Deck 9: How Chemicals React
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/150
Play
Full screen (f)
Deck 9: How Chemicals React
1
Four units of monoatomic substance A combines with one unit diatomic substance B to make the triatomic substance C. Two units of triatomic substance C combine with one unit of diatomic substance B to make substance D. Place the letter of each substance next to the symbol that best describes its atomic or molecular structure. 
A)A, D, B, C
B)C, A, B, D
C)D, B, A, C
D)B, D, A, C

A)A, D, B, C
B)C, A, B, D
C)D, B, A, C
D)B, D, A, C
D, B, A, C
2
Steel wool wetted with vinegar is sealed within a balloon inflated with air. After several hours, what happens to the volume of the balloon?
A)The balloon inflates.
B)The balloon deflates.
C)The balloon dissolves.
D)Nothing because the vinegar is acting on the steel wool, not upon the balloon.
A)The balloon inflates.
B)The balloon deflates.
C)The balloon dissolves.
D)Nothing because the vinegar is acting on the steel wool, not upon the balloon.
The balloon deflates.
3
For the following balanced reaction, which of the following is a gas? 2 Na(l) + Cl2(g) → 2 NaCl(s)
A)Na
B)2 Na
C)Cl2
D)Cl
E)NaCl
A)Na
B)2 Na
C)Cl2
D)Cl
E)NaCl
Cl2
4
Given the following generic chemical reaction, which is the reactant? X → Y
A)Y is the reactant.
B)X is the reactant.
C)→ is the reactant.
D)Both X and Y are the products.
E)Both X and Y are the reactants.
A)Y is the reactant.
B)X is the reactant.
C)→ is the reactant.
D)Both X and Y are the products.
E)Both X and Y are the reactants.

Unlock Deck
Unlock for access to all 150 flashcards in this deck.
Unlock Deck
k this deck
5
Given the following generic chemical reaction, which is the product? X → Y
A)Y is the product.
B)X is the product.
C)→ is the product.
D)Both X and Y are the products.
E)Both X and Y are the reactants.
A)Y is the product.
B)X is the product.
C)→ is the product.
D)Both X and Y are the products.
E)Both X and Y are the reactants.

Unlock Deck
Unlock for access to all 150 flashcards in this deck.
Unlock Deck
k this deck
6
What is a chemical equation?
A)It is a shorthand notation for illustrating a chemical reaction.
B)It is the sum of the masses of the products and reactants.
C)It is the chemical combination of equal numbers of reactants and products.
D)It is a picture of the atoms undergoing a chemical equalization.
E)It is any type of reaction that takes place at the equator.
A)It is a shorthand notation for illustrating a chemical reaction.
B)It is the sum of the masses of the products and reactants.
C)It is the chemical combination of equal numbers of reactants and products.
D)It is a picture of the atoms undergoing a chemical equalization.
E)It is any type of reaction that takes place at the equator.

Unlock Deck
Unlock for access to all 150 flashcards in this deck.
Unlock Deck
k this deck
7
What is wrong with the following depiction of a chemical reaction? 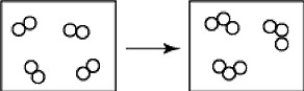
A)These boxes contain only molecules but no atoms.
B)One box contains more molecules than the other.
C)One box contains more atoms than the other.
D)all of the above

A)These boxes contain only molecules but no atoms.
B)One box contains more molecules than the other.
C)One box contains more atoms than the other.
D)all of the above

Unlock Deck
Unlock for access to all 150 flashcards in this deck.
Unlock Deck
k this deck
8
In a chemical equation the coefficients ________.
A)of the reactants should always sum up to those of the products
B)appear as subscripts
C)appear before the chemical formulas
D)Two of the above are correct.
A)of the reactants should always sum up to those of the products
B)appear as subscripts
C)appear before the chemical formulas
D)Two of the above are correct.

Unlock Deck
Unlock for access to all 150 flashcards in this deck.
Unlock Deck
k this deck
9
What coefficient is needed in front of the O2 molecule to balance the following equation? 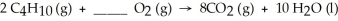
A)8
B)13
C)5
D)1
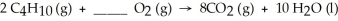
A)8
B)13
C)5
D)1

Unlock Deck
Unlock for access to all 150 flashcards in this deck.
Unlock Deck
k this deck
10
Balance the following chemical equation. ____ N2 + ____ H2 → ____ NH3
A)1, 3, 2
B)1, 2, 3
C)3, 2, 1
D)2, 6, 4
E)1/2, 3/2, 1
A)1, 3, 2
B)1, 2, 3
C)3, 2, 1
D)2, 6, 4
E)1/2, 3/2, 1

Unlock Deck
Unlock for access to all 150 flashcards in this deck.
Unlock Deck
k this deck
11
What is a chemical reaction?
A)when one or more new compounds are formed by rearranging atoms
B)when a new element is formed by rearranging nucleons
C)when two solids mix together to form a heterogeneous mixture
D)when two liquids mix to form a homogeneous mixture
E)when a liquid undergoes a phase change and produces a solid
A)when one or more new compounds are formed by rearranging atoms
B)when a new element is formed by rearranging nucleons
C)when two solids mix together to form a heterogeneous mixture
D)when two liquids mix to form a homogeneous mixture
E)when a liquid undergoes a phase change and produces a solid

Unlock Deck
Unlock for access to all 150 flashcards in this deck.
Unlock Deck
k this deck
12
Steel wool wetted with vinegar is stuffed into a narrow mouth round glass bottle. A rubber balloon is then sealed over the mouth of the bottle. After several hours, the balloon inflates into the bottle in an inverted manner. What happened?
A)Vinegar fumes are diamagnetic and as they accumulate above the liquid the steel wool is attracted thus inflating the balloon into the mouth of the bottle in an inverted manner.
B)The caustic vinegar fumes get past the steel wool and deteriorate the balloon, which begins to sag into the bottle and inflate it in an inverted manner.
C)The vinegar reacts with the steel wool by absorbing oxygen within the bottle thus decreasing the pressure. The greater outside pressure causes the balloon to inflate in an inverted manner.
D)False! The balloon inflates above the mouth of the bottle because the reaction between the vinegar and steel wool produces a gas which is forced upward because of increased pressure inside the sealed bottle.
A)Vinegar fumes are diamagnetic and as they accumulate above the liquid the steel wool is attracted thus inflating the balloon into the mouth of the bottle in an inverted manner.
B)The caustic vinegar fumes get past the steel wool and deteriorate the balloon, which begins to sag into the bottle and inflate it in an inverted manner.
C)The vinegar reacts with the steel wool by absorbing oxygen within the bottle thus decreasing the pressure. The greater outside pressure causes the balloon to inflate in an inverted manner.
D)False! The balloon inflates above the mouth of the bottle because the reaction between the vinegar and steel wool produces a gas which is forced upward because of increased pressure inside the sealed bottle.

Unlock Deck
Unlock for access to all 150 flashcards in this deck.
Unlock Deck
k this deck
13
For the following balanced equation, which has the highest coefficient? 4 H2 + 2 C → 2 CH4
A)H2
B)C
C)CH4
D)H4
E)none of the above
A)H2
B)C
C)CH4
D)H4
E)none of the above

Unlock Deck
Unlock for access to all 150 flashcards in this deck.
Unlock Deck
k this deck
14
A friend argues that if mass were really conserved he would never need to refill his gas tank. What explanation do you offer your friend?
A)The atoms (mass)of gasoline are converted into energy by the engine according to E=m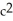 .
.
B)The Law of Conservation of Mass does not apply to reactions involving combustion or explosion of matter.
C)The atoms (mass)of gasoline are converted into exhaust fumes.
D)The oil companies make gasoline in a way that it gets used up so that we are always required to replenish it.
A)The atoms (mass)of gasoline are converted into energy by the engine according to E=m
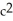 .
.B)The Law of Conservation of Mass does not apply to reactions involving combustion or explosion of matter.
C)The atoms (mass)of gasoline are converted into exhaust fumes.
D)The oil companies make gasoline in a way that it gets used up so that we are always required to replenish it.

Unlock Deck
Unlock for access to all 150 flashcards in this deck.
Unlock Deck
k this deck
15
Balance the following equation. ____ NO → ____ N2O + ____ NO2
A)3, 1, 1
B)3, 0, 0
C)4, 4, 8
D)1, 2, 4
E)6, 2, 1
A)3, 1, 1
B)3, 0, 0
C)4, 4, 8
D)1, 2, 4
E)6, 2, 1

Unlock Deck
Unlock for access to all 150 flashcards in this deck.
Unlock Deck
k this deck
16
Which of the following is a correctly balanced equation?
A)P4 + 6 H2 → 4 PH3
B)1 P4 + 6 H2 → 4 PH3
C)0 P4 + 6 H2 → 4 PH3
D)2 P4 + 12 H2 → 8 PH3
E)P4 + 3 H2 → PH3
A)P4 + 6 H2 → 4 PH3
B)1 P4 + 6 H2 → 4 PH3
C)0 P4 + 6 H2 → 4 PH3
D)2 P4 + 12 H2 → 8 PH3
E)P4 + 3 H2 → PH3

Unlock Deck
Unlock for access to all 150 flashcards in this deck.
Unlock Deck
k this deck
17
Which equations are balanced?
a)Mg (s)+ 2HCl (aq)→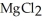 (aq)+
(aq)+ 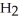 (g)
(g)
b)3Al (s)+ 3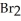 (l)→
(l)→ 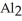
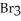 (s)
(s)
c)2HgO (s)→ 2 Hg (l)+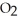 (g)
(g)
A)Only equation "c" is balanced.
B)Equations "a" and "c" are balanced.
C)Equations "b" and "c" are balanced.
D)All of them are balanced.
a)Mg (s)+ 2HCl (aq)→
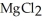 (aq)+
(aq)+ 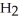 (g)
(g)b)3Al (s)+ 3
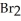 (l)→
(l)→ 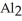
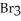 (s)
(s)c)2HgO (s)→ 2 Hg (l)+
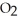 (g)
(g)A)Only equation "c" is balanced.
B)Equations "a" and "c" are balanced.
C)Equations "b" and "c" are balanced.
D)All of them are balanced.

Unlock Deck
Unlock for access to all 150 flashcards in this deck.
Unlock Deck
k this deck
18
For the following balanced reaction, which of the following is a solid? 2 Na(l) + Cl2(g) → 2 NaCl(s)
A)Na
B)2 Na
C)Cl2
D)Cl
E)NaCl
A)Na
B)2 Na
C)Cl2
D)Cl
E)NaCl

Unlock Deck
Unlock for access to all 150 flashcards in this deck.
Unlock Deck
k this deck
19
Balance these equations. ____ 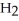 (g)+ ____
(g)+ ____ 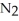 (g)→ ____ N
(g)→ ____ N 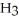 (g)
(g)
A)2, 2, 3
B)2, 2, 5
C)3, 3, 2
D)3, 1, 2
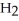 (g)+ ____
(g)+ ____ 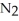 (g)→ ____ N
(g)→ ____ N 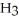 (g)
(g)A)2, 2, 3
B)2, 2, 5
C)3, 3, 2
D)3, 1, 2

Unlock Deck
Unlock for access to all 150 flashcards in this deck.
Unlock Deck
k this deck
20
What coefficients balance the following equation? ____ 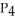 (s)+ ____
(s)+ ____ 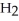 (g)→ ____ P
(g)→ ____ P 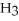 (g)
(g)
A)4, 2, 3
B)1, 6, 4
C)1, 4, 4
D)2, 10, 8
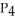 (s)+ ____
(s)+ ____ 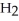 (g)→ ____ P
(g)→ ____ P 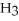 (g)
(g)A)4, 2, 3
B)1, 6, 4
C)1, 4, 4
D)2, 10, 8

Unlock Deck
Unlock for access to all 150 flashcards in this deck.
Unlock Deck
k this deck
21
The following diagram depicts the reaction between gaseous oxygen, 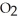 , and gaseous hydrogen,
, and gaseous hydrogen, 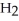 , to form water vapor,
, to form water vapor, 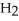 O. What molecules and how many of them should be drawn in the empty box? How many grams of water are formed under these conditions? How many grams of which chemical remain unreacted?
O. What molecules and how many of them should be drawn in the empty box? How many grams of water are formed under these conditions? How many grams of which chemical remain unreacted? 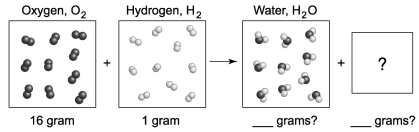
A)five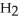 molecules in empty box; 9 grams of
molecules in empty box; 9 grams of 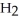 O + 0.5 grams of
O + 0.5 grams of 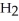
B)five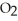 molecules in empty box; 9 grams of
molecules in empty box; 9 grams of 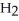 O + 8 grams of
O + 8 grams of 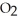
C)five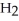 O molecules in empty box; 11.3 grams of
O molecules in empty box; 11.3 grams of 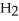 O + 5.7 grams of
O + 5.7 grams of 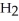 O
O
D)ten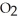 molecules in empty box; 9 grams of
molecules in empty box; 9 grams of 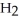 O + 8 grams of
O + 8 grams of 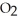
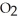 , and gaseous hydrogen,
, and gaseous hydrogen, 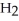 , to form water vapor,
, to form water vapor, 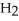 O. What molecules and how many of them should be drawn in the empty box? How many grams of water are formed under these conditions? How many grams of which chemical remain unreacted?
O. What molecules and how many of them should be drawn in the empty box? How many grams of water are formed under these conditions? How many grams of which chemical remain unreacted? 
A)five
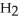 molecules in empty box; 9 grams of
molecules in empty box; 9 grams of 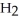 O + 0.5 grams of
O + 0.5 grams of 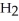
B)five
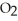 molecules in empty box; 9 grams of
molecules in empty box; 9 grams of 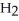 O + 8 grams of
O + 8 grams of 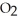
C)five
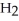 O molecules in empty box; 11.3 grams of
O molecules in empty box; 11.3 grams of 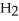 O + 5.7 grams of
O + 5.7 grams of 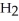 O
OD)ten
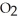 molecules in empty box; 9 grams of
molecules in empty box; 9 grams of 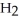 O + 8 grams of
O + 8 grams of 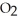

Unlock Deck
Unlock for access to all 150 flashcards in this deck.
Unlock Deck
k this deck
22
The atomic masses appearing in the periodic table are ________.
A)absolute masses
B)relative masses
C)standard deviation masses
D)formula masses
A)absolute masses
B)relative masses
C)standard deviation masses
D)formula masses

Unlock Deck
Unlock for access to all 150 flashcards in this deck.
Unlock Deck
k this deck
23
Which equation best describes the reaction represented in the illustration above?
A)2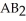 + 2
+ 2 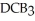 +
+ 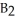 → 2
→ 2 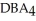 + 2
+ 2 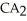
B)2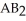 + 2
+ 2 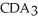 +
+ 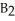 → 2
→ 2 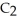
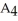 + 2 DBA
+ 2 DBA
C)2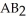 + 2
+ 2 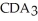 +
+ 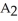 → 2
→ 2 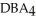 + 2
+ 2 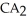
D)2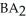 + 2
+ 2 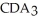 +
+ 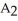 → 2
→ 2 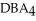 + 2
+ 2 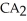
A)2
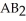 + 2
+ 2 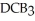 +
+ 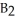 → 2
→ 2 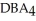 + 2
+ 2 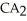
B)2
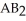 + 2
+ 2 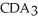 +
+ 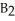 → 2
→ 2 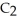
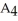 + 2 DBA
+ 2 DBAC)2
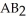 + 2
+ 2 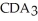 +
+ 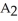 → 2
→ 2 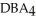 + 2
+ 2 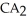
D)2
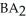 + 2
+ 2 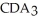 +
+ 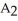 → 2
→ 2 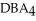 + 2
+ 2 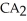

Unlock Deck
Unlock for access to all 150 flashcards in this deck.
Unlock Deck
k this deck
24
If the relative mass of a hydrogen atom is 1/4 that of a helium atom, how many helium atoms would you need to equal the mass of 200 hydrogen atoms?
A)50
B)200
C)800
D)4
E)100
A)50
B)200
C)800
D)4
E)100

Unlock Deck
Unlock for access to all 150 flashcards in this deck.
Unlock Deck
k this deck
25
Which is greater: 1.01 amu of hydrogen or 1.01 grams of hydrogen?
A)1.01 amu of hydrogen is greater than 1.01 grams of hydrogen.
B)1.01 grams of hydrogen is greater than 1.01 amu of hydrogen
C)1.01 grams of hydrogen and 1.01 amu of hydrogen have the same mass.
D)Not enough information information is provided.
A)1.01 amu of hydrogen is greater than 1.01 grams of hydrogen.
B)1.01 grams of hydrogen is greater than 1.01 amu of hydrogen
C)1.01 grams of hydrogen and 1.01 amu of hydrogen have the same mass.
D)Not enough information information is provided.

Unlock Deck
Unlock for access to all 150 flashcards in this deck.
Unlock Deck
k this deck
26
If the relative mass of a hydrogen atom is 1/4 that of a helium atom, how many hydrogen atoms would you need to equal the mass of four helium atoms?
A)16
B)4
C)1/4
D)25
E)6.022 × 1023
A)16
B)4
C)1/4
D)25
E)6.022 × 1023

Unlock Deck
Unlock for access to all 150 flashcards in this deck.
Unlock Deck
k this deck
27
How many oxygen molecules are needed to make 10 carbon dioxide molecules according to the following balanced chemical equation? 2 CO + O2 → 2 CO2
A)5
B)1
C)4
D)10
E)2
A)5
B)1
C)4
D)10
E)2

Unlock Deck
Unlock for access to all 150 flashcards in this deck.
Unlock Deck
k this deck
28
The reactants shown schematically below represent iron oxide, 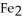
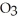 and carbon monoxide, CO. Which of the following is the correct full balanced chemical equation for what is depicted?
and carbon monoxide, CO. Which of the following is the correct full balanced chemical equation for what is depicted? 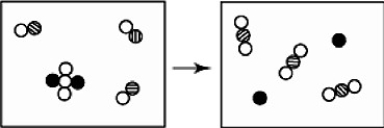
A)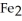
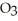 + 3 CO → 2 Fe + 3
+ 3 CO → 2 Fe + 3 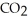
B)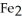
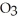 + 3 CO → 3 FeO + 2 C
+ 3 CO → 3 FeO + 2 C
C)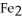
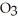 + 3 CO → 3 Fe
+ 3 CO → 3 Fe 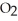 + 2C
+ 2C
D)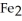
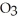 + 3 CO → 2 Fe + 3
+ 3 CO → 2 Fe + 3 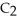 O
O
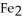
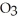 and carbon monoxide, CO. Which of the following is the correct full balanced chemical equation for what is depicted?
and carbon monoxide, CO. Which of the following is the correct full balanced chemical equation for what is depicted? 
A)
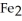
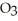 + 3 CO → 2 Fe + 3
+ 3 CO → 2 Fe + 3 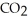
B)
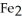
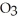 + 3 CO → 3 FeO + 2 C
+ 3 CO → 3 FeO + 2 CC)
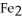
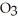 + 3 CO → 3 Fe
+ 3 CO → 3 Fe 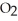 + 2C
+ 2CD)
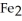
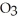 + 3 CO → 2 Fe + 3
+ 3 CO → 2 Fe + 3 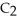 O
O
Unlock Deck
Unlock for access to all 150 flashcards in this deck.
Unlock Deck
k this deck
29
If it takes three carbon atoms to equal the mass of one chlorine atom, what mass of chlorine do you need to equal the number of atoms in one kilogram of carbon?
A)1/3 of a kg
B)30 kg
C)1 kg
D)3 kg
E)6 kg
A)1/3 of a kg
B)30 kg
C)1 kg
D)3 kg
E)6 kg

Unlock Deck
Unlock for access to all 150 flashcards in this deck.
Unlock Deck
k this deck
30
If it takes 200 golf balls to equal the mass of four bowling balls, what is the relative mass of bowling balls to golf balls?
A)1/50
B)1/20
C)20 times
D)100 times
E)6.022 × 1023
A)1/50
B)1/20
C)20 times
D)100 times
E)6.022 × 1023

Unlock Deck
Unlock for access to all 150 flashcards in this deck.
Unlock Deck
k this deck
31
The above diagram represents a chemical reaction where the box before the arrow shows the reactants and the box after the arrow shows the products. How many diatomic molecules are represented?
A)1
B)2
C)3
D)4
A)1
B)2
C)3
D)4

Unlock Deck
Unlock for access to all 150 flashcards in this deck.
Unlock Deck
k this deck
32
If it takes three golf balls to equal the mass of one tennis ball, what mass of tennis balls do you need to equal the number of golf balls in one kilogram of golf balls?
A)1/3 of a kg
B)30 kg
C)1 kg
D)3 kg
E)6 kg
A)1/3 of a kg
B)30 kg
C)1 kg
D)3 kg
E)6 kg

Unlock Deck
Unlock for access to all 150 flashcards in this deck.
Unlock Deck
k this deck
33
If the relative mass of a pingpong ball is 1/20 that of a golf ball, how many golf balls would you need to equal the mass of 200 Ping-Pong balls?
A)10
B)200
C)100
D)20
E)6.022 × 1023
A)10
B)200
C)100
D)20
E)6.022 × 1023

Unlock Deck
Unlock for access to all 150 flashcards in this deck.
Unlock Deck
k this deck
34
What is the formula mass of sulfur dioxide, S 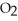 ?
?
A)about 16 amu
B)about 32 amu
C)about 60 amu
D)about 64 amu
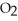 ?
?A)about 16 amu
B)about 32 amu
C)about 60 amu
D)about 64 amu

Unlock Deck
Unlock for access to all 150 flashcards in this deck.
Unlock Deck
k this deck
35
Is it possible to have a macroscopic sample of oxygen that has a mass of 14 atomic mass units?
A)Yes, but it would need to be made of oxygen atoms that each had less than the normal number of neutrons.
B)No, this is less than than the mass of a single oxygen atom.
C)Yes, but it would have the same density as nitrogen.
D)No, because oxygen is a gas at room temperature.
A)Yes, but it would need to be made of oxygen atoms that each had less than the normal number of neutrons.
B)No, this is less than than the mass of a single oxygen atom.
C)Yes, but it would have the same density as nitrogen.
D)No, because oxygen is a gas at room temperature.

Unlock Deck
Unlock for access to all 150 flashcards in this deck.
Unlock Deck
k this deck
36
Gas A is composed of diatomic molecules (two atoms per molecule)of a pure element. Gas B is composed of triatomic molecules (three atoms per molecule)of another pure element. A volume of gas B is found to be three times more massive than an equal volume of gas A. How does the mass of an atom of gas B compare with the mass of an atom of gas A? 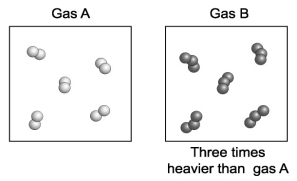
A)An atom of gas B is six times the mass of an atom of gas A.
B)An atom of gas B is three times the mass of an atom of gas A.
C)An atom of gas B is two times the mass of an an atom of gas A.
D)An atom of gas B is equivalent to the mass of an atom of gas A.

A)An atom of gas B is six times the mass of an atom of gas A.
B)An atom of gas B is three times the mass of an atom of gas A.
C)An atom of gas B is two times the mass of an an atom of gas A.
D)An atom of gas B is equivalent to the mass of an atom of gas A.

Unlock Deck
Unlock for access to all 150 flashcards in this deck.
Unlock Deck
k this deck
37
What are the formula masses of water, 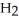 O; propene,
O; propene, 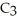
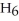 ; and 2-propanol,
; and 2-propanol, 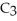
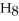 O?
O?
A)water: 18 amu; propene: 40 amu; 2-propanol: 58 amu
B)water: 18 amu; propene: 42 amu; 2-propanol: 62 amu
C)water: 18 amu; propene: 42 amu; 2-propanol: 60 amu
D)water: 18 amu; propene: 44 amu; 2-propanol: 64 amu
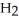 O; propene,
O; propene, 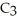
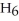 ; and 2-propanol,
; and 2-propanol, 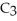
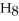 O?
O?A)water: 18 amu; propene: 40 amu; 2-propanol: 58 amu
B)water: 18 amu; propene: 42 amu; 2-propanol: 62 amu
C)water: 18 amu; propene: 42 amu; 2-propanol: 60 amu
D)water: 18 amu; propene: 44 amu; 2-propanol: 64 amu

Unlock Deck
Unlock for access to all 150 flashcards in this deck.
Unlock Deck
k this deck
38
If it takes 20 beryllium atoms to equal the mass of two krypton atoms, what is the relative mass of beryllium compared to krypton?
A)1/10
B)1/20
C)40 times
D)100 times
E)10 times
A)1/10
B)1/20
C)40 times
D)100 times
E)10 times

Unlock Deck
Unlock for access to all 150 flashcards in this deck.
Unlock Deck
k this deck
39
If the relative mass of a pingpong ball is 1/20 that of a golf ball, how many Ping-Pong balls would you need to equal the mass of two golf balls?
A)40
B)20
C)24
D)100
E)6.022 × 1023
A)40
B)20
C)24
D)100
E)6.022 × 1023

Unlock Deck
Unlock for access to all 150 flashcards in this deck.
Unlock Deck
k this deck
40
Why is it important for a chemist to know the relative masses of atoms?
A)There are not that many different kinds of atoms and so it's important to know how they relate to one another.
B)It provides information about how many atoms two samples have relative to each other
C)It provides an indication of how the different atoms will interact
D)Because the mass of an atom is directly related to its chemical properties.
A)There are not that many different kinds of atoms and so it's important to know how they relate to one another.
B)It provides information about how many atoms two samples have relative to each other
C)It provides an indication of how the different atoms will interact
D)Because the mass of an atom is directly related to its chemical properties.

Unlock Deck
Unlock for access to all 150 flashcards in this deck.
Unlock Deck
k this deck
41
How many grams of water can be formed from the reaction between 10 grams of oxygen and 1 gram of hydrogen?
A)11 grams of water are formed since mass must be conserved.
B)10 grams of water are formed since you can't get a greater mass of water produced than oxygen reacting.
C)9 grams of water are formed because oxygen and hydrogen react in an 8:1 ratio.
D)No water is formed because there is insufficient hydrogen to react with the oxygen.
A)11 grams of water are formed since mass must be conserved.
B)10 grams of water are formed since you can't get a greater mass of water produced than oxygen reacting.
C)9 grams of water are formed because oxygen and hydrogen react in an 8:1 ratio.
D)No water is formed because there is insufficient hydrogen to react with the oxygen.

Unlock Deck
Unlock for access to all 150 flashcards in this deck.
Unlock Deck
k this deck
42
What is the formula mass of a molecule of CO2?
A)44 amu
B)56 amu
C)58.9 amu
D)118 amu
E)none of the above
A)44 amu
B)56 amu
C)58.9 amu
D)118 amu
E)none of the above

Unlock Deck
Unlock for access to all 150 flashcards in this deck.
Unlock Deck
k this deck
43
The following diagrams depict the reaction between gaseous chlorine, 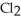 , and gaseous hydrogen,
, and gaseous hydrogen, 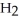 , to form gaseous hydrogen chloride, HCl. What should be drawn in the empty box and how many grams of products are formed?
, to form gaseous hydrogen chloride, HCl. What should be drawn in the empty box and how many grams of products are formed? 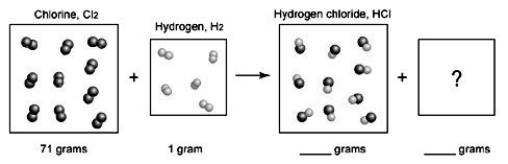
A)five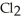 molecules in empty box; 37 grams of HCl + 36 grams of
molecules in empty box; 37 grams of HCl + 36 grams of 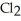
B)five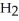 molecules in empty box; 71 grams of HCl + 1 gram of
molecules in empty box; 71 grams of HCl + 1 gram of 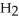
C)five HCl molecules in empty box; 50 grams of HCl + 22 grams of HCl
D)five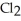 molecules in empty box; 71 grams of HCl + 1 gram of
molecules in empty box; 71 grams of HCl + 1 gram of 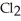
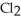 , and gaseous hydrogen,
, and gaseous hydrogen, 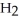 , to form gaseous hydrogen chloride, HCl. What should be drawn in the empty box and how many grams of products are formed?
, to form gaseous hydrogen chloride, HCl. What should be drawn in the empty box and how many grams of products are formed? 
A)five
 molecules in empty box; 37 grams of HCl + 36 grams of
molecules in empty box; 37 grams of HCl + 36 grams of 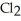
B)five
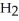 molecules in empty box; 71 grams of HCl + 1 gram of
molecules in empty box; 71 grams of HCl + 1 gram of 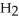
C)five HCl molecules in empty box; 50 grams of HCl + 22 grams of HCl
D)five
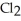 molecules in empty box; 71 grams of HCl + 1 gram of
molecules in empty box; 71 grams of HCl + 1 gram of 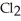

Unlock Deck
Unlock for access to all 150 flashcards in this deck.
Unlock Deck
k this deck
44
What is the number of moles of H2O produced if you combust 0.5 mole of CH4 according to the following balanced equation? CH4 + 2 O2 → CO2 + 2 H2O
A)2 moles
B)4 moles
C)6 moles
D)8 moles
E)1 mole
A)2 moles
B)4 moles
C)6 moles
D)8 moles
E)1 mole

Unlock Deck
Unlock for access to all 150 flashcards in this deck.
Unlock Deck
k this deck
45
The relative mass of carbon is 3/8 that of an oxygen molecule. How many grams of carbon are needed to have the same number of particles as found in 32 grams of oxygen molecules?
A)12 g
B)32 g
C)3 g
D)8 g
E)3/8 g
A)12 g
B)32 g
C)3 g
D)8 g
E)3/8 g

Unlock Deck
Unlock for access to all 150 flashcards in this deck.
Unlock Deck
k this deck
46
How many grams of water, H2O, can be produced by the reaction of 8 grams of oxygen, O2, and 8 grams of hydrogen, H2?
A)16 grams
B)10 grams
C)9 grams
D)8 grams
A)16 grams
B)10 grams
C)9 grams
D)8 grams

Unlock Deck
Unlock for access to all 150 flashcards in this deck.
Unlock Deck
k this deck
47
You are given two samples of elements, and each sample has a mass of 10 grams. If the number of atoms in each of these samples is the same, what must be true of the two elements?
A)The density of the two elements are the same.
B)The elements are likely be located in the same position in the periodic table.
C)Their spectral patterns will likely be identical.
D)all of the above
A)The density of the two elements are the same.
B)The elements are likely be located in the same position in the periodic table.
C)Their spectral patterns will likely be identical.
D)all of the above

Unlock Deck
Unlock for access to all 150 flashcards in this deck.
Unlock Deck
k this deck
48
According to the following balanced chemical equation, if you want to generate two moles of H2O, how many moles of O2 do you need? 2 H2 + O2 → 2 H2O
A)1
B)2
C)1/2
D)4
E)6.022 × 1023
A)1
B)2
C)1/2
D)4
E)6.022 × 1023

Unlock Deck
Unlock for access to all 150 flashcards in this deck.
Unlock Deck
k this deck
49
How does formula mass differ from atomic mass?
A)They represent the same thing.
B)The formula mass of a substance is the sum of the atomic masses of the elements is its chemical formula. The atomic mass is the mass of a single atom.
C)The atomic mass of a substance is the sum of the formula masses of the elements is its chemical formula. The atomic mass is the mass of a single atom.
D)The formula mass is the mass of the chemical formula and the atomic mass is the mass of the molecule.
A)They represent the same thing.
B)The formula mass of a substance is the sum of the atomic masses of the elements is its chemical formula. The atomic mass is the mass of a single atom.
C)The atomic mass of a substance is the sum of the formula masses of the elements is its chemical formula. The atomic mass is the mass of a single atom.
D)The formula mass is the mass of the chemical formula and the atomic mass is the mass of the molecule.

Unlock Deck
Unlock for access to all 150 flashcards in this deck.
Unlock Deck
k this deck
50
How many grams of water, H2O, can be produced from the reaction of 25.0 grams of hydrogen, H2, and 225 grams of oxygen, O2?
A)250 grams
B)225 grams
C)200 grams
D)25 grams
A)250 grams
B)225 grams
C)200 grams
D)25 grams

Unlock Deck
Unlock for access to all 150 flashcards in this deck.
Unlock Deck
k this deck
51
What is the mass of one mole of H2?
A)2 g
B)1 g
C)20 g
D)6.022 × 1023 g
E)none of the above
A)2 g
B)1 g
C)20 g
D)6.022 × 1023 g
E)none of the above

Unlock Deck
Unlock for access to all 150 flashcards in this deck.
Unlock Deck
k this deck
52
Fourteen grams of nitrogen, N2, (N, atomic mass 14 amu)contains ________.
A)1 mole of nitrogen atoms
B)0.5 moles of nitrogen atoms
C)0.25 moles of nitrogen atoms
D)Not enough information is given
A)1 mole of nitrogen atoms
B)0.5 moles of nitrogen atoms
C)0.25 moles of nitrogen atoms
D)Not enough information is given

Unlock Deck
Unlock for access to all 150 flashcards in this deck.
Unlock Deck
k this deck
53
According to the following balanced chemical equation, if you want to generate two moles of H2O , how many molecules of O2 do you need? 2 H2 + O2 → 2 H2O
A)1
B)2
C)1/2
D)4
E)6.022 × 1023
A)1
B)2
C)1/2
D)4
E)6.022 × 1023

Unlock Deck
Unlock for access to all 150 flashcards in this deck.
Unlock Deck
k this deck
54
What is the formula mass of a molecule of C6H12O6?
A)180 amu
B)24 amu
C)29 amu
D)168 amu
E)none of the above
A)180 amu
B)24 amu
C)29 amu
D)168 amu
E)none of the above

Unlock Deck
Unlock for access to all 150 flashcards in this deck.
Unlock Deck
k this deck
55
According to the following balanced chemical equation, if you want to generate two moles of H2O how many grams of O2 do you need? 2 H2 + O2 → 2 H2O
A)32
B)16
C)8
D)4
E)6.022 × 1023
A)32
B)16
C)8
D)4
E)6.022 × 1023

Unlock Deck
Unlock for access to all 150 flashcards in this deck.
Unlock Deck
k this deck
56
How is Avogadro's number related to the numbers on the periodic table?
A)The atomic mass listed is the mass of Avogadro's number's worth of atoms.
B)The masses are all divisible by Avogadro's number, which gives you the weight of one mole.
C)The periodic table tells you the mass of one atom. From that, and Avogadro's number you know the number of moles.
D)The periodic table only gives us atomic numbers, not atomic mass.
E)The mass listed is Avogadro's number.
A)The atomic mass listed is the mass of Avogadro's number's worth of atoms.
B)The masses are all divisible by Avogadro's number, which gives you the weight of one mole.
C)The periodic table tells you the mass of one atom. From that, and Avogadro's number you know the number of moles.
D)The periodic table only gives us atomic numbers, not atomic mass.
E)The mass listed is Avogadro's number.

Unlock Deck
Unlock for access to all 150 flashcards in this deck.
Unlock Deck
k this deck
57
What is the number of moles of H2O produced if you combust one mole of CH4 according to the following balanced equation? CH4 + 2 O2 → CO2 + 2 H2O
A)2 moles
B)4 moles
C)6 moles
D)8 moles
E)1 mole
A)2 moles
B)4 moles
C)6 moles
D)8 moles
E)1 mole

Unlock Deck
Unlock for access to all 150 flashcards in this deck.
Unlock Deck
k this deck
58
Which of the following has the greatest number of particles?
A)1 mole of Na
B)22.990 g of Na
C)1 mole of Be
D)9.012 g of Be
E)All are the same.
A)1 mole of Na
B)22.990 g of Na
C)1 mole of Be
D)9.012 g of Be
E)All are the same.

Unlock Deck
Unlock for access to all 150 flashcards in this deck.
Unlock Deck
k this deck
59
Which of the following has the greatest mass?
A)1 mole of Pb
B)1 mole of H2
C)1 mole of Be
D)1 mole of Na
E)All have the same mass.
A)1 mole of Pb
B)1 mole of H2
C)1 mole of Be
D)1 mole of Na
E)All have the same mass.

Unlock Deck
Unlock for access to all 150 flashcards in this deck.
Unlock Deck
k this deck
60
Seven grams of nitrogen, N2, (N, atomic mass 14 amu)contains ________.
A)1 mole of nitrogen atoms
B)0.5 moles of nitrogen atoms
C)0.25 moles of nitrogen atoms
D)Not enough information is given
A)1 mole of nitrogen atoms
B)0.5 moles of nitrogen atoms
C)0.25 moles of nitrogen atoms
D)Not enough information is given

Unlock Deck
Unlock for access to all 150 flashcards in this deck.
Unlock Deck
k this deck
61
Which has more atoms: 17.031 g of ammonia, N 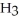 (17.031 amu), or 72.922 g of hydrogen chloride, HCl (36.461 amu)?
(17.031 amu), or 72.922 g of hydrogen chloride, HCl (36.461 amu)?
A)17.031 g of N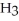 has more atoms than 72.922 g of HCl
has more atoms than 72.922 g of HCl
B)72.922 g of HCl has more atoms than 17.031 g of N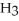
C)72.922 g of HCl and 17.031 g of N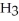 have about the same number of atoms.
have about the same number of atoms.
D)Not enough information is given.
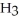 (17.031 amu), or 72.922 g of hydrogen chloride, HCl (36.461 amu)?
(17.031 amu), or 72.922 g of hydrogen chloride, HCl (36.461 amu)?A)17.031 g of N
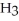 has more atoms than 72.922 g of HCl
has more atoms than 72.922 g of HClB)72.922 g of HCl has more atoms than 17.031 g of N
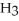
C)72.922 g of HCl and 17.031 g of N
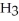 have about the same number of atoms.
have about the same number of atoms.D)Not enough information is given.

Unlock Deck
Unlock for access to all 150 flashcards in this deck.
Unlock Deck
k this deck
62
Two amu equals how many grams? (1 amu = 1.661 × 10-24 g)
A)2 grams
B)1.661 ×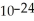 grams
grams
C)3.322 ×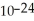 grams
grams
D)1.204 ×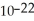 grams
grams
A)2 grams
B)1.661 ×
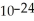 grams
gramsC)3.322 ×
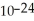 grams
gramsD)1.204 ×
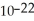 grams
grams
Unlock Deck
Unlock for access to all 150 flashcards in this deck.
Unlock Deck
k this deck
63
Three identical candles are placed in the jars shown below and lit at the same time. Jar x is left open while jars y and z are sealed with lids. The candle in which jar goes out first? 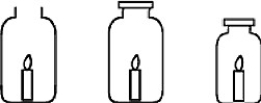 x y z
x y z
A)The candle in jar x will go out first.
B)The candle in jar y will go out first.
C)The candle in jar z will go out first.
D)All the candles go out at the same time.
 x y z
x y zA)The candle in jar x will go out first.
B)The candle in jar y will go out first.
C)The candle in jar z will go out first.
D)All the candles go out at the same time.

Unlock Deck
Unlock for access to all 150 flashcards in this deck.
Unlock Deck
k this deck
64
Assume air has an average molar mass of 28 grams/mole, and determine how many moles of air molecules there are 1.0 liters of air, which contains 1.26 grams of air molecules.
A)28 moles
B)0.45 mole
C)0.045 mole
D)22.4 moles
A)28 moles
B)0.45 mole
C)0.045 mole
D)22.4 moles

Unlock Deck
Unlock for access to all 150 flashcards in this deck.
Unlock Deck
k this deck
65
What is the mass of a water molecule, 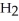 O, in atomic mass units?
O, in atomic mass units?
A)2 amu
B)3 amu
C)16 amu
D)18 amu
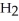 O, in atomic mass units?
O, in atomic mass units?A)2 amu
B)3 amu
C)16 amu
D)18 amu

Unlock Deck
Unlock for access to all 150 flashcards in this deck.
Unlock Deck
k this deck
66
Two candles of the same mass, one wide and short and the other thin and tall, are placed in identical jars as shown below and lit at the same time. Lids are then placed on each jar. Which candle goes out first? 
A)the short candle
B)the tall candle
C)Both candles go out at the same time.
D)There is no way to know which candle will go out first.

A)the short candle
B)the tall candle
C)Both candles go out at the same time.
D)There is no way to know which candle will go out first.

Unlock Deck
Unlock for access to all 150 flashcards in this deck.
Unlock Deck
k this deck
67
What is the mass of an oxygen atom, O, in grams? (1 amu = 1.661 × 10-24 g)
A)16 grams
B)1.661 × grams
grams
C)2.66 ×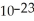 grams
grams
D)none of the above
A)16 grams
B)1.661 ×
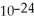 grams
gramsC)2.66 ×
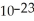 grams
gramsD)none of the above

Unlock Deck
Unlock for access to all 150 flashcards in this deck.
Unlock Deck
k this deck
68
Which has the greater mass, 1.204 × 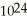 molecules of molecular hydrogen,
molecules of molecular hydrogen, 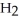 , or 1.204 ×
, or 1.204 × 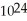 molecules of water,
molecules of water, 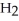 O?
O?
A)1.204 ×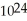 molecules of molecular hydrogen has a greater mass than 1.204 ×
molecules of molecular hydrogen has a greater mass than 1.204 × 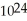 molecules of water
molecules of water
B)1.204 ×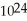 molecules of water has a greater mass than 1.204 ×
molecules of water has a greater mass than 1.204 × 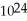 molecules of molecular hydrogen.
molecules of molecular hydrogen.
C)The mass of this many molecules of water and molecules are molecular hydrogen are the same.
D)Not enough information is provided.
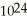 molecules of molecular hydrogen,
molecules of molecular hydrogen, 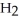 , or 1.204 ×
, or 1.204 × 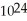 molecules of water,
molecules of water, 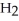 O?
O?A)1.204 ×
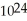 molecules of molecular hydrogen has a greater mass than 1.204 ×
molecules of molecular hydrogen has a greater mass than 1.204 × 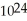 molecules of water
molecules of waterB)1.204 ×
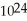 molecules of water has a greater mass than 1.204 ×
molecules of water has a greater mass than 1.204 × 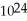 molecules of molecular hydrogen.
molecules of molecular hydrogen.C)The mass of this many molecules of water and molecules are molecular hydrogen are the same.
D)Not enough information is provided.

Unlock Deck
Unlock for access to all 150 flashcards in this deck.
Unlock Deck
k this deck
69
What is the mass of an oxygen atom, O, in atomic mass units?
A)12 amu
B)16 amu
C)18 amu
D)32 amu
A)12 amu
B)16 amu
C)18 amu
D)32 amu

Unlock Deck
Unlock for access to all 150 flashcards in this deck.
Unlock Deck
k this deck
70
What is the mass of a water molecule, 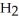 O, in grams? (1 amu = 1.661 × 10-24 g)
O, in grams? (1 amu = 1.661 × 10-24 g)
A)18 grams
B)1.661 ×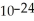 grams
grams
C)2.99 × 10-23 grams
D)none of the above
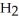 O, in grams? (1 amu = 1.661 × 10-24 g)
O, in grams? (1 amu = 1.661 × 10-24 g)A)18 grams
B)1.661 ×
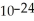 grams
gramsC)2.99 × 10-23 grams
D)none of the above

Unlock Deck
Unlock for access to all 150 flashcards in this deck.
Unlock Deck
k this deck
71
A 1.00 carat pure diamond has a mass of 0.20 grams. How many carbon atoms are there within this diamond?
A)6.0 ×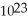 carbon atoms
carbon atoms
B)2.0 ×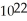 carbon atoms
carbon atoms
C)1.0 ×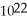 carbon atoms
carbon atoms
D)6.0 ×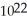 carbon atoms
carbon atoms
A)6.0 ×
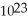 carbon atoms
carbon atomsB)2.0 ×
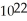 carbon atoms
carbon atomsC)1.0 ×
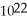 carbon atoms
carbon atomsD)6.0 ×
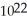 carbon atoms
carbon atoms
Unlock Deck
Unlock for access to all 150 flashcards in this deck.
Unlock Deck
k this deck
72
How many molecules of aspirin (formula mass aspirin = 180.0 amu)are there in a 0.250-gram sample?
A)6.02 ×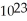
B)8.36 ×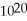
C)1.51 ×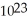
D)More information is needed.
A)6.02 ×
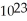
B)8.36 ×
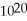
C)1.51 ×
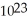
D)More information is needed.

Unlock Deck
Unlock for access to all 150 flashcards in this deck.
Unlock Deck
k this deck
73
Which has the greatest number of molecules?
A)28 g of nitrogen,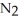
B)32 g of oxygen,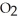
C)32 g of methane, C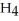
D)38 g of fluorine,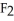
A)28 g of nitrogen,
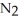
B)32 g of oxygen,
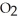
C)32 g of methane, C
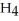
D)38 g of fluorine,
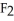

Unlock Deck
Unlock for access to all 150 flashcards in this deck.
Unlock Deck
k this deck
74
Which has the greatest number of atoms?
A)28 g of nitrogen,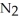
B)32 g of oxygen,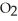
C)16 g of methane, C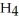
D)38 g of fluorine,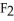
A)28 g of nitrogen,
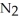
B)32 g of oxygen,
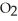
C)16 g of methane, C
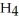
D)38 g of fluorine,
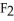

Unlock Deck
Unlock for access to all 150 flashcards in this deck.
Unlock Deck
k this deck
75
What is the number of molecules of O2 consumed if you combust one mole of CH4 according to the following balanced equation? CH4 + 2 O2 → CO2 + 2 H2O
A)1 molecule
B)2 molecules
C)6.022 × 1023 molecules
D)1.204 × 024 molecules
E)1 g of molecules
A)1 molecule
B)2 molecules
C)6.022 × 1023 molecules
D)1.204 × 024 molecules
E)1 g of molecules

Unlock Deck
Unlock for access to all 150 flashcards in this deck.
Unlock Deck
k this deck
76
There are 1000 liters in 1 cubic meter and 1000 grams in 1 kilogram. How many grams of air are there in 1.00 liter of air? (Assume a density of 1.25 kg/ 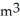 .)
.)
A)0.00125 gram
B)0.125 gram
C)1.25 grams
D)12.5 grams
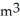 .)
.)A)0.00125 gram
B)0.125 gram
C)1.25 grams
D)12.5 grams

Unlock Deck
Unlock for access to all 150 flashcards in this deck.
Unlock Deck
k this deck
77
Which has more atoms: 64.058 g of sulfur dioxide, S 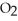 (64.058 amu), or 72.922 g of hydrogen chloride, HCl (36.461 amu)?
(64.058 amu), or 72.922 g of hydrogen chloride, HCl (36.461 amu)?
A)64.058 g of S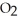 has more atoms than 72.922 g of HCl
has more atoms than 72.922 g of HCl
B)72.922 g of HCl has more atoms than 64.058 g of S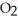
C)72.922 g of HCl and 64.058 g of S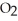 have about the same number atoms.
have about the same number atoms.
D)Not enough information is given.
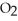 (64.058 amu), or 72.922 g of hydrogen chloride, HCl (36.461 amu)?
(64.058 amu), or 72.922 g of hydrogen chloride, HCl (36.461 amu)?A)64.058 g of S
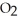 has more atoms than 72.922 g of HCl
has more atoms than 72.922 g of HClB)72.922 g of HCl has more atoms than 64.058 g of S
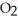
C)72.922 g of HCl and 64.058 g of S
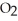 have about the same number atoms.
have about the same number atoms.D)Not enough information is given.

Unlock Deck
Unlock for access to all 150 flashcards in this deck.
Unlock Deck
k this deck
78
How many grams of gallium are there in a 145 gram sample of gallium arenside, GaAs?
A)74.9 g
B)69.7 g
C)145 g
D)6.02 × 1023 g
A)74.9 g
B)69.7 g
C)145 g
D)6.02 × 1023 g

Unlock Deck
Unlock for access to all 150 flashcards in this deck.
Unlock Deck
k this deck
79
What is the number of grams of CO2 produced if you combust 0.50 mole of CH4 according to the following balanced equation? CH4 + 2 O2 → CO2 + 2 H2O
A)22 g
B)10 g
C)44 g
D)32 g
E)1 g
A)22 g
B)10 g
C)44 g
D)32 g
E)1 g

Unlock Deck
Unlock for access to all 150 flashcards in this deck.
Unlock Deck
k this deck
80
Small samples of oxygen gas needed in the laboratory can be generated by any number of simple chemical reactions, such as 2 KCl 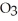 (s)→ 2 KCl (s)+ 3
(s)→ 2 KCl (s)+ 3 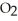 (g)
(g)
What mass of oxygen (in grams)is produced when 122.6 g of KCl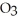 (formula mass = 122.6 amu)takes part in this reaction?
(formula mass = 122.6 amu)takes part in this reaction?
A)32.00 grams
B)48.00 grams
C)96.00 grams
D)More information is needed.
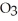 (s)→ 2 KCl (s)+ 3
(s)→ 2 KCl (s)+ 3 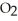 (g)
(g)What mass of oxygen (in grams)is produced when 122.6 g of KCl
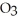 (formula mass = 122.6 amu)takes part in this reaction?
(formula mass = 122.6 amu)takes part in this reaction?A)32.00 grams
B)48.00 grams
C)96.00 grams
D)More information is needed.

Unlock Deck
Unlock for access to all 150 flashcards in this deck.
Unlock Deck
k this deck


