gas trapped in the apparatus shown below when the atmospheric pressure is 695 mmHg. 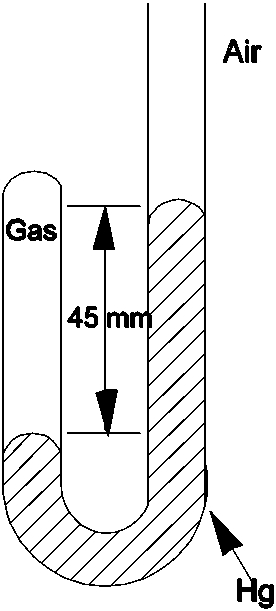
A)45 mmHg
B)650 mmHg
C)695 mmHg
D)740 mmHg
E)760 mmHg

A)45 mmHg
B)650 mmHg
C)695 mmHg
D)740 mmHg
E)760 mmHg

Unlock Deck
Unlock for access to all 109 flashcards in this deck.
Unlock Deck
k this deck
78
What is the pressure (in atmospheres)of the sample of gas trapped in the closed-tube mercury manometer shown below if h = 23.6 cm? 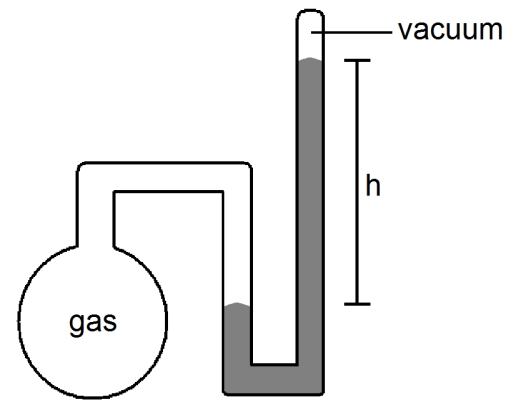


Unlock Deck
Unlock for access to all 109 flashcards in this deck.
Unlock Deck
k this deck
79
The mole fraction of oxygen molecules in dry air is 0.2095. What volume of dry air at 1.00 atm and 25°C is required for burning 1.00 L of hexane (C6H14, density = 0.660 g/mL)completely, yielding carbon dioxide and water?
A)187 L
B)712 L
C)1780 L
D)894 L
E)8490
A)187 L
B)712 L
C)1780 L
D)894 L
E)8490

Unlock Deck
Unlock for access to all 109 flashcards in this deck.
Unlock Deck
k this deck
80
The mole fraction of oxygen molecules in dry air is 0.2095. What volume of dry air at 1.00 atm and 25°C is required for burning 1.00 L of octane (C8H18, density = 0.7025 g/mL)completely, yielding carbon dioxide and water?
A)718 L
B)367 L
C)8980 L
D)1880 L
E)150 L
A)718 L
B)367 L
C)8980 L
D)1880 L
E)150 L

Unlock Deck
Unlock for access to all 109 flashcards in this deck.
Unlock Deck
k this deck


