Deck 7: How Molecules Mix
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/157
Play
Full screen (f)
Deck 7: How Molecules Mix
1
In which of the following molecules will water induce a temporary dipole?
A)CO2
B)O2
C)N2
D)all of the above
E)none of the above
A)CO2
B)O2
C)N2
D)all of the above
E)none of the above
all of the above
2
Fluorine is a relatively ________.
A)large atom
B)soft atom
C)small atom
D)greenish atom
A)large atom
B)soft atom
C)small atom
D)greenish atom
small atom
3
What is a hydrogen bond?
A)a special type of dipole-dipole attraction involving hydrogen bound to a highly electronegative atom
B)a special type of dipole-dipole attraction involving hydrogen bound to any other atom
C)a special type of dipole-dipole attraction involving hydrogen bound to another hydrogen atom
D)a special type of attraction involving any molecules that contain hydrogens
E)none of the above
A)a special type of dipole-dipole attraction involving hydrogen bound to a highly electronegative atom
B)a special type of dipole-dipole attraction involving hydrogen bound to any other atom
C)a special type of dipole-dipole attraction involving hydrogen bound to another hydrogen atom
D)a special type of attraction involving any molecules that contain hydrogens
E)none of the above
a special type of dipole-dipole attraction involving hydrogen bound to a highly electronegative atom
4
If an ionic bond is stronger than a dipole-dipole interaction, how can water dissolve an ionic compound?
A)The ion-dipole interactions of a bunch of water molecules gang up on the strong ionic bond and pull it into the solution.
B)The ionic bond is weakened by the ion-dipole interactions and ionic repulsion ejects the ions from the crystal.
C)The ion-dipole interaction causes the ions to heat up and vibrate free of the crystal.
D)The ions never overcome their interatomic attraction and therefore are not soluble.
E)none of the above
A)The ion-dipole interactions of a bunch of water molecules gang up on the strong ionic bond and pull it into the solution.
B)The ionic bond is weakened by the ion-dipole interactions and ionic repulsion ejects the ions from the crystal.
C)The ion-dipole interaction causes the ions to heat up and vibrate free of the crystal.
D)The ions never overcome their interatomic attraction and therefore are not soluble.
E)none of the above

Unlock Deck
Unlock for access to all 157 flashcards in this deck.
Unlock Deck
k this deck
5
Which of the following molecules would you expect to be the most strongly attracted to a Cl- ion?
A)H-F
B)H3C-CH3
C)Cl-Cl
D)F-F
E)CCl4
A)H-F
B)H3C-CH3
C)Cl-Cl
D)F-F
E)CCl4

Unlock Deck
Unlock for access to all 157 flashcards in this deck.
Unlock Deck
k this deck
6
What is happening at the molecular level when a polar molecule like water interacts with a typical sodium ion?
A)The water molecule aligns such that the oxygen interacts with the sodium.
B)The water molecule aligns such that the hydrogens interact with the sodium.
C)The polarity of the water molecule is altered making the oxygen more positively charged.
D)The polarity of the water molecule is altered making the hydrogens more negatively charged.
E)none of the above
A)The water molecule aligns such that the oxygen interacts with the sodium.
B)The water molecule aligns such that the hydrogens interact with the sodium.
C)The polarity of the water molecule is altered making the oxygen more positively charged.
D)The polarity of the water molecule is altered making the hydrogens more negatively charged.
E)none of the above

Unlock Deck
Unlock for access to all 157 flashcards in this deck.
Unlock Deck
k this deck
7
A dipole is a ________.
A)separation of charges
B)molecule with parallel bonds
C)nonpolar entity
D)form of electronegativity
A)separation of charges
B)molecule with parallel bonds
C)nonpolar entity
D)form of electronegativity

Unlock Deck
Unlock for access to all 157 flashcards in this deck.
Unlock Deck
k this deck
8
What is the difference between a dipole-dipole interaction and an ion-dipole interaction?
A)one involves dipole attraction between neutral molecules while the other involves dipole interactions with ions
B)one involves hydrogen bonding while the other does not
C)one involves salts and water while the other doesn't involve water
D)one involves ionic molecules interacting with other ionic molecules while the other deals with polar molecules
E)none of the above
A)one involves dipole attraction between neutral molecules while the other involves dipole interactions with ions
B)one involves hydrogen bonding while the other does not
C)one involves salts and water while the other doesn't involve water
D)one involves ionic molecules interacting with other ionic molecules while the other deals with polar molecules
E)none of the above

Unlock Deck
Unlock for access to all 157 flashcards in this deck.
Unlock Deck
k this deck
9
Which of the following molecules is most likely to show a dipole-dipole interaction?
A)CH3OH
B)CH3SH
C)CH4
D)H-C C-H
C-H
E)A and B
A)CH3OH
B)CH3SH
C)CH4
D)H-C
 C-H
C-HE)A and B

Unlock Deck
Unlock for access to all 157 flashcards in this deck.
Unlock Deck
k this deck
10
Which of the following is the weakest form of interatomic attraction?
A)a chemical bond
B)an ion-dipole interaction
C)a dipole-dipole interaction
D)a dipole-induced dipole interaction
E)an induced dipole-induced dipole interaction
A)a chemical bond
B)an ion-dipole interaction
C)a dipole-dipole interaction
D)a dipole-induced dipole interaction
E)an induced dipole-induced dipole interaction

Unlock Deck
Unlock for access to all 157 flashcards in this deck.
Unlock Deck
k this deck
11
Which of the following molecules would you expect to be the least attracted to a Na+ ion?
A)H-F
B)H3C-CH3
C)Cl2CH2
D)F-
E)HO-
A)H-F
B)H3C-CH3
C)Cl2CH2
D)F-
E)HO-

Unlock Deck
Unlock for access to all 157 flashcards in this deck.
Unlock Deck
k this deck
12
Which of the following is the strongest form of interatomic attraction?
A)a chemical bond
B)an ion-dipole interaction
C)a dipole-dipole interaction
D)a dipole-induced dipole interaction
E)an induced dipole-induced dipole interaction
A)a chemical bond
B)an ion-dipole interaction
C)a dipole-dipole interaction
D)a dipole-induced dipole interaction
E)an induced dipole-induced dipole interaction

Unlock Deck
Unlock for access to all 157 flashcards in this deck.
Unlock Deck
k this deck
13
Given the following diagram, describe what happens electronically between these two molecules. 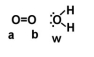
A)Oxygen B becomes slightly positively charged due to the electrons on the water molecule.
B)Oxygen A becomes slightly positively charged due to the electrons on the water molecule.
C)Oxygen W becomes slightly negatively charged due to the oxygen molecule.
D)Oxygen W becomes slightly positively charged due to the oxygen molecule.
E)none of the above

A)Oxygen B becomes slightly positively charged due to the electrons on the water molecule.
B)Oxygen A becomes slightly positively charged due to the electrons on the water molecule.
C)Oxygen W becomes slightly negatively charged due to the oxygen molecule.
D)Oxygen W becomes slightly positively charged due to the oxygen molecule.
E)none of the above

Unlock Deck
Unlock for access to all 157 flashcards in this deck.
Unlock Deck
k this deck
14
Which of the following substances is pure?
A)an ionic compound consisting of sodium and chlorine ions
B)a covalent compound consisting of one type of molecule
C)an element
D)all of the above
E)none of the above
A)an ionic compound consisting of sodium and chlorine ions
B)a covalent compound consisting of one type of molecule
C)an element
D)all of the above
E)none of the above

Unlock Deck
Unlock for access to all 157 flashcards in this deck.
Unlock Deck
k this deck
15
Which of the following is the strongest form of intermolecular attraction in a water molecule?
A)hydrogen bonding
B)induced dipole-induced dipole
C)covalent bonding
D)ion-dipole
E)polar-induced polar
A)hydrogen bonding
B)induced dipole-induced dipole
C)covalent bonding
D)ion-dipole
E)polar-induced polar

Unlock Deck
Unlock for access to all 157 flashcards in this deck.
Unlock Deck
k this deck
16
The separation of charges within a polar molecule is called a(n)________.
A)dipole
B)dipole-dipole
C)ionic bond
D)strong attraction
E)polar bond
A)dipole
B)dipole-dipole
C)ionic bond
D)strong attraction
E)polar bond

Unlock Deck
Unlock for access to all 157 flashcards in this deck.
Unlock Deck
k this deck
17
Which of the following describes an aqueous solution?
A)a mixture of some compound dissolved in water
B)a mixture of polar molecules dissolved in a nonpolar solvent
C)a mixture of water dispersed in an ionic compound
D)a mixture of nonpolar molecules dissolved in a polar solvent
E)none of the above
A)a mixture of some compound dissolved in water
B)a mixture of polar molecules dissolved in a nonpolar solvent
C)a mixture of water dispersed in an ionic compound
D)a mixture of nonpolar molecules dissolved in a polar solvent
E)none of the above

Unlock Deck
Unlock for access to all 157 flashcards in this deck.
Unlock Deck
k this deck
18
Which of the following molecules is most likely to show a hydrogen bonding interaction?
A)CH3OH
B)CH3SH
C)CH4
D)H-C C-H
C-H
E)A, B and C
A)CH3OH
B)CH3SH
C)CH4
D)H-C
 C-H
C-HE)A, B and C

Unlock Deck
Unlock for access to all 157 flashcards in this deck.
Unlock Deck
k this deck
19
Which of the following molecules is most likely to show a dipole-dipole interaction?
A)SO2
B)CO2
C)CH4
D)H-C C-H
C-H
E)none of the above
A)SO2
B)CO2
C)CH4
D)H-C
 C-H
C-HE)none of the above

Unlock Deck
Unlock for access to all 157 flashcards in this deck.
Unlock Deck
k this deck
20
Given the following diagram, describe what happens electronically between these two molecules. 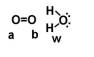
A)Oxygen A becomes slightly positively charged due to the protons on the water molecule.
B)Oxygen B becomes slightly positively charged due to the protons on the water molecule.
C)Oxygen A becomes slightly negatively charged due to the oxygen molecule.
D)Hydrogens on oxygen W becomes slightly positively charged due to the oxygen molecule.
E)none of the above

A)Oxygen A becomes slightly positively charged due to the protons on the water molecule.
B)Oxygen B becomes slightly positively charged due to the protons on the water molecule.
C)Oxygen A becomes slightly negatively charged due to the oxygen molecule.
D)Hydrogens on oxygen W becomes slightly positively charged due to the oxygen molecule.
E)none of the above

Unlock Deck
Unlock for access to all 157 flashcards in this deck.
Unlock Deck
k this deck
21
How are oxygen molecules attracted to water molecules?
A)The attraction between oxygen and water molecules is a classic example of dipole-dipole interaction.
B)The hydrogen bonding in water causes the attraction of the oxygen atoms in the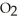 molecule to water.
molecule to water.
C)As a water molecule is brought close to an oxygen molecule an induced dipole results in the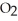 molecule causing the attraction.
molecule causing the attraction.
D)The attraction of oxygen and water molecules for one another is part of the common atom effect. Since both molecules contain oxygen, there is a built-in attraction.
A)The attraction between oxygen and water molecules is a classic example of dipole-dipole interaction.
B)The hydrogen bonding in water causes the attraction of the oxygen atoms in the
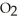 molecule to water.
molecule to water.C)As a water molecule is brought close to an oxygen molecule an induced dipole results in the
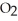 molecule causing the attraction.
molecule causing the attraction.D)The attraction of oxygen and water molecules for one another is part of the common atom effect. Since both molecules contain oxygen, there is a built-in attraction.

Unlock Deck
Unlock for access to all 157 flashcards in this deck.
Unlock Deck
k this deck
22
Chlorine, 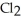 , is a gas at room temperature, but bromine,
, is a gas at room temperature, but bromine, 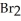 , is a liquid. Explain.
, is a liquid. Explain.
A)Chlorine atoms are larger and this makes the formation of induced dipole-induced dipole attractions more favorable.
B)Bromine atoms are larger and this makes the formation of induced dipole-induced dipole attractions more favorable.
C)The smaller chlorine molecules are able to pack together in a tighter physical orientation.
D)The bromine ions are held together by ionic bonds.
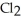 , is a gas at room temperature, but bromine,
, is a gas at room temperature, but bromine, 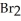 , is a liquid. Explain.
, is a liquid. Explain.A)Chlorine atoms are larger and this makes the formation of induced dipole-induced dipole attractions more favorable.
B)Bromine atoms are larger and this makes the formation of induced dipole-induced dipole attractions more favorable.
C)The smaller chlorine molecules are able to pack together in a tighter physical orientation.
D)The bromine ions are held together by ionic bonds.

Unlock Deck
Unlock for access to all 157 flashcards in this deck.
Unlock Deck
k this deck
23
What is the main difference between a dipole-dipole interaction and a dipole-induced dipole interaction?
A)Both are similar, but one involves a temporary dipole created by a permanent dipole.
B)Dipole-dipole interactions are weaker because the dipoles are permanent.
C)Dipole-induced dipole interactions are stronger because the induced dipoles can be formed at any time.
D)Both are identical.
E)none of the above
A)Both are similar, but one involves a temporary dipole created by a permanent dipole.
B)Dipole-dipole interactions are weaker because the dipoles are permanent.
C)Dipole-induced dipole interactions are stronger because the induced dipoles can be formed at any time.
D)Both are identical.
E)none of the above

Unlock Deck
Unlock for access to all 157 flashcards in this deck.
Unlock Deck
k this deck
24
Which of the following would have the highest boiling point?
A)Cl2
B)Br2
C)F2
D)I2
E)not enough information given
A)Cl2
B)Br2
C)F2
D)I2
E)not enough information given

Unlock Deck
Unlock for access to all 157 flashcards in this deck.
Unlock Deck
k this deck
25
The charges with sodium chloride are all balanced-for every positive sodium ion there is a corresponding negative chloride ion. Since its charges are balanced, how can sodium chloride be attracted to water, and vice versa?
A)Dispersion forces come into play as the sodium chloride and water come into close proximity.
B)Hydrogen bonding in water allows the sodium chloride molecule to be attracted to the water molecule.
C)As a water molecule gets close to the sodium chloride it can distinguish the various ions and it is thus attracted to an individual ion by ion-dipole forces.
D)This is not a matter of attraction. Sodium chloride dissolves in water because water provides a medium in which the individual sodium and chloride ions can disperse.
A)Dispersion forces come into play as the sodium chloride and water come into close proximity.
B)Hydrogen bonding in water allows the sodium chloride molecule to be attracted to the water molecule.
C)As a water molecule gets close to the sodium chloride it can distinguish the various ions and it is thus attracted to an individual ion by ion-dipole forces.
D)This is not a matter of attraction. Sodium chloride dissolves in water because water provides a medium in which the individual sodium and chloride ions can disperse.

Unlock Deck
Unlock for access to all 157 flashcards in this deck.
Unlock Deck
k this deck
26
Why are ion-dipole attractions stronger than dipole-dipole attractions?
A)The chemical bond in an ion-dipole molecule is similar also a covalent bond.
B)The magnitude of the electric charge associated with an ion is much greater.
C)Dipole areas are subject to changing from positive to negative regions on the molecule.
D)Like charge (dipole)does not attract like charge (another dipole.)
A)The chemical bond in an ion-dipole molecule is similar also a covalent bond.
B)The magnitude of the electric charge associated with an ion is much greater.
C)Dipole areas are subject to changing from positive to negative regions on the molecule.
D)Like charge (dipole)does not attract like charge (another dipole.)

Unlock Deck
Unlock for access to all 157 flashcards in this deck.
Unlock Deck
k this deck
27
A thin stream of water is pulled to a statically charged balloon. Might a small ice cube also be pulled to a statically charged balloon?
A)No, this behavior is unique to water while in it liquid state only.
B)Yes, the same forces that cause the attraction in the liquid state are present in the solid state of water as well.
C)Yes and No. While the ice remains completely solid, no attraction occurs. Once the ice begins to melt so that both phases are present, the attraction becomes apparent.
D)Yes and No. Like the thin stream of water, the ice must be in motion in order for the attraction to be observed.
A)No, this behavior is unique to water while in it liquid state only.
B)Yes, the same forces that cause the attraction in the liquid state are present in the solid state of water as well.
C)Yes and No. While the ice remains completely solid, no attraction occurs. Once the ice begins to melt so that both phases are present, the attraction becomes apparent.
D)Yes and No. Like the thin stream of water, the ice must be in motion in order for the attraction to be observed.

Unlock Deck
Unlock for access to all 157 flashcards in this deck.
Unlock Deck
k this deck
28
Which of the following is most likely to have the weakest induced dipole-induced dipole interaction?
A)Cl2
B)Br2
C)F2
D)I2
E)All of the above have the same interactions.
A)Cl2
B)Br2
C)F2
D)I2
E)All of the above have the same interactions.

Unlock Deck
Unlock for access to all 157 flashcards in this deck.
Unlock Deck
k this deck
29
Plastic wrap is made of nonpolar molecules and is able to stick well to polar surfaces, such as glass, by way of dipole/induced dipole molecular attractions. How is it that plastic wrap also sticks to itself so well?
A)by way of dipole-dipole molecular attractions
B)by way of dipole-induced dipole molecular attractions
C)Ions are formed as the plastic rubs against itself.
D)by way of induced dipole-induced dipole molecular attractions
A)by way of dipole-dipole molecular attractions
B)by way of dipole-induced dipole molecular attractions
C)Ions are formed as the plastic rubs against itself.
D)by way of induced dipole-induced dipole molecular attractions

Unlock Deck
Unlock for access to all 157 flashcards in this deck.
Unlock Deck
k this deck
30
List the following compounds in order of increasing boiling point: 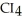 ,
, 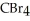 ,
, 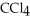 ,
, 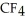 .
.
A)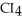 ,
, 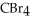 ,
, 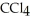 ,
, 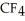
B)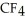 ,
, 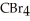 ,
, 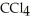 ,
, 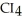
C)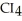 ,
, 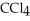 ,
,
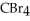 ,
, 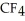
D)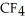 ,
,
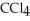 ,
, 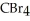 ,
, 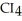
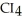 ,
, 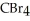 ,
, 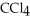 ,
, 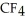 .
.A)
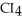 ,
, 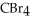 ,
, 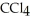 ,
, 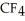
B)
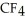 ,
, 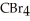 ,
, 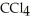 ,
, 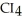
C)
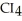 ,
, 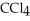 ,
, 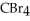 ,
, 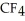
D)
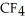 ,
, 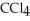 ,
, 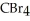 ,
, 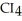

Unlock Deck
Unlock for access to all 157 flashcards in this deck.
Unlock Deck
k this deck
31
Dipole-induced dipole forces of attraction exist between water and gasoline, and yet these two substances do not mix because water has such a strong attraction for itself. Which of the following compounds might best help to make these two substances mix into a single liquid phase? 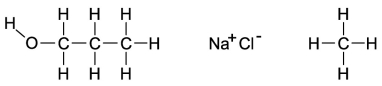
A)the molecule on the far left because the O-H bond is polar and the carbon and hydrogen bonds are nonpolar
B)the molecule in the middle because when the salts mix into the water, it will help separate the water and decrease the attraction for itself
C)The molecule on the right will form attractions with the polar ends of the water, allowing the gasoline a chance to mix with the water.
D)All of these molecules would be equally effective at increasing the mixing of gasoline and water.
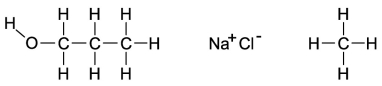
A)the molecule on the far left because the O-H bond is polar and the carbon and hydrogen bonds are nonpolar
B)the molecule in the middle because when the salts mix into the water, it will help separate the water and decrease the attraction for itself
C)The molecule on the right will form attractions with the polar ends of the water, allowing the gasoline a chance to mix with the water.
D)All of these molecules would be equally effective at increasing the mixing of gasoline and water.

Unlock Deck
Unlock for access to all 157 flashcards in this deck.
Unlock Deck
k this deck
32
Which of the following intermolecular forces best describes why molecules like sucrose (which has many OH groups)are very water soluble?
A)dipole-dipole
B)induced dipole-induced dipole
C)dipole-induced dipole
D)ion-dipole
E)hydrogen bonding
A)dipole-dipole
B)induced dipole-induced dipole
C)dipole-induced dipole
D)ion-dipole
E)hydrogen bonding

Unlock Deck
Unlock for access to all 157 flashcards in this deck.
Unlock Deck
k this deck
33
Why is the surface area of a gecko's foot so extensive?
A)A gecko, like all amphibians, needs extensive surface area under foot for stability on land as well as mobility in water.
B)A gecko's foot acts like a large dipole and thus allows for ion-dipole interaction in water.
C)The greater the surface area the greater the number of induced dipole-induced dipole forces of attraction that can occur between the gecko's foot and the surface.
D)The extensive surface area, once charged by the gecko's body, allows for the dipole-dipole attraction of every contact surface.
A)A gecko, like all amphibians, needs extensive surface area under foot for stability on land as well as mobility in water.
B)A gecko's foot acts like a large dipole and thus allows for ion-dipole interaction in water.
C)The greater the surface area the greater the number of induced dipole-induced dipole forces of attraction that can occur between the gecko's foot and the surface.
D)The extensive surface area, once charged by the gecko's body, allows for the dipole-dipole attraction of every contact surface.

Unlock Deck
Unlock for access to all 157 flashcards in this deck.
Unlock Deck
k this deck
34
Which of the following would have the highest boiling point?
A)C6H14
B)C8H18
C)C10H22
D)C12H26
E)not enough information given
A)C6H14
B)C8H18
C)C10H22
D)C12H26
E)not enough information given

Unlock Deck
Unlock for access to all 157 flashcards in this deck.
Unlock Deck
k this deck
35
Friends on a crowded ice skating rink tend to stay together. Strangers, however, tend to disperse. Is this behavior typical of atoms forming chemical bonds or molecules interacting? Explain.
A)No, atoms forming chemical bonds are just the opposite. Chemical bonds forming or molecules reacting would be more analogous to strangers tending to stay together and friends dispersing.
B)Yes, atoms forming chemical bonds are analogous to friends in the rink. Atoms held together by covalent bonds represent a molecule.
C)No, opposites (like strangers)attract to form stable covalent bonds in the world of atoms and molecules.
D)Yes and No; No, because this behavior is not typical of atoms forming chemical bonds, but yes since it is typical of molecules interacting.
A)No, atoms forming chemical bonds are just the opposite. Chemical bonds forming or molecules reacting would be more analogous to strangers tending to stay together and friends dispersing.
B)Yes, atoms forming chemical bonds are analogous to friends in the rink. Atoms held together by covalent bonds represent a molecule.
C)No, opposites (like strangers)attract to form stable covalent bonds in the world of atoms and molecules.
D)Yes and No; No, because this behavior is not typical of atoms forming chemical bonds, but yes since it is typical of molecules interacting.

Unlock Deck
Unlock for access to all 157 flashcards in this deck.
Unlock Deck
k this deck
36
Which of the following would have the smallest number of induced dipole-induced dipole interactions?
A)C6H14
B)C8H18
C)C10H22
D)C12H26
E)not enough information given
A)C6H14
B)C8H18
C)C10H22
D)C12H26
E)not enough information given

Unlock Deck
Unlock for access to all 157 flashcards in this deck.
Unlock Deck
k this deck
37
Which of the following would have the lowest melting point?
A)CCl4
B)CBr4
C)CF4
D)CI4
E)not enough information given
A)CCl4
B)CBr4
C)CF4
D)CI4
E)not enough information given

Unlock Deck
Unlock for access to all 157 flashcards in this deck.
Unlock Deck
k this deck
38
Why is calcium fluoride, Ca 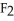 , a high melting point crystalline solid while tin (IV)chloride,
, a high melting point crystalline solid while tin (IV)chloride, 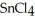 , is a volatile liquid?
, is a volatile liquid?
A)There is no theory to predict the physical property of melting point. Melting point temperatures are empirically determined.
B)Actually, we would predict these results to be the opposite. Since each metal is combined with a group 17 halogen, the heavier metal (tin)combination should have the higher melting point.
C)Ca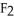 is a small, linear, non-polar molecule, while
is a small, linear, non-polar molecule, while 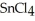 is a huge tetrahedral structure. Therefore the bonds in calcium fluoride tend to give it a higher melting point temperature.
is a huge tetrahedral structure. Therefore the bonds in calcium fluoride tend to give it a higher melting point temperature.
D)Ionic compounds formed by elements on opposite sides of the periodic table, like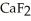 , tend to have higher melting points than more covalently bonded structures, like
, tend to have higher melting points than more covalently bonded structures, like 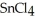 .
.
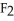 , a high melting point crystalline solid while tin (IV)chloride,
, a high melting point crystalline solid while tin (IV)chloride, 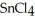 , is a volatile liquid?
, is a volatile liquid?A)There is no theory to predict the physical property of melting point. Melting point temperatures are empirically determined.
B)Actually, we would predict these results to be the opposite. Since each metal is combined with a group 17 halogen, the heavier metal (tin)combination should have the higher melting point.
C)Ca
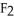 is a small, linear, non-polar molecule, while
is a small, linear, non-polar molecule, while 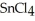 is a huge tetrahedral structure. Therefore the bonds in calcium fluoride tend to give it a higher melting point temperature.
is a huge tetrahedral structure. Therefore the bonds in calcium fluoride tend to give it a higher melting point temperature.D)Ionic compounds formed by elements on opposite sides of the periodic table, like
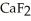 , tend to have higher melting points than more covalently bonded structures, like
, tend to have higher melting points than more covalently bonded structures, like 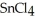 .
.
Unlock Deck
Unlock for access to all 157 flashcards in this deck.
Unlock Deck
k this deck
39
Which of the following intermolecular forces best describes why nonpolar molecules like gasoline (C8H18)have only limited solubility in water?
A)dipole-dipole
B)induced dipole-induced dipole
C)dipole-induced dipole
D)ion-dipole
E)Both A and B
A)dipole-dipole
B)induced dipole-induced dipole
C)dipole-induced dipole
D)ion-dipole
E)Both A and B

Unlock Deck
Unlock for access to all 157 flashcards in this deck.
Unlock Deck
k this deck
40
Which of the following intermolecular forces best describes why molecules like CF3CF3 are soluble in liquid CO2?
A)dipole-dipole
B)induced dipole-induced dipole
C)dipole-induced dipole
D)ion-dipole
E)hydrogen bonding
A)dipole-dipole
B)induced dipole-induced dipole
C)dipole-induced dipole
D)ion-dipole
E)hydrogen bonding

Unlock Deck
Unlock for access to all 157 flashcards in this deck.
Unlock Deck
k this deck
41
Two chemical structures are shown, one of a typical gasoline molecule and the other of a typical motor oil molecule. Which is which? 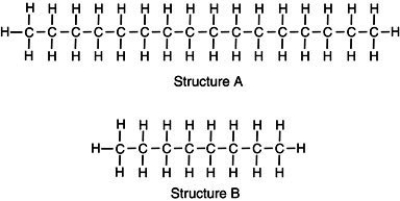 Base your reasoning not on memorization but rather upon what you know about molecular interactions and the various physical properties of gasoline and motor oil.
Base your reasoning not on memorization but rather upon what you know about molecular interactions and the various physical properties of gasoline and motor oil.
A)Structure A represents the gas molecule because there are more bonds to gain energy from, giving it a higher energy content than oil.
B)Structure A represents motor oil, illustrating a molecule with greater induced dipole-induced dipole molecular interactions thus, the molecules are strongly attracted to one another.
C)Structure B represents the oil molecule. Because oil molecules are smaller, they can compact closer together, giving the appearance of a thicker solution than gasoline.
D)Structure B represents crude oil which is processed to generate longer molecules of gasoline to prevent toxic vapors from harming consumers.
 Base your reasoning not on memorization but rather upon what you know about molecular interactions and the various physical properties of gasoline and motor oil.
Base your reasoning not on memorization but rather upon what you know about molecular interactions and the various physical properties of gasoline and motor oil.A)Structure A represents the gas molecule because there are more bonds to gain energy from, giving it a higher energy content than oil.
B)Structure A represents motor oil, illustrating a molecule with greater induced dipole-induced dipole molecular interactions thus, the molecules are strongly attracted to one another.
C)Structure B represents the oil molecule. Because oil molecules are smaller, they can compact closer together, giving the appearance of a thicker solution than gasoline.
D)Structure B represents crude oil which is processed to generate longer molecules of gasoline to prevent toxic vapors from harming consumers.

Unlock Deck
Unlock for access to all 157 flashcards in this deck.
Unlock Deck
k this deck
42
Which has the most atoms?
A)a mole of gold
B)a mole of helium
C)a mole of lead
D)All of the above have the same number of atoms.
E)none of the above
A)a mole of gold
B)a mole of helium
C)a mole of lead
D)All of the above have the same number of atoms.
E)none of the above

Unlock Deck
Unlock for access to all 157 flashcards in this deck.
Unlock Deck
k this deck
43
In a solution of 77 percent nitrogen and 23 percent oxygen, which is the solvent?
A)nitrogen
B)oxygen
C)both
D)neither
E)Gases cannot form solutions.
A)nitrogen
B)oxygen
C)both
D)neither
E)Gases cannot form solutions.

Unlock Deck
Unlock for access to all 157 flashcards in this deck.
Unlock Deck
k this deck
44
In a solution made from one teaspoon of sugar and one liter of water, which is the solute?
A)sugar
B)water
C)the teaspoon
D)both sugar and water
E)none of the above
A)sugar
B)water
C)the teaspoon
D)both sugar and water
E)none of the above

Unlock Deck
Unlock for access to all 157 flashcards in this deck.
Unlock Deck
k this deck
45
Which of the following solutions is the most dilute?
A)one liter of water with 1 gram of sugar
B)one liter of water with 2 grams of sugar
C)one liter of water with 5 grams of sugar
D)one liter of water with 10 grams of sugar
E)They all have the same volume.
A)one liter of water with 1 gram of sugar
B)one liter of water with 2 grams of sugar
C)one liter of water with 5 grams of sugar
D)one liter of water with 10 grams of sugar
E)They all have the same volume.

Unlock Deck
Unlock for access to all 157 flashcards in this deck.
Unlock Deck
k this deck
46
Which of the following describes the term concentration?
A)It is what you are doing now to answer this question.
B)It is the amount of solute in a given amount of solution.
C)It is the amount of solvent in a given amount of solution.
D)It is the given amount of solution in a given container.
E)It is the given amount of solvent per amount of solute.
A)It is what you are doing now to answer this question.
B)It is the amount of solute in a given amount of solution.
C)It is the amount of solvent in a given amount of solution.
D)It is the given amount of solution in a given container.
E)It is the given amount of solvent per amount of solute.

Unlock Deck
Unlock for access to all 157 flashcards in this deck.
Unlock Deck
k this deck
47
What statement best describes a mole?
A)a little furry mammal that lives in the ground
B)a very small number chemists use to count atoms or molecules
C)the amount of molecules or atoms in 1 gram of something
D)It is a very large number chemists use to count atoms or molecules.
E)none of the above
A)a little furry mammal that lives in the ground
B)a very small number chemists use to count atoms or molecules
C)the amount of molecules or atoms in 1 gram of something
D)It is a very large number chemists use to count atoms or molecules.
E)none of the above

Unlock Deck
Unlock for access to all 157 flashcards in this deck.
Unlock Deck
k this deck
48
Which of the following solutions is the most dilute?
A)0.1 liter of water with 1 gram of sugar
B)0.2 liter of water with 2 grams of sugar
C)0.5 liter of water with 5 grams of sugar
D)1 liter of water with 10 grams of sugar
E)They all have the same concentration.
A)0.1 liter of water with 1 gram of sugar
B)0.2 liter of water with 2 grams of sugar
C)0.5 liter of water with 5 grams of sugar
D)1 liter of water with 10 grams of sugar
E)They all have the same concentration.

Unlock Deck
Unlock for access to all 157 flashcards in this deck.
Unlock Deck
k this deck
49
Which of the following statements describes a saturated solution?
A)a solution where the solvent cannot dissolve any more solute
B)a solution of salt water with salt at the bottom
C)a carbonated beverage with bubbles
D)all of the above
E)none of the above
A)a solution where the solvent cannot dissolve any more solute
B)a solution of salt water with salt at the bottom
C)a carbonated beverage with bubbles
D)all of the above
E)none of the above

Unlock Deck
Unlock for access to all 157 flashcards in this deck.
Unlock Deck
k this deck
50
What is molarity?
A)the number of moles of solute per liter of solution
B)the number of grams of solute per liter of solution
C)the number of moles of solute per liter of solvent
D)the number of liters of solute per mole of solution
E)none of the above
A)the number of moles of solute per liter of solution
B)the number of grams of solute per liter of solution
C)the number of moles of solute per liter of solvent
D)the number of liters of solute per mole of solution
E)none of the above

Unlock Deck
Unlock for access to all 157 flashcards in this deck.
Unlock Deck
k this deck
51
Why are the melting temperatures of most ionic compounds far greater than the melting temperatures of most covalent compounds?
A)Ionic bonds are so much stronger than the intermolecular attractions between covalently bonded compounds.
B)Covalent bonds are not as strong as ionic bonds.
C)As a solid, salts have a very organized crystalline structure which takes a lot of energy to break apart.
D)Most covalent compounds have at least one weak bond in their structure that is easily broken when heat is added.
A)Ionic bonds are so much stronger than the intermolecular attractions between covalently bonded compounds.
B)Covalent bonds are not as strong as ionic bonds.
C)As a solid, salts have a very organized crystalline structure which takes a lot of energy to break apart.
D)Most covalent compounds have at least one weak bond in their structure that is easily broken when heat is added.

Unlock Deck
Unlock for access to all 157 flashcards in this deck.
Unlock Deck
k this deck
52
A 1 molar solution of sugar water contains ________.
A)1 mole of sucrose
B)342 grams of sucrose
C)6.02 × 1023 molecules of sucrose
D)all of the above
E)Not enough information is given
A)1 mole of sucrose
B)342 grams of sucrose
C)6.02 × 1023 molecules of sucrose
D)all of the above
E)Not enough information is given

Unlock Deck
Unlock for access to all 157 flashcards in this deck.
Unlock Deck
k this deck
53
Which of the following material phases cannot form a solution?
A)solids
B)liquids
C)gases
D)All of the above can form solutions.
E)None of the above can form solutions.
A)solids
B)liquids
C)gases
D)All of the above can form solutions.
E)None of the above can form solutions.

Unlock Deck
Unlock for access to all 157 flashcards in this deck.
Unlock Deck
k this deck
54
Consider the boiling points of the following compounds and their solubilities in room-temperature water. Why does the solubilities in water go down as the boiling points of these alcohols go up. 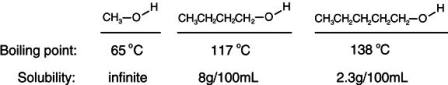
A)Larger molecules are less attracted to one another by induced dipole-induced dipole as well as by dipole-dipole and dipole-induced dipole attractions.
B)As the boiling increases, it is more difficult to keep the alcohol from evaporating out of solution.
C)As the boiling point increases, the size of the alcohol molecules decreases.
D)Larger molecules are more attracted to one another by induced dipole-induced dipole as well as by dipole-dipole and dipole-induced dipole attractions.

A)Larger molecules are less attracted to one another by induced dipole-induced dipole as well as by dipole-dipole and dipole-induced dipole attractions.
B)As the boiling increases, it is more difficult to keep the alcohol from evaporating out of solution.
C)As the boiling point increases, the size of the alcohol molecules decreases.
D)Larger molecules are more attracted to one another by induced dipole-induced dipole as well as by dipole-dipole and dipole-induced dipole attractions.

Unlock Deck
Unlock for access to all 157 flashcards in this deck.
Unlock Deck
k this deck
55
An inventor claims to have developed a new perfume that lasts a long time because it doesn't evaporate. Comment on this claim.
A)A perfume that does not evaporate could be toxic since the molecules never leave the skin.
B)In order to smell something, the molecules must evaporate and reach your nose. If the new perfume doesn't evaporate, it will not have an odor.
C)This would be impossible to make because the perfume would have to be pressurized in order to not evaporate.
D)This product is sure to sweep the market making many happy customers.
A)A perfume that does not evaporate could be toxic since the molecules never leave the skin.
B)In order to smell something, the molecules must evaporate and reach your nose. If the new perfume doesn't evaporate, it will not have an odor.
C)This would be impossible to make because the perfume would have to be pressurized in order to not evaporate.
D)This product is sure to sweep the market making many happy customers.

Unlock Deck
Unlock for access to all 157 flashcards in this deck.
Unlock Deck
k this deck
56
Which of the following solutions is the most concentrated?
A)0.1 liter of water with 1 gram of sugar
B)2 liters of water with 0.2 gram of sugar
C)0.5 liter of water with 50 grams of sugar
D)3 liters of water with 30 grams of sugar
E)They all have the same concentration.
A)0.1 liter of water with 1 gram of sugar
B)2 liters of water with 0.2 gram of sugar
C)0.5 liter of water with 50 grams of sugar
D)3 liters of water with 30 grams of sugar
E)They all have the same concentration.

Unlock Deck
Unlock for access to all 157 flashcards in this deck.
Unlock Deck
k this deck
57
A sample of steel is composed of 5 percent carbon and 95 percent iron. Which is the solvent?
A)iron
B)carbon
C)steel
D)Steel is not a solution, it is a mixture.
E)A solid cannot be a solvent.
A)iron
B)carbon
C)steel
D)Steel is not a solution, it is a mixture.
E)A solid cannot be a solvent.

Unlock Deck
Unlock for access to all 157 flashcards in this deck.
Unlock Deck
k this deck
58
Which of the following solutions is the most concentrated?
A)one liter of water with 1 gram of sugar
B)one liter of water with 2 grams of sugar
C)one liter of water with 5 grams of sugar
D)one liter of water with 10 grams of sugar
E)They all have the same volume.
A)one liter of water with 1 gram of sugar
B)one liter of water with 2 grams of sugar
C)one liter of water with 5 grams of sugar
D)one liter of water with 10 grams of sugar
E)They all have the same volume.

Unlock Deck
Unlock for access to all 157 flashcards in this deck.
Unlock Deck
k this deck
59
A gemstone is an example of a ________.
A)pure material
B)solid solution
C)suspension
D)saturated solution
A)pure material
B)solid solution
C)suspension
D)saturated solution

Unlock Deck
Unlock for access to all 157 flashcards in this deck.
Unlock Deck
k this deck
60
The boiling point of 1,4-butanediol is 230°C. Would you expect this compound to be soluble or insoluble in room-temperature water? 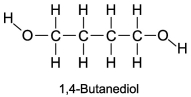
A)Since there are no polar areas on this molecule, it is insoluble in water at room temperature.
B)A high boiling point means that the substance interacts with itself quite strongly. Therefore this molecule is not soluble in water.
C)Since there are polar areas on this molecule, it is insoluble in water at room temperature.
D)Water would be attracted to both ends of 1,4 butanediol, and it is infinitely soluble in water.

A)Since there are no polar areas on this molecule, it is insoluble in water at room temperature.
B)A high boiling point means that the substance interacts with itself quite strongly. Therefore this molecule is not soluble in water.
C)Since there are polar areas on this molecule, it is insoluble in water at room temperature.
D)Water would be attracted to both ends of 1,4 butanediol, and it is infinitely soluble in water.

Unlock Deck
Unlock for access to all 157 flashcards in this deck.
Unlock Deck
k this deck
61
What is the sum of the atomic masses of all the atoms in sucrose, 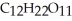 ?
?
A)342 amu
B)182 amu
C)270 amu
D)none of the above
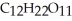 ?
?A)342 amu
B)182 amu
C)270 amu
D)none of the above

Unlock Deck
Unlock for access to all 157 flashcards in this deck.
Unlock Deck
k this deck
62
If you need 3.01 × 1023 molecules of sucrose, how many liters of a 4.00 molar solution would you need?
A)0.125 L
B)0.250 L
C)4.00 L
D)1.00 L
E)none of the above
A)0.125 L
B)0.250 L
C)4.00 L
D)1.00 L
E)none of the above

Unlock Deck
Unlock for access to all 157 flashcards in this deck.
Unlock Deck
k this deck
63
How many grams of sodium chloride are needed to make 15 L of a solution that has a concentration of 3.0 g per liter of solution?
A)30. g
B)141 g
C)5.0 g
D)45 g
A)30. g
B)141 g
C)5.0 g
D)45 g

Unlock Deck
Unlock for access to all 157 flashcards in this deck.
Unlock Deck
k this deck
64
If you need 10. moles of sucrose, how many liters of a 4.0 molar solution would you need?
A)2.5 L
B)0.25 L
C)25 L
D)10. L
E)none of the above
A)2.5 L
B)0.25 L
C)25 L
D)10. L
E)none of the above

Unlock Deck
Unlock for access to all 157 flashcards in this deck.
Unlock Deck
k this deck
65
Which of the following solutions is the most concentrated?
A)0.5 L of a 3 molar solution
B)3.0 L of a 0.5 molar solution
C)2.0 L of a 1 molar solution
D)0.5 L of a 1 molar solution
E)2.0 L of a 2 molar solution
A)0.5 L of a 3 molar solution
B)3.0 L of a 0.5 molar solution
C)2.0 L of a 1 molar solution
D)0.5 L of a 1 molar solution
E)2.0 L of a 2 molar solution

Unlock Deck
Unlock for access to all 157 flashcards in this deck.
Unlock Deck
k this deck
66
A student is told to use 10.00 grams of sodium chloride to make an aqueous solution that has a concentration of 10.00 grams of sodium chloride per liter of solution. About how much water will she use in making this solution?
A)10.08 L
B)10.00 L
C)9.992 L
A)10.08 L
B)10.00 L
C)9.992 L

Unlock Deck
Unlock for access to all 157 flashcards in this deck.
Unlock Deck
k this deck
67
How many moles of sugar, C12H22O11, are there in 200. grams?
A)0.585 moles
B)68,400 moles
C)1.71 moles
D)0.684 moles
A)0.585 moles
B)68,400 moles
C)1.71 moles
D)0.684 moles

Unlock Deck
Unlock for access to all 157 flashcards in this deck.
Unlock Deck
k this deck
68
Which of the following best describes a two-molar sucrose solution?
A)one liter of solution that contains 2 moles of sucrose
B)one liter of solution that contains 2 moles of water
C)one liter of solution that contains 6.02 × 1023 molecules of sucrose
D)two liters of solution that contains 1 mole of sucrose
E)one mole of sucrose dissolved in 2 liters of solution
A)one liter of solution that contains 2 moles of sucrose
B)one liter of solution that contains 2 moles of water
C)one liter of solution that contains 6.02 × 1023 molecules of sucrose
D)two liters of solution that contains 1 mole of sucrose
E)one mole of sucrose dissolved in 2 liters of solution

Unlock Deck
Unlock for access to all 157 flashcards in this deck.
Unlock Deck
k this deck
69
A sealed plastic bottle is filled with enough sand so that the bottle floats just beneath the surface in ocean water. Some sand is then removed and the bottle is then placed in some fresh water where it floats just beneath the surface. What is true about the amount of sand that was removed from the bottle?
A)The mass of sand removed divided by the volume of the original plastic bottle equals the density of the ocean water.
B)The mass of the sand removed divided by the mass of the sand remaining in the plastic bottle is the same ratio as the density of fresh water to the density of ocean water.
C)The amount of sand removed from the plastic bottle equals the amount of salt dissolved in the ocean water.
D)The mass of the sand removed multiplied by the density of ocean water equals the volume of the plastic bottle.
A)The mass of sand removed divided by the volume of the original plastic bottle equals the density of the ocean water.
B)The mass of the sand removed divided by the mass of the sand remaining in the plastic bottle is the same ratio as the density of fresh water to the density of ocean water.
C)The amount of sand removed from the plastic bottle equals the amount of salt dissolved in the ocean water.
D)The mass of the sand removed multiplied by the density of ocean water equals the volume of the plastic bottle.

Unlock Deck
Unlock for access to all 157 flashcards in this deck.
Unlock Deck
k this deck
70
How many molecules of sucrose are in 0.5 00L of a 2.00 molar solution of sucrose?
A)3.01 × 1023 molecules of sucrose
B)6.02 × 1023 molecules of sucrose
C)12.04 × 1023 molecules of sucrose
D)0.5
E)1 gram
A)3.01 × 1023 molecules of sucrose
B)6.02 × 1023 molecules of sucrose
C)12.04 × 1023 molecules of sucrose
D)0.5
E)1 gram

Unlock Deck
Unlock for access to all 157 flashcards in this deck.
Unlock Deck
k this deck
71
What is the molarity when water is added to 2 moles of sodium chloride to make 0.5 liter of solution?
A)8 M
B)4 M
C)5 M
D)2.5 M
A)8 M
B)4 M
C)5 M
D)2.5 M

Unlock Deck
Unlock for access to all 157 flashcards in this deck.
Unlock Deck
k this deck
72
Many solvents expand to occupy greater volumes with increasing temperature. What happens to the concentration of a solution made with such a solvent as its temperature is increased?
A)Since concentration depends on how much mass is dissolved in a given volume, as the volume increases, the concentration decreases.
B)The concentration of a solution increases as the solute fits into the new spaces between the molecules.
C)Since it has a greater ability to dissolve more solute at a higher temperature, its concentration has decreased.
D)Since it has a greater ability to dissolve more solute at a higher temperature, its concentration has increased.
A)Since concentration depends on how much mass is dissolved in a given volume, as the volume increases, the concentration decreases.
B)The concentration of a solution increases as the solute fits into the new spaces between the molecules.
C)Since it has a greater ability to dissolve more solute at a higher temperature, its concentration has decreased.
D)Since it has a greater ability to dissolve more solute at a higher temperature, its concentration has increased.

Unlock Deck
Unlock for access to all 157 flashcards in this deck.
Unlock Deck
k this deck
73
What is the molarity of 0.50 liters of a solution with five moles of sucrose in it?
A)10. molar
B)0.5 molar
C)5 molar
D)2.5 molar
E)1 molar
A)10. molar
B)0.5 molar
C)5 molar
D)2.5 molar
E)1 molar

Unlock Deck
Unlock for access to all 157 flashcards in this deck.
Unlock Deck
k this deck
74
How are intermolecular forces and solubility related?
A)Solubility depends on the solvent's ability to overcome the intermolecular forces in a solid.
B)Solubility depends on the solute's ability to overcome the intermolecular forces in the solvent.
C)Solubility is a measure of how strong a solvent's intermolecular forces are.
D)Solubility is a measure of how weak the intermolecular forces in the solute are.
E)none of the above
A)Solubility depends on the solvent's ability to overcome the intermolecular forces in a solid.
B)Solubility depends on the solute's ability to overcome the intermolecular forces in the solvent.
C)Solubility is a measure of how strong a solvent's intermolecular forces are.
D)Solubility is a measure of how weak the intermolecular forces in the solute are.
E)none of the above

Unlock Deck
Unlock for access to all 157 flashcards in this deck.
Unlock Deck
k this deck
75
How many molecules of sucrose are in a 0.500 moles of sucrose?
A)3.01 × 1023 molecules of sucrose
B)6.02 × 1023 molecules of sucrose
C)12.04 × 1023 molecules of sucrose
D)0.5
E)1 gram
A)3.01 × 1023 molecules of sucrose
B)6.02 × 1023 molecules of sucrose
C)12.04 × 1023 molecules of sucrose
D)0.5
E)1 gram

Unlock Deck
Unlock for access to all 157 flashcards in this deck.
Unlock Deck
k this deck
76
How many molecules of sucrose are in 0.500 L of a 1.00 molar solution of sucrose?
A)3.01 × 1023 molecules of sucrose
B)6.02 × 1023 molecules of sucrose
C)12.04 × 1023 molecules of sucrose
D)0.5
E)1 gram
A)3.01 × 1023 molecules of sucrose
B)6.02 × 1023 molecules of sucrose
C)12.04 × 1023 molecules of sucrose
D)0.5
E)1 gram

Unlock Deck
Unlock for access to all 157 flashcards in this deck.
Unlock Deck
k this deck
77
How many grams of sugar (sucrose)are there in 5.0 liters of sugar water that has a concentration of 0.50 grams per liter of solution?
A)50 g
B)25 g
C)2.5 g
D)1.5 g
A)50 g
B)25 g
C)2.5 g
D)1.5 g

Unlock Deck
Unlock for access to all 157 flashcards in this deck.
Unlock Deck
k this deck
78
How many moles of water are there in 100. grams of water?
A)1800 moles
B)100 moles
C)0.018 moles
D)5.55 moles
A)1800 moles
B)100 moles
C)0.018 moles
D)5.55 moles

Unlock Deck
Unlock for access to all 157 flashcards in this deck.
Unlock Deck
k this deck
79
The warming of a saturated solution of calcium carbonate, CaCO3, produces ________.
A)a precipitate
B)a clear solution
C)effervescence
D)a counter cooling effect
A)a precipitate
B)a clear solution
C)effervescence
D)a counter cooling effect

Unlock Deck
Unlock for access to all 157 flashcards in this deck.
Unlock Deck
k this deck
80
Does a plastic bottle of fresh water sink or float in the ocean? Why?
A)Sinks; The combined density of the plastic bottle plus the fresh water inside is greater than the density of the ocean water.
B)Floats; The bottle filled with fresh water floats in ocean water because it is less dense than the ocean water.
C)Floats then sinks; The bottle filled with fresh water floats in ocean water until the fresh water reaches the same temperature as the ocean water, at which point it sinks.
D)Sinks slightly; The added density of the plastic bottle will cause the bottle to sink slightly. It would most likely sink less than a foot where the density of the ocean water would be equal to or greater than the bottle of fresh water.
A)Sinks; The combined density of the plastic bottle plus the fresh water inside is greater than the density of the ocean water.
B)Floats; The bottle filled with fresh water floats in ocean water because it is less dense than the ocean water.
C)Floats then sinks; The bottle filled with fresh water floats in ocean water until the fresh water reaches the same temperature as the ocean water, at which point it sinks.
D)Sinks slightly; The added density of the plastic bottle will cause the bottle to sink slightly. It would most likely sink less than a foot where the density of the ocean water would be equal to or greater than the bottle of fresh water.

Unlock Deck
Unlock for access to all 157 flashcards in this deck.
Unlock Deck
k this deck


