Deck 5: Thermochemistry
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/154
Play
Full screen (f)
Deck 5: Thermochemistry
1
Which one of the following conditions would always result in an increase in the internal energy of a system?
A)The system loses heat and does work on the surroundings.
B)The system gains heat and does work on the surroundings.
C)The system loses heat and has work done on it by the surroundings.
D)The system gains heat and has work done on it by the surroundings.
E)None of the above is correct.
A)The system loses heat and does work on the surroundings.
B)The system gains heat and does work on the surroundings.
C)The system loses heat and has work done on it by the surroundings.
D)The system gains heat and has work done on it by the surroundings.
E)None of the above is correct.
The system gains heat and has work done on it by the surroundings.
2
Of the following, which one is a state function?
A)H
B)q
C)w
D)heat
E)none of the above
A)H
B)q
C)w
D)heat
E)none of the above
H
3
Which of the following is a statement of the first law of thermodynamics?
A)Ek = m
m 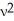
B)A negative ΔH corresponds to an exothermic process.
C)ΔE = Efinal - Einitial
D)Energy lost by the system must be gained by the surroundings.
E)1 cal = 4.184 J (exactly)
A)Ek =
 m
m 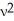
B)A negative ΔH corresponds to an exothermic process.
C)ΔE = Efinal - Einitial
D)Energy lost by the system must be gained by the surroundings.
E)1 cal = 4.184 J (exactly)
Energy lost by the system must be gained by the surroundings.
4
Under what condition(s)is the enthalpy change of a process equal to the amount of heat transferred into or out of the system? (a)temperature is constant
(b)pressure is constant
(c)volume is constant
A)a only
B)b only
C)c only
D)a and b
E)b and c
(b)pressure is constant
(c)volume is constant
A)a only
B)b only
C)c only
D)a and b
E)b and c

Unlock Deck
Unlock for access to all 154 flashcards in this deck.
Unlock Deck
k this deck
5
The internal energy can be increased by ________. (a)transferring heat from the surroundings to the system
(b)transferring heat from the system to the surroundings
(c)doing work on the system
A)a only
B)b only
C)c only
D)a and c
E)b and c
(b)transferring heat from the system to the surroundings
(c)doing work on the system
A)a only
B)b only
C)c only
D)a and c
E)b and c

Unlock Deck
Unlock for access to all 154 flashcards in this deck.
Unlock Deck
k this deck
6
Which one of the following statements is true?
A)Enthalpy is an intensive property.
B)The enthalpy change for a reaction is independent of the state of the reactants and products.
C)Enthalpy is a state function.
D)H is the value of q measured under conditions of constant volume.
E)The enthalpy change of a reaction is the reciprocal of the ΔH of the reverse reaction.
A)Enthalpy is an intensive property.
B)The enthalpy change for a reaction is independent of the state of the reactants and products.
C)Enthalpy is a state function.
D)H is the value of q measured under conditions of constant volume.
E)The enthalpy change of a reaction is the reciprocal of the ΔH of the reverse reaction.

Unlock Deck
Unlock for access to all 154 flashcards in this deck.
Unlock Deck
k this deck
7
A chemical reaction that releases heat to the surroundings is said to be ________ and has a ________ ΔH at constant pressure.
A)endothermic, positive
B)endothermic, negative
C)exothermic, negative
D)exothermic, positive
E)exothermic, neutral
A)endothermic, positive
B)endothermic, negative
C)exothermic, negative
D)exothermic, positive
E)exothermic, neutral

Unlock Deck
Unlock for access to all 154 flashcards in this deck.
Unlock Deck
k this deck
8
Which one of the following is an endothermic process?
A)ice melting
B)water freezing
C)boiling soup
D)Hydrochloric acid and barium hydroxide are mixed at 25 °C: the temperature increases.
E)Both A and C
A)ice melting
B)water freezing
C)boiling soup
D)Hydrochloric acid and barium hydroxide are mixed at 25 °C: the temperature increases.
E)Both A and C

Unlock Deck
Unlock for access to all 154 flashcards in this deck.
Unlock Deck
k this deck
9
ΔH for an endothermic process is ________ while ΔH for an exothermic process is ________.
A)zero, positive
B)zero, negative
C)positive, zero
D)negative, positive
E)positive, negative
A)zero, positive
B)zero, negative
C)positive, zero
D)negative, positive
E)positive, negative

Unlock Deck
Unlock for access to all 154 flashcards in this deck.
Unlock Deck
k this deck
10
The internal energy of a system is always increased by ________.
A)adding heat to the system
B)having the system do work on the surroundings
C)withdrawing heat from the system
D)adding heat to the system and having the system do work on the surroundings
E)a volume compression
A)adding heat to the system
B)having the system do work on the surroundings
C)withdrawing heat from the system
D)adding heat to the system and having the system do work on the surroundings
E)a volume compression

Unlock Deck
Unlock for access to all 154 flashcards in this deck.
Unlock Deck
k this deck
11
The reaction 4Al (s) + 3O2 (g) → 2Al2O3 (s) ΔH° = -3351 kJ
Is ________, and therefore heat is ________ by the reaction.
A)endothermic, released
B)endothermic, absorbed
C)exothermic, released
D)exothermic, absorbed
E)thermoneutral, neither released nor absorbed
Is ________, and therefore heat is ________ by the reaction.
A)endothermic, released
B)endothermic, absorbed
C)exothermic, released
D)exothermic, absorbed
E)thermoneutral, neither released nor absorbed

Unlock Deck
Unlock for access to all 154 flashcards in this deck.
Unlock Deck
k this deck
12
Which one of the following is an exothermic process?
A)ice melting
B)water evaporating
C)boiling soup
D)condensation of water vapor
E)Ammonium thiocyanate and barium hydroxide are mixed at 25 °C: the temperature drops.
A)ice melting
B)water evaporating
C)boiling soup
D)condensation of water vapor
E)Ammonium thiocyanate and barium hydroxide are mixed at 25 °C: the temperature drops.

Unlock Deck
Unlock for access to all 154 flashcards in this deck.
Unlock Deck
k this deck
13
For a given process at constant pressure, w is positive. This means that the process involves ________.
A)work being done by the system on the surroundings
B)work being done by the surroundings on the system
C)no work being done
D)an equal amount of work done on the system and by the system
E)work being done against a vacuum
A)work being done by the system on the surroundings
B)work being done by the surroundings on the system
C)no work being done
D)an equal amount of work done on the system and by the system
E)work being done against a vacuum

Unlock Deck
Unlock for access to all 154 flashcards in this deck.
Unlock Deck
k this deck
14
When a system ________, ΔE is always negative.
A)absorbs heat and does work
B)gives off heat and does work
C)absorbs heat and has work done on it
D)gives off heat and has work done on it
E)None of the above is always negative.
A)absorbs heat and does work
B)gives off heat and does work
C)absorbs heat and has work done on it
D)gives off heat and has work done on it
E)None of the above is always negative.

Unlock Deck
Unlock for access to all 154 flashcards in this deck.
Unlock Deck
k this deck
15
A ________ ΔH corresponds to an ________ process.
A)negative, endothermic
B)negative, exothermic
C)positive, exothermic
D)zero, exothermic
E)zero, endothermic
A)negative, endothermic
B)negative, exothermic
C)positive, exothermic
D)zero, exothermic
E)zero, endothermic

Unlock Deck
Unlock for access to all 154 flashcards in this deck.
Unlock Deck
k this deck
16
Objects can possess energy as ________. (a)endothermic energy
(b)potential energy
(c)kinetic energy
A)a only
B)b only
C)c only
D)a and c
E)b and c
(b)potential energy
(c)kinetic energy
A)a only
B)b only
C)c only
D)a and c
E)b and c

Unlock Deck
Unlock for access to all 154 flashcards in this deck.
Unlock Deck
k this deck
17
Which of the following statements is false?
A)Internal energy is a state function.
B)Enthalpy is an intensive property.
C)The enthalpy change for a reaction is equal in magnitude, but opposite in sign, to the enthalpy change for the reverse reaction.
D)The enthalpy change for a reaction depends on the state of the reactants and products.
E)The enthalpy of a reaction is equal to the heat of the reaction.
A)Internal energy is a state function.
B)Enthalpy is an intensive property.
C)The enthalpy change for a reaction is equal in magnitude, but opposite in sign, to the enthalpy change for the reverse reaction.
D)The enthalpy change for a reaction depends on the state of the reactants and products.
E)The enthalpy of a reaction is equal to the heat of the reaction.

Unlock Deck
Unlock for access to all 154 flashcards in this deck.
Unlock Deck
k this deck
18
A chemical reaction that absorbs heat from the surroundings is said to be ________ and has a ________ ΔH at constant pressure.
A)endothermic, positive
B)endothermic, negative
C)exothermic, negative
D)exothermic, positive
E)exothermic, neutral
A)endothermic, positive
B)endothermic, negative
C)exothermic, negative
D)exothermic, positive
E)exothermic, neutral

Unlock Deck
Unlock for access to all 154 flashcards in this deck.
Unlock Deck
k this deck
19
The internal energy of a system ________.
A)is the sum of the kinetic energy of all of its components
B)is the sum of the rotational, vibrational, and translational energies of all of its components
C)refers only to the energies of the nuclei of the atoms of the component molecules
D)is the sum of the potential and kinetic energies of the components
E)none of the above
A)is the sum of the kinetic energy of all of its components
B)is the sum of the rotational, vibrational, and translational energies of all of its components
C)refers only to the energies of the nuclei of the atoms of the component molecules
D)is the sum of the potential and kinetic energies of the components
E)none of the above

Unlock Deck
Unlock for access to all 154 flashcards in this deck.
Unlock Deck
k this deck
20
A ________ ΔH corresponds to an ________ process.
A)negative, endothermic
B)positive, exothermic
C)positive, endothermic
D)zero, exothermic
E)zero, endothermic
A)negative, endothermic
B)positive, exothermic
C)positive, endothermic
D)zero, exothermic
E)zero, endothermic

Unlock Deck
Unlock for access to all 154 flashcards in this deck.
Unlock Deck
k this deck
21
Of the following, ΔH°f is not zero for ________.
A)O2 (g)
B)C (graphite)
C)N2 (g)
D)F2 (s)
E)Cl2 (g)
A)O2 (g)
B)C (graphite)
C)N2 (g)
D)F2 (s)
E)Cl2 (g)

Unlock Deck
Unlock for access to all 154 flashcards in this deck.
Unlock Deck
k this deck
22
For which one of the following reactions is the value of ΔH°rxn equal to ΔH°f for the product?
A)2Ca (s) + O2 (g) → 2CaO (s)
B)C2H2 (g) + H2 (g) → C2H4 (g)
C)2C (graphite) + O2 (g) → 2CO (g)
D)3Mg (s) + N2 (g) → Mg3N2 (s)
E)C (diamond) + O2 (g) → CO2 (g)
A)2Ca (s) + O2 (g) → 2CaO (s)
B)C2H2 (g) + H2 (g) → C2H4 (g)
C)2C (graphite) + O2 (g) → 2CO (g)
D)3Mg (s) + N2 (g) → Mg3N2 (s)
E)C (diamond) + O2 (g) → CO2 (g)

Unlock Deck
Unlock for access to all 154 flashcards in this deck.
Unlock Deck
k this deck
23
In the reaction below, ΔH°f is zero for ________. Ni (s) + 2CO (g) + 2PF3 (g) → Ni(CO)2(PF3)2 (l)
A)Ni (s)
B)CO (g)
C)F3 (g)
D)Ni(CO)2(PF3)2 (l)
E)both CO (g)and PF3 (g)
A)Ni (s)
B)CO (g)
C)F3 (g)
D)Ni(CO)2(PF3)2 (l)
E)both CO (g)and PF3 (g)

Unlock Deck
Unlock for access to all 154 flashcards in this deck.
Unlock Deck
k this deck
24
For the species in the reaction below, ΔH°f is zero for ________. 2Co (s) + H2 (g) + 8PF3 (g) → 2HCo(PF3)4 (l)
A)Co (s)
B)H2 (g)
C)PF3 (g)
D)HCo(PF3)4 (l)
E)both Co(s)and H2 (g)
A)Co (s)
B)H2 (g)
C)PF3 (g)
D)HCo(PF3)4 (l)
E)both Co(s)and H2 (g)

Unlock Deck
Unlock for access to all 154 flashcards in this deck.
Unlock Deck
k this deck
25
For which one of the following reactions is the value of ΔH°rxn equal to ΔH°f for the product?
A)H2O (l) + 1/2 O2 (g) → H2O2 (l)
B)N2 (g) + O2 (g) → 2NO (g)
C)2H2 (g) + O2 (g) → 2H2O (l)
D)2H2 (g) + O2 (g) → 2H2O (g)
E)none of the above
A)H2O (l) + 1/2 O2 (g) → H2O2 (l)
B)N2 (g) + O2 (g) → 2NO (g)
C)2H2 (g) + O2 (g) → 2H2O (l)
D)2H2 (g) + O2 (g) → 2H2O (g)
E)none of the above

Unlock Deck
Unlock for access to all 154 flashcards in this deck.
Unlock Deck
k this deck
26
The units of specific heat are ________.
A)K/J or °C/J
B)J/K or J/°C
C)J/g-K or J/g-°C
D)J/mol
E)g-K/J or g-°C/J
A)K/J or °C/J
B)J/K or J/°C
C)J/g-K or J/g-°C
D)J/mol
E)g-K/J or g-°C/J

Unlock Deck
Unlock for access to all 154 flashcards in this deck.
Unlock Deck
k this deck
27
The units of heat capacity are ________.
A)K/J or °C/J
B)J/K or J/°C
C)J/g-K or J/g-°C
D)J/mol
E)g-K/J or g-°C/J
A)K/J or °C/J
B)J/K or J/°C
C)J/g-K or J/g-°C
D)J/mol
E)g-K/J or g-°C/J

Unlock Deck
Unlock for access to all 154 flashcards in this deck.
Unlock Deck
k this deck
28
Fuel values of hydrocarbons increase as the H/C atomic ratio increases. Which of the following compounds has the highest fuel value?
A)C2H6
B)C2H4
C)C2H2
D)CH4
E)C6H6
A)C2H6
B)C2H4
C)C2H2
D)CH4
E)C6H6

Unlock Deck
Unlock for access to all 154 flashcards in this deck.
Unlock Deck
k this deck
29
The energy released by combustion of 1 g of a substance is called the ________ of the substance.
A)specific heat
B)fuel value
C)nutritional calorie content
D)heat capacity
E)enthalpy
A)specific heat
B)fuel value
C)nutritional calorie content
D)heat capacity
E)enthalpy

Unlock Deck
Unlock for access to all 154 flashcards in this deck.
Unlock Deck
k this deck
30
With reference to enthalpy changes, the term standard conditions means ________. (a)P = 1 atm
(b)some common temperature, usually 298 K
(c)V = 1 L
A)a only
B)b only
C)c only
D)a and c
E)a and b
(b)some common temperature, usually 298 K
(c)V = 1 L
A)a only
B)b only
C)c only
D)a and c
E)a and b

Unlock Deck
Unlock for access to all 154 flashcards in this deck.
Unlock Deck
k this deck
31
For which one of the following equations is ΔH°rxn equal to ΔH°f for the product?
A)Xe (g) + 2F2 (g) → XeF4 (g)
B)CH4 (g) + 2Cl2 (g) → CH2Cl2 (l) + 2HCl (g)
C)N2 (g) + O3 (g) → N2O3 (g)
D)2CO (g) + O2 (g) → 2CO2 (g)
E)C (diamond) + O2 (g) → CO2 (g)
A)Xe (g) + 2F2 (g) → XeF4 (g)
B)CH4 (g) + 2Cl2 (g) → CH2Cl2 (l) + 2HCl (g)
C)N2 (g) + O3 (g) → N2O3 (g)
D)2CO (g) + O2 (g) → 2CO2 (g)
E)C (diamond) + O2 (g) → CO2 (g)

Unlock Deck
Unlock for access to all 154 flashcards in this deck.
Unlock Deck
k this deck
32
Which of the following is a statement of Hess's law?
A)If a reaction is carried out in a series of steps, the ΔH for the reaction will equal the sum of the enthalpy changes for the individual steps.
B)If a reaction is carried out in a series of steps, the ΔH for the reaction will equal the product of the enthalpy changes for the individual steps.
C)The ΔH for a process in the forward direction is equal in magnitude and opposite in sign to the ΔH for the process in the reverse direction.
D)The ΔH for a process in the forward direction is equal to the ΔH for the process in the reverse direction.
E)The ΔH of a reaction depends on the physical states of the reactants and products.
A)If a reaction is carried out in a series of steps, the ΔH for the reaction will equal the sum of the enthalpy changes for the individual steps.
B)If a reaction is carried out in a series of steps, the ΔH for the reaction will equal the product of the enthalpy changes for the individual steps.
C)The ΔH for a process in the forward direction is equal in magnitude and opposite in sign to the ΔH for the process in the reverse direction.
D)The ΔH for a process in the forward direction is equal to the ΔH for the process in the reverse direction.
E)The ΔH of a reaction depends on the physical states of the reactants and products.

Unlock Deck
Unlock for access to all 154 flashcards in this deck.
Unlock Deck
k this deck
33
Consider the following two reactions: A → 2B ΔH°rxn = 456.7 kJ/mol
A → C ΔH°rxn = -22.1 kJ/mol
Determine the enthalpy change for the process:
2B → C
A)-478.8 kJ/mol
B)-434.6 kJ/mol
C)434.6 kJ/mol
D)478.8 kJ/mol
E)More information is needed to solve the problem.
A → C ΔH°rxn = -22.1 kJ/mol
Determine the enthalpy change for the process:
2B → C
A)-478.8 kJ/mol
B)-434.6 kJ/mol
C)434.6 kJ/mol
D)478.8 kJ/mol
E)More information is needed to solve the problem.

Unlock Deck
Unlock for access to all 154 flashcards in this deck.
Unlock Deck
k this deck
34
The British thermal unit (Btu)is commonly used in engineering applications. A Btu is defined as the amount of heat required to raise the temperature of 1 lb of water by 1 °F. There are ________ Btu in one Joule. 1 lb = 453.59 g; °C = (5/9)(°F - 32°); specific heat of H2O (l)= 4.184 J/g-K.
A)1056 Btu
B)1.896 × 10-3 Btu
C)9.278 × 10-4 Btu
D)5.120 × 10-3 Btu
E)Additional information is needed to complete the calculation.
A)1056 Btu
B)1.896 × 10-3 Btu
C)9.278 × 10-4 Btu
D)5.120 × 10-3 Btu
E)Additional information is needed to complete the calculation.

Unlock Deck
Unlock for access to all 154 flashcards in this deck.
Unlock Deck
k this deck
35
Of the following, ΔH°f is not zero for ________.
A)Sc (g)
B)Si (s)
C)P4 (s, white)
D)Br2 (l)
E)Ca (s)
A)Sc (g)
B)Si (s)
C)P4 (s, white)
D)Br2 (l)
E)Ca (s)

Unlock Deck
Unlock for access to all 154 flashcards in this deck.
Unlock Deck
k this deck
36
Of the substances below, the highest fuel value is obtained from ________.
A)charcoal
B)bituminous coal
C)natural gas
D)hydrogen
E)wood
A)charcoal
B)bituminous coal
C)natural gas
D)hydrogen
E)wood

Unlock Deck
Unlock for access to all 154 flashcards in this deck.
Unlock Deck
k this deck
37
Which one of the choices below is not considered a fossil fuel?
A)anthracite coal
B)crude oil
C)natural gas
D)hydrogen
E)petroleum
A)anthracite coal
B)crude oil
C)natural gas
D)hydrogen
E)petroleum

Unlock Deck
Unlock for access to all 154 flashcards in this deck.
Unlock Deck
k this deck
38
For which one of the following reactions is ΔH°rxn equal to the heat of formation of the product?
A)N2 (g) + 3H2 (g) → 2NH3 (g)
B)(1/2)N2 (g) + O2 (g) → NO2 (g)
C)6C (s) + 6H (g) → C6H6 (l)
D)P (g) + 4H (g) + Br (g) → PH4Br (l)
E)12C (g) + 11H2 (g) + 11O (g) → C6H22O11 (g)
A)N2 (g) + 3H2 (g) → 2NH3 (g)
B)(1/2)N2 (g) + O2 (g) → NO2 (g)
C)6C (s) + 6H (g) → C6H6 (l)
D)P (g) + 4H (g) + Br (g) → PH4Br (l)
E)12C (g) + 11H2 (g) + 11O (g) → C6H22O11 (g)

Unlock Deck
Unlock for access to all 154 flashcards in this deck.
Unlock Deck
k this deck
39
For which one of the following reactions is the value of ΔH°rxn equal to ΔH°f for the product?
A)H2 (g) + 1/2 O2 (g) → H2O (l)
B)H2 (g) + O2 (g) → H2O2 (l)
C)2C (s, graphite) + 2H2 (g) → C2H4 (g)
D)1/2 N2 (g) + O2 (g) → NO2 (g)
E)all of the above
A)H2 (g) + 1/2 O2 (g) → H2O (l)
B)H2 (g) + O2 (g) → H2O2 (l)
C)2C (s, graphite) + 2H2 (g) → C2H4 (g)
D)1/2 N2 (g) + O2 (g) → NO2 (g)
E)all of the above

Unlock Deck
Unlock for access to all 154 flashcards in this deck.
Unlock Deck
k this deck
40
For which one of the following reactions is the value of ΔH°rxn equal to ΔH°f for the product?
A)2C (s, graphite) + 2H2 (g) → C2H4 (g)
B)N2 (g) + O2 (g) → 2NO (g)
C)2H2 (g) + O2 (g) → 2H2O (l)
D)2H2 (g) + O2 (g) → 2H2O (g)
E)H2O (l) + 1/2 O2 (g) → H2O2 (l)
A)2C (s, graphite) + 2H2 (g) → C2H4 (g)
B)N2 (g) + O2 (g) → 2NO (g)
C)2H2 (g) + O2 (g) → 2H2O (l)
D)2H2 (g) + O2 (g) → 2H2O (g)
E)H2O (l) + 1/2 O2 (g) → H2O2 (l)

Unlock Deck
Unlock for access to all 154 flashcards in this deck.
Unlock Deck
k this deck
41
Calculate the kinetic energy in joules of an 80.0 g bullet traveling at 300.0 m/s.
A)3.60 × 106 J
B)1.20 × 104 J
C)3.60 × 103 J
D)12.0 J
E)80.0 J
A)3.60 × 106 J
B)1.20 × 104 J
C)3.60 × 103 J
D)12.0 J
E)80.0 J

Unlock Deck
Unlock for access to all 154 flashcards in this deck.
Unlock Deck
k this deck
42
The ΔE of a system that absorbs 12.4 J of heat and does 4.2 J of work on the surroundings is ________ J.
A)16.6
B)12.4
C)4.2
D)-16.6
E)8.2
A)16.6
B)12.4
C)4.2
D)-16.6
E)8.2

Unlock Deck
Unlock for access to all 154 flashcards in this deck.
Unlock Deck
k this deck
43
Calculate the kinetic energy in J of an electron moving at 6.00 × 106 m/s. The mass of an electron is 9.11 × 10-28 g.
A)4.98 × 10-48 J
B)3.28 × 10-14 J
C)1.64 × 10-17 J
D)2.49 × 10-48 J
E)6.56 × 10-14 J
A)4.98 × 10-48 J
B)3.28 × 10-14 J
C)1.64 × 10-17 J
D)2.49 × 10-48 J
E)6.56 × 10-14 J

Unlock Deck
Unlock for access to all 154 flashcards in this deck.
Unlock Deck
k this deck
44
Calculate the kinetic energy in joules of an automobile weighing 4345 lb and traveling at 75 mph. (1 mile = 1.6093 km, 1lb = 453.59 g).
A)5.5 × 105 J
B)5.5 × 10-5 J
C)1.1 × 106 J
D)2.2 × 106 J
E)2.2 × 10-6 J
A)5.5 × 105 J
B)5.5 × 10-5 J
C)1.1 × 106 J
D)2.2 × 106 J
E)2.2 × 10-6 J

Unlock Deck
Unlock for access to all 154 flashcards in this deck.
Unlock Deck
k this deck
45
The kinetic energy of a 23.2-g object moving at a speed of 81.9 km/hr is ________ kcal.
A)1.43 × 10-3
B)6.00
C)1900
D)454
E)0.0251
A)1.43 × 10-3
B)6.00
C)1900
D)454
E)0.0251

Unlock Deck
Unlock for access to all 154 flashcards in this deck.
Unlock Deck
k this deck
46
The value of ΔH° for the reaction below is -6535 kJ. ________ kJ of heat are released in the combustion of 16.0 g of C6H6 (l)? 2C6H6 (l) + 15O2 (g) → 12CO2 (g) + 6 H2O (l)
A)1.34 × 103
B)5.23 × 104
C)669
D)2.68 × 103
E)-6535
A)1.34 × 103
B)5.23 × 104
C)669
D)2.68 × 103
E)-6535

Unlock Deck
Unlock for access to all 154 flashcards in this deck.
Unlock Deck
k this deck
47
The value of ΔH° for the reaction below is -482 kJ. Calculate the heat (kJ)released to the surroundings when 38.5 g of O2 (g)reacts with excess CO. 2CO (g) + O2 (g) → 2CO2 (g)
A)2.65 × 103 kJ
B)482 kJ
C)580. kJ
D)65.7 kJ
E)210. kJ
A)2.65 × 103 kJ
B)482 kJ
C)580. kJ
D)65.7 kJ
E)210. kJ

Unlock Deck
Unlock for access to all 154 flashcards in this deck.
Unlock Deck
k this deck
48
Calculate the kinetic energy in joules of an automobile weighing 2135 lb and traveling at 55 mph. (1 mile = 1.6093 km, 1lb = 453.59 g).
A)1.2 × 104 J
B)2.9 × 105 J
C)5.9 × 105 J
D)3.2 × 106 J
E)3.2 × 10-6 J
A)1.2 × 104 J
B)2.9 × 105 J
C)5.9 × 105 J
D)3.2 × 106 J
E)3.2 × 10-6 J

Unlock Deck
Unlock for access to all 154 flashcards in this deck.
Unlock Deck
k this deck
49
The value of ΔH° for the reaction below is -790 kJ. The enthalpy change accompanying the reaction of 0.95 g of S is ________ kJ. 2S (s) + 3O2 (g) → 2SO3 (g)
A)23
B)-23
C)-12
D)12
E)-790
A)23
B)-23
C)-12
D)12
E)-790

Unlock Deck
Unlock for access to all 154 flashcards in this deck.
Unlock Deck
k this deck
50
The most abundant fossil fuel is ________.
A)natural gas
B)petroleum
C)coal
D)uranium
E)hydrogen
A)natural gas
B)petroleum
C)coal
D)uranium
E)hydrogen

Unlock Deck
Unlock for access to all 154 flashcards in this deck.
Unlock Deck
k this deck
51
A 100-watt electric incandescent light bulb consumes ________ J of energy in 24 hours. [1 Watt (W)= 1 J/sec]
A)2.40 × 103
B)8.64 × 103
C)4.17
D)2.10 × 103
E) 8.64 × 106
A)2.40 × 103
B)8.64 × 103
C)4.17
D)2.10 × 103
E) 8.64 × 106

Unlock Deck
Unlock for access to all 154 flashcards in this deck.
Unlock Deck
k this deck
52
Calculate the kinetic energy in joules of a 150 lb jogger (68.1 kg)traveling at 12.0 mile/hr (5.36 m/s).
A)1.96 × 103 J
B)365 J
C)978 J
D)183 J
E)68.1 J
A)1.96 × 103 J
B)365 J
C)978 J
D)183 J
E)68.1 J

Unlock Deck
Unlock for access to all 154 flashcards in this deck.
Unlock Deck
k this deck
53
The kinetic energy of a 10.3 g golf ball traveling at 48.0 m/s is ________ J.
A)1.20 × 103
B)66
C)11.9
D)1.3 × 102
E)23.7
A)1.20 × 103
B)66
C)11.9
D)1.3 × 102
E)23.7

Unlock Deck
Unlock for access to all 154 flashcards in this deck.
Unlock Deck
k this deck
54
The kinetic energy of a 7.3 kg steel ball traveling at 18.0 m/s is ________ J.
A)1.2 × 103
B)66
C)2.4 × 103
D)1.3 × 102
E)7.3
A)1.2 × 103
B)66
C)2.4 × 103
D)1.3 × 102
E)7.3

Unlock Deck
Unlock for access to all 154 flashcards in this deck.
Unlock Deck
k this deck
55
The change in the internal energy of a system that releases 2,500 J of heat and that does 7,655 J of work on the surroundings is ________ J.
A)-10,155
B)-5,155
C)-1.91 × 107
D)10,155
E)5,155
A)-10,155
B)-5,155
C)-1.91 × 107
D)10,155
E)5,155

Unlock Deck
Unlock for access to all 154 flashcards in this deck.
Unlock Deck
k this deck
56
The value of ΔH° for the reaction below is -126 kJ. ________ kj are released when 2.00 mol of NaOH is formed in the reaction? 2Na2O2 (s) + 2H2O (l) → 4NaOH (s) + O2 (g)
A)252
B)63
C)3.9
D)7.8
E)-126
A)252
B)63
C)3.9
D)7.8
E)-126

Unlock Deck
Unlock for access to all 154 flashcards in this deck.
Unlock Deck
k this deck
57
The value of ΔH° for the reaction below is -72 kJ. ________ kJ of heat are released when 80.9 grams of HBr is formed in this reaction. H2 (g) + Br2 (g) → 2HBr (g)
A)144
B)72
C)0.44
D)36
E)-72
A)144
B)72
C)0.44
D)36
E)-72

Unlock Deck
Unlock for access to all 154 flashcards in this deck.
Unlock Deck
k this deck
58
The ΔE of a system that releases 12.4 J of heat and does 4.2 J of work on the surroundings is ________ J.
A)16.6
B)12.4
C)4.2
D)-16.6
E)-8.2
A)16.6
B)12.4
C)4.2
D)-16.6
E)-8.2

Unlock Deck
Unlock for access to all 154 flashcards in this deck.
Unlock Deck
k this deck
59
The kinetic energy of a 23.2-g object moving at a speed of 81.9 km/hr is ________ J.
A)1900
B)77.8
C)145
D)1.43 × 10-3
E)6.00
A)1900
B)77.8
C)145
D)1.43 × 10-3
E)6.00

Unlock Deck
Unlock for access to all 154 flashcards in this deck.
Unlock Deck
k this deck
60
The change in the internal energy of a system that absorbs 2,500 J of heat and that does 7,655 J of work on the surroundings is ________ J.
A)10,155
B)5,155
C)-5,155
D)-10,155
E)1.91 ×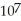
A)10,155
B)5,155
C)-5,155
D)-10,155
E)1.91 ×

Unlock Deck
Unlock for access to all 154 flashcards in this deck.
Unlock Deck
k this deck
61
The specific heat of bromine liquid is 0.226 J/g-K. The molar heat capacity (in J/mol-K)of bromine liquid is ________.
A)707 J/mol-K
B)36.1 J/mol-K
C)18.1 J/mol-K
D)9.05 J/mol-K
E)0.226 J/mol-K
A)707 J/mol-K
B)36.1 J/mol-K
C)18.1 J/mol-K
D)9.05 J/mol-K
E)0.226 J/mol-K

Unlock Deck
Unlock for access to all 154 flashcards in this deck.
Unlock Deck
k this deck
62
The value of ΔH° for the reaction below is -1107 kJ: 2Ba (s) + O2 (g) → 2BaO (s)
How many kJ of heat are released when 5.75 g of Ba (s)reacts completely with oxygen to form BaO (s)?
A)96.3 kJ
B)26.3 kJ
C)46.4 kJ
D)23.2 kJ
E)193 kJ
How many kJ of heat are released when 5.75 g of Ba (s)reacts completely with oxygen to form BaO (s)?
A)96.3 kJ
B)26.3 kJ
C)46.4 kJ
D)23.2 kJ
E)193 kJ

Unlock Deck
Unlock for access to all 154 flashcards in this deck.
Unlock Deck
k this deck
63
The specific heat of liquid bromine is 0.226 J/g-K. How much heat (J)is required to raise the temperature of 10.0 mL of bromine from 25.00 °C to 27.30 °C? The density of liquid bromine: 3.12 g/mL.
A)5.20 J
B)16.2 J
C)300 J
D)32.4 J
E)10.4 J
A)5.20 J
B)16.2 J
C)300 J
D)32.4 J
E)10.4 J

Unlock Deck
Unlock for access to all 154 flashcards in this deck.
Unlock Deck
k this deck
64
The value of ΔH° for the reaction below is -1107 kJ: 2Ba (s) + O2 (g) → 2BaO (s)
How many kJ of heat are released when 5.75 g of BaO (s)is produced?
A)56.9 kJ
B)23.2 kJ
C)20.8 kJ
D)193 kJ
E)96.3 kJ
How many kJ of heat are released when 5.75 g of BaO (s)is produced?
A)56.9 kJ
B)23.2 kJ
C)20.8 kJ
D)193 kJ
E)96.3 kJ

Unlock Deck
Unlock for access to all 154 flashcards in this deck.
Unlock Deck
k this deck
65
The value of ΔH° for the reaction below is +128.1 kJ: CH3OH (l) → CO (g) + 2H2 (g)
How many kJ of heat are consumed when 5.10 g of CO (g)is formed as shown in the equation?
A)0.182 kJ
B)162 kJ
C)8.31 kJ
D)23.3 kJ
E)62.0 kJ
How many kJ of heat are consumed when 5.10 g of CO (g)is formed as shown in the equation?
A)0.182 kJ
B)162 kJ
C)8.31 kJ
D)23.3 kJ
E)62.0 kJ

Unlock Deck
Unlock for access to all 154 flashcards in this deck.
Unlock Deck
k this deck
66
The value of ΔH° for the reaction below is -336 kJ. Calculate the heat (kJ)released to the surroundings when 23.0 g of HCl is formed. CH4 (g) + 3Cl2 (g) → CHCl3 (l) + 3HCl (g)
A)177 kJ
B)2.57 × 103 kJ
C)70.7 kJ
D)211 kJ
E)-336 kJ
A)177 kJ
B)2.57 × 103 kJ
C)70.7 kJ
D)211 kJ
E)-336 kJ

Unlock Deck
Unlock for access to all 154 flashcards in this deck.
Unlock Deck
k this deck
67
The enthalpy change for the following reaction is -483.6 kJ: 2H2 (g) + O2 (g) → 2H2O (g)
Therefore, the enthalpy change for the following reaction is ________ kJ.
4H2 (g) + 2O2 (g) → 4H2O (g)
A)-483.6
B)-967.2
C)2.34 × 105
D)483.6
E)967.2
Therefore, the enthalpy change for the following reaction is ________ kJ.
4H2 (g) + 2O2 (g) → 4H2O (g)
A)-483.6
B)-967.2
C)2.34 × 105
D)483.6
E)967.2

Unlock Deck
Unlock for access to all 154 flashcards in this deck.
Unlock Deck
k this deck
68
The value of ΔH° for the reaction below is +128.1 kJ: CH3OH (l) → CO (g) + 2H2 (g)
How many kJ of heat are consumed when 5.10 g of H2 (g)is formed as shown in the equation?
A)162 kJ
B)62.0 kJ
C)128 kJ
D)653 kJ
E)326 kJ
How many kJ of heat are consumed when 5.10 g of H2 (g)is formed as shown in the equation?
A)162 kJ
B)62.0 kJ
C)128 kJ
D)653 kJ
E)326 kJ

Unlock Deck
Unlock for access to all 154 flashcards in this deck.
Unlock Deck
k this deck
69
The value of ΔH° for the reaction below is +128.1 kJ: CH3OH (l) → CO (g) + 2H2 (g)
How many kJ of heat are consumed when 5.75 g of CO (g)is formed as shown in the equation?
A)23.3 kJ
B)62.0 kJ
C)26.3 kJ
D)162 kJ
E)8.3 kJ
How many kJ of heat are consumed when 5.75 g of CO (g)is formed as shown in the equation?
A)23.3 kJ
B)62.0 kJ
C)26.3 kJ
D)162 kJ
E)8.3 kJ

Unlock Deck
Unlock for access to all 154 flashcards in this deck.
Unlock Deck
k this deck
70
Given the following reactions N2 (g) + 2O2 (g) → 2NO2 (g)ΔH = 66.4 kJ
2NO (g) + O2 (g) → 2NO2 (g) ΔH = -114.2 kJ
The enthalpy of the reaction of the nitrogen to produce nitric oxide
N2 (g) + O2 (g) → 2NO (g)
Is ________ kJ.
A)180.6
B)-47.8
C)47.8
D)90.3
E)-180.6
2NO (g) + O2 (g) → 2NO2 (g) ΔH = -114.2 kJ
The enthalpy of the reaction of the nitrogen to produce nitric oxide
N2 (g) + O2 (g) → 2NO (g)
Is ________ kJ.
A)180.6
B)-47.8
C)47.8
D)90.3
E)-180.6

Unlock Deck
Unlock for access to all 154 flashcards in this deck.
Unlock Deck
k this deck
71
Given the following reactions Fe2O3 (s) + 3CO (s) → 2Fe (s)+ 3CO2 (g)ΔH = -28.0 kJ
3Fe (s) + 4CO2 (s) → 4CO (g) + Fe3O4 (s)ΔH = +12.5 kJ
The enthalpy of the reaction of Fe2O3 with CO
3Fe2O3 (s) + CO (g) → CO2 (g) + 2 Fe3O4 (s)
Is ________ kJ.
A)-59.0
B)40.5
C)-15.5
D)-109
E)+109
3Fe (s) + 4CO2 (s) → 4CO (g) + Fe3O4 (s)ΔH = +12.5 kJ
The enthalpy of the reaction of Fe2O3 with CO
3Fe2O3 (s) + CO (g) → CO2 (g) + 2 Fe3O4 (s)
Is ________ kJ.
A)-59.0
B)40.5
C)-15.5
D)-109
E)+109

Unlock Deck
Unlock for access to all 154 flashcards in this deck.
Unlock Deck
k this deck
72
The value of ΔH° for the reaction below is +128.1 kJ: CH3OH (l) → CO (g) + 2H2 (g)
How many kJ of heat are consumed when 15.5 g of CH3OH (l)decomposes as shown in the equation?
A)0.48 kJ
B)62.0 kJ
C)1.3 × 102 kJ
D)32 kJ
E)8.3 kJ
How many kJ of heat are consumed when 15.5 g of CH3OH (l)decomposes as shown in the equation?
A)0.48 kJ
B)62.0 kJ
C)1.3 × 102 kJ
D)32 kJ
E)8.3 kJ

Unlock Deck
Unlock for access to all 154 flashcards in this deck.
Unlock Deck
k this deck
73
ΔH for the reaction IF5 (g) → IF3 (g) + F2 (g)
Is ________ kJ, give the data below.
IF (g) + F2 (g) → IF3 (g)ΔH = -390 kJ
IF (g) + 2F2 (g) → IF5 (g)ΔH = -745 kJ
A)+355
B)-1135
C)+1135
D)+35
E)-35
Is ________ kJ, give the data below.
IF (g) + F2 (g) → IF3 (g)ΔH = -390 kJ
IF (g) + 2F2 (g) → IF5 (g)ΔH = -745 kJ
A)+355
B)-1135
C)+1135
D)+35
E)-35

Unlock Deck
Unlock for access to all 154 flashcards in this deck.
Unlock Deck
k this deck
74
The molar heat capacity of a compound with the formula C2H6 SO is 88.0 J/mol-K. The specific heat of this substance is ________ J/g-K.
A)88.0
B)1.13
C)4.89
D)6.88 × 103
E)-88.0
A)88.0
B)1.13
C)4.89
D)6.88 × 103
E)-88.0

Unlock Deck
Unlock for access to all 154 flashcards in this deck.
Unlock Deck
k this deck
75
The value of ΔH° for the reaction below is -186 kJ. Calculate the heat (kJ)released from the reaction of 25 g of Cl2. H2 (g) + Cl2 (g) → 2HCl (g)
A)66 kJ
B)5.3 × 102 kJ
C)33 kJ
D)47 kJ
E)-186 kJ
A)66 kJ
B)5.3 × 102 kJ
C)33 kJ
D)47 kJ
E)-186 kJ

Unlock Deck
Unlock for access to all 154 flashcards in this deck.
Unlock Deck
k this deck
76
Given the following reactions 2NO → N2 + O2 ΔH = -180 kJ
2NO + O2 → 2NO2 ΔH = -112 kJ
The enthalpy of the reaction of nitrogen with oxygen to produce nitrogen dioxide
N2 + 2O2 → 2NO2
Is ________ kJ.
A)68
B)-68
C)-292
D)292
E)-146
2NO + O2 → 2NO2 ΔH = -112 kJ
The enthalpy of the reaction of nitrogen with oxygen to produce nitrogen dioxide
N2 + 2O2 → 2NO2
Is ________ kJ.
A)68
B)-68
C)-292
D)292
E)-146

Unlock Deck
Unlock for access to all 154 flashcards in this deck.
Unlock Deck
k this deck
77
The temperature of a 15-g sample of lead metal increases from 22 °C to 37 °C upon the addition of 29.0 J of heat. The specific heat capacity of the lead is ________ J/g-K.
A)7.8
B)1.9
C)29
D)0.13
E)-29
A)7.8
B)1.9
C)29
D)0.13
E)-29

Unlock Deck
Unlock for access to all 154 flashcards in this deck.
Unlock Deck
k this deck
78
The specific heat capacity of lead is 0.13 J/g-K. How much heat (in J)is required to raise the temperature of 15 g of lead from 22 °C to 37 °C?
A)2.0 J
B)-0.13 J
C)5.8 × 10-4 J
D)29 J
E)0.13 J
A)2.0 J
B)-0.13 J
C)5.8 × 10-4 J
D)29 J
E)0.13 J

Unlock Deck
Unlock for access to all 154 flashcards in this deck.
Unlock Deck
k this deck
79
Given the following reactions 2S (s) + 3O2 (g) → 2SO3 (g) ΔH = -790 kJ
S (s) + O2 (g) → SO2 (g)ΔH = -297 kJ
The enthalpy of the reaction in which sulfur dioxide is oxidized to sulfur trioxide
2SO2 (g) + O2 (g) → 2SO3 (g)
Is ________ kJ.
A)196
B)-196
C)1087
D)-1384
E)-543
S (s) + O2 (g) → SO2 (g)ΔH = -297 kJ
The enthalpy of the reaction in which sulfur dioxide is oxidized to sulfur trioxide
2SO2 (g) + O2 (g) → 2SO3 (g)
Is ________ kJ.
A)196
B)-196
C)1087
D)-1384
E)-543

Unlock Deck
Unlock for access to all 154 flashcards in this deck.
Unlock Deck
k this deck
80
The value of ΔH° for the reaction below is -1107 kJ: 2Ba (s) + O2 (g) → 2BaO (s)
How many kJ of heat are released when 15.75 g of Ba (s)reacts completely with oxygen to form BaO (s)?
A)20.8 kJ
B)63.5 kJ
C)114 kJ
D)70.3 kJ
E)35.1 kJ
How many kJ of heat are released when 15.75 g of Ba (s)reacts completely with oxygen to form BaO (s)?
A)20.8 kJ
B)63.5 kJ
C)114 kJ
D)70.3 kJ
E)35.1 kJ

Unlock Deck
Unlock for access to all 154 flashcards in this deck.
Unlock Deck
k this deck


