Deck 10: Gases
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/175
Play
Full screen (f)
Deck 10: Gases
1
Which of the following statements about gases is false?
A)Gases are highly compressible.
B)Distances between molecules of gas are very large compared to bond distances within molecules.
C)Non-reacting gas mixtures are homogeneous.
D)Gases expand spontaneously to fill the container they are placed in.
E)All gases are colorless and odorless at room temperature.
A)Gases are highly compressible.
B)Distances between molecules of gas are very large compared to bond distances within molecules.
C)Non-reacting gas mixtures are homogeneous.
D)Gases expand spontaneously to fill the container they are placed in.
E)All gases are colorless and odorless at room temperature.
All gases are colorless and odorless at room temperature.
2
Which of the following equations shows an incorrect relationship between pressures given in terms of different units?
A)1.20 atm = 122 kPa
B)152 mm Hg = 2.03 × 104 Pa
C)0.760 atm = 578 mm Hg
D)1.0 torr = 2.00 mm Hg
E)1.00 atm = 760 torr
A)1.20 atm = 122 kPa
B)152 mm Hg = 2.03 × 104 Pa
C)0.760 atm = 578 mm Hg
D)1.0 torr = 2.00 mm Hg
E)1.00 atm = 760 torr
1.0 torr = 2.00 mm Hg
3
Of the following, ________ has a slight odor of bitter almonds and is toxic.
A)NH3
B)N2O
C)CO
D)CH4
E)HCN
A)NH3
B)N2O
C)CO
D)CH4
E)HCN
HCN
4
Gaseous mixtures ________.
A)can only contain molecules
B)are all heterogeneous
C)can only contain isolated atoms
D)are all homogeneous
E)must contain both isolated atoms and molecules
A)can only contain molecules
B)are all heterogeneous
C)can only contain isolated atoms
D)are all homogeneous
E)must contain both isolated atoms and molecules

Unlock Deck
Unlock for access to all 175 flashcards in this deck.
Unlock Deck
k this deck
5
The first person to investigate the relationship between the pressure of a gas and its volume was ________.
A)Amadeo Avogadro
B)Lord Kelvin
C)Jacques Charles
D)Robert Boyle
E)Joseph Louis Gay-Lussac
A)Amadeo Avogadro
B)Lord Kelvin
C)Jacques Charles
D)Robert Boyle
E)Joseph Louis Gay-Lussac

Unlock Deck
Unlock for access to all 175 flashcards in this deck.
Unlock Deck
k this deck
6
Which statement about atmospheric pressure is false?
A)As air becomes thinner, its density decreases.
B)Air actually has weight.
C)With an increase in altitude, atmospheric pressure increases as well.
D)The warmer the air, the lower the atmospheric pressure.
E)Atmospheric pressure prevents water in lakes, rivers, and oceans from boiling away.
A)As air becomes thinner, its density decreases.
B)Air actually has weight.
C)With an increase in altitude, atmospheric pressure increases as well.
D)The warmer the air, the lower the atmospheric pressure.
E)Atmospheric pressure prevents water in lakes, rivers, and oceans from boiling away.

Unlock Deck
Unlock for access to all 175 flashcards in this deck.
Unlock Deck
k this deck
7
In ideal gas equation calculations, expressing pressure in Pascals (Pa), necessitates the use of the gas constant, R, equal to ________.
A)0.08206 atm L mol-1K-1
B)8.314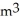 -Pa/mol-K
-Pa/mol-K
C)62.36 L torr mol-1K-1
D)1.987 cal mol-1K-1
E)none of the above
A)0.08206 atm L mol-1K-1
B)8.314
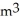 -Pa/mol-K
-Pa/mol-KC)62.36 L torr mol-1K-1
D)1.987 cal mol-1K-1
E)none of the above

Unlock Deck
Unlock for access to all 175 flashcards in this deck.
Unlock Deck
k this deck
8
The pressure exerted by a column of liquid is equal to the product of the height of the column times the gravitational constant times the density of the liquid, P = ghd. How high a column of water (d = 1.0 g/mL)would be supported by a pressure that supports a 713 mm column of mercury (d = 13.6 g/mL)?
A)14 mm
B)52 mm
C)713 mm
D)1.2 × 104 mm
E)9.7 × 103 mm
A)14 mm
B)52 mm
C)713 mm
D)1.2 × 104 mm
E)9.7 × 103 mm

Unlock Deck
Unlock for access to all 175 flashcards in this deck.
Unlock Deck
k this deck
9
One significant difference between gases and liquids is that ________.
A)a gas is made up of molecules
B)a gas expands to fill its container
C)a gas may consist of both elements and compounds
D)gases are always mixtures
E)All of the above answers are correct.
A)a gas is made up of molecules
B)a gas expands to fill its container
C)a gas may consist of both elements and compounds
D)gases are always mixtures
E)All of the above answers are correct.

Unlock Deck
Unlock for access to all 175 flashcards in this deck.
Unlock Deck
k this deck
10
Which one of the following is a valid statement of Avogadro's law?
A) = constant
= constant
B) = constant
= constant
C)PV = constant
D)V = constant × n
E)V = constant × P
A)
 = constant
= constantB)
 = constant
= constantC)PV = constant
D)V = constant × n
E)V = constant × P

Unlock Deck
Unlock for access to all 175 flashcards in this deck.
Unlock Deck
k this deck
11
The pressure exerted by a column of liquid is equal to the product of the height of the column times the gravitational constant times the density of the liquid, P = ghd. How high a column of methanol (d = 0.79 g/mL)would be supported by a pressure that supports a 713 mm column of mercury
(d = 13.6 g/mL)?
A)713 mm
B)41 mm
C)1.2 × 104 mm
D)9.7 × 103 mm
E)17 mm
(d = 13.6 g/mL)?
A)713 mm
B)41 mm
C)1.2 × 104 mm
D)9.7 × 103 mm
E)17 mm

Unlock Deck
Unlock for access to all 175 flashcards in this deck.
Unlock Deck
k this deck
12
Of the following, ________ has the odor of rotten eggs.
A)NH3
B)H2S
C)CO
D)NO2
E)HCN
A)NH3
B)H2S
C)CO
D)NO2
E)HCN

Unlock Deck
Unlock for access to all 175 flashcards in this deck.
Unlock Deck
k this deck
13
Standard temperature and pressure (STP), in the context of gases, refers to ________.
A)298.15 K and 1 atm
B)273.15 K and 1 atm
C)298.15 K and 1 torr
D)273.15 K and 1 pascal
E)273.15 K and 1 torr
A)298.15 K and 1 atm
B)273.15 K and 1 atm
C)298.15 K and 1 torr
D)273.15 K and 1 pascal
E)273.15 K and 1 torr

Unlock Deck
Unlock for access to all 175 flashcards in this deck.
Unlock Deck
k this deck
14
Sodium bicarbonate is reacted with concentrated hydrochloric acid at 37.0 °C and 1.00 atm. The reaction of 6.00 kg of bicarbonate with excess hydrochloric acid under these conditions will produce ________ L of CO2.
A)1.09 × 102
B)2.85 × 104
C)1.82 × 104
D)8.70 × 102
E)1.82 × 103
A)1.09 × 102
B)2.85 × 104
C)1.82 × 104
D)8.70 × 102
E)1.82 × 103

Unlock Deck
Unlock for access to all 175 flashcards in this deck.
Unlock Deck
k this deck
15
Of the following, ________ is a correct statement of Boyle's law.
A)PV = constant
B)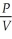 = constant
= constant
C)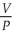 = constant
= constant
D) = constant
= constant
E)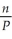 = constant
= constant
A)PV = constant
B)
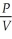 = constant
= constantC)
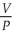 = constant
= constantD)
 = constant
= constantE)
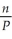 = constant
= constant
Unlock Deck
Unlock for access to all 175 flashcards in this deck.
Unlock Deck
k this deck
16
Of the following, only ________ is impossible for an ideal gas.
A)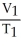 =
= 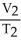
B)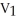
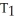 =
= 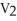
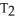
C)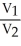 =
= 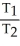
D)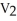 =
= 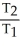
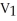
E)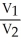 =
= 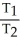 = 0
= 0
A)
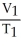 =
= 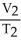
B)
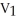
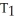 =
= 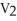
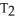
C)
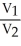 =
= 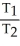
D)
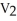 =
= 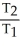
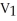
E)
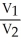 =
= 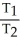 = 0
= 0
Unlock Deck
Unlock for access to all 175 flashcards in this deck.
Unlock Deck
k this deck
17
Of the following, ________ is a valid statement of Charles' law.
A) = constant
= constant
B) = constant
= constant
C)PV = constant
D)V = constant × n
E)V = constant × P
A)
 = constant
= constantB)
 = constant
= constantC)PV = constant
D)V = constant × n
E)V = constant × P

Unlock Deck
Unlock for access to all 175 flashcards in this deck.
Unlock Deck
k this deck
18
The volume of an ideal gas is zero at ________.
A)0 °C
B)-45 °F
C)-273 K
D)-363 K
E)-273 °C
A)0 °C
B)-45 °F
C)-273 K
D)-363 K
E)-273 °C

Unlock Deck
Unlock for access to all 175 flashcards in this deck.
Unlock Deck
k this deck
19
"Isothermal" means ________.
A)at constant pressure
B)at constant temperature
C)at variable temperature and pressure conditions
D)at ideal temperature and pressure conditions
E)that ΔHrxn = 0
A)at constant pressure
B)at constant temperature
C)at variable temperature and pressure conditions
D)at ideal temperature and pressure conditions
E)that ΔHrxn = 0

Unlock Deck
Unlock for access to all 175 flashcards in this deck.
Unlock Deck
k this deck
20
The molar volume of a gas at STP is ________ L.
A)0.08206
B)62.36
C)1.00
D)22.4
E)14.7
A)0.08206
B)62.36
C)1.00
D)22.4
E)14.7

Unlock Deck
Unlock for access to all 175 flashcards in this deck.
Unlock Deck
k this deck
21
The volume of a sample of gas (2.49 g)was 752 mL at 1.98 atm and 62 °C. The gas is ________.
A)SO2
B)SO3
C)NH3
D)NO2
E)Ne
A)SO2
B)SO3
C)NH3
D)NO2
E)Ne

Unlock Deck
Unlock for access to all 175 flashcards in this deck.
Unlock Deck
k this deck
22
Sodium bicarbonate is reacted with concentrated hydrochloric acid at 25.0 °C and 1.50 atm. The reaction of 7.75 kg of bicarbonate with excess hydrochloric acid under these conditions will produce ________ L of CO2.
A)1.82 × 103
B)2.85 × 104
C)1.82 × 104
D)1.50 × 103
E)8.70 × 102
A)1.82 × 103
B)2.85 × 104
C)1.82 × 104
D)1.50 × 103
E)8.70 × 102

Unlock Deck
Unlock for access to all 175 flashcards in this deck.
Unlock Deck
k this deck
23
Arrange the following gases in order of increasing average molecular speed at 25 °C. He, O2, CO2, N2
A)He < N2 < O2 < CO2
B)He < O2 < N2 < CO2
C)CO2 < O2 < N2 < He
D)CO2 < N2 < O2 < He
E)CO2 < He < N2 < O2
A)He < N2 < O2 < CO2
B)He < O2 < N2 < CO2
C)CO2 < O2 < N2 < He
D)CO2 < N2 < O2 < He
E)CO2 < He < N2 < O2

Unlock Deck
Unlock for access to all 175 flashcards in this deck.
Unlock Deck
k this deck
24
The density of NO2 in a 4.50 L tank at 760.0 torr and 25.0 °C is ________ g/L.
A)1.64
B)9.30
C)1.68
D)1.88
E)3.27
A)1.64
B)9.30
C)1.68
D)1.88
E)3.27

Unlock Deck
Unlock for access to all 175 flashcards in this deck.
Unlock Deck
k this deck
25
The average kinetic energy of the particles of a gas is directly proportional to ________.
A)the rms speed
B)the square of the rms speed
C)the square root of the rms speed
D)the square of the particle mass
E)the particle mass
A)the rms speed
B)the square of the rms speed
C)the square root of the rms speed
D)the square of the particle mass
E)the particle mass

Unlock Deck
Unlock for access to all 175 flashcards in this deck.
Unlock Deck
k this deck
26
According to kinetic-molecular theory, if the temperature of a gas is raised from 100 °C to 200 °C, the average kinetic energy of the gas will ________.
A)double
B)increase by a factor of 1.27
C)increase by a factor of 100
D)decrease by half
E)decrease by a factor of 100
A)double
B)increase by a factor of 1.27
C)increase by a factor of 100
D)decrease by half
E)decrease by a factor of 100

Unlock Deck
Unlock for access to all 175 flashcards in this deck.
Unlock Deck
k this deck
27
A sample of oxygen gas (O2)was found to effuse at a rate equal to three times that of an unknown gas. The molecular weight of the unknown gas is ________ g/mol.
A)288
B)96
C)55
D)4
E)10.7
A)288
B)96
C)55
D)4
E)10.7

Unlock Deck
Unlock for access to all 175 flashcards in this deck.
Unlock Deck
k this deck
28
A tank containing both HF and HBr gases developed a leak. The ratio of the rate of effusion of HF to the rate of effusion of HBr is ________.
A)4.04
B)0.247
C)2.01
D)0.497
E)16.3
A)4.04
B)0.247
C)2.01
D)0.497
E)16.3

Unlock Deck
Unlock for access to all 175 flashcards in this deck.
Unlock Deck
k this deck
29
A sample of oxygen gas was found to effuse at a rate equal to two times that of an unknown gas. The molecular weight of the unknown gas is ________ g/mol.
A)64
B)128
C)8
D)16
E)8.0
A)64
B)128
C)8
D)16
E)8.0

Unlock Deck
Unlock for access to all 175 flashcards in this deck.
Unlock Deck
k this deck
30
The kinetic-molecular theory predicts that pressure rises as the temperature of a gas increases because ________.
A)the average kinetic energy of the gas molecules decreases
B)the gas molecules collide more frequently with the wall
C)the gas molecules collide less frequently with the wall
D)the gas molecules collide more energetically with the wall
E)both the gas molecules collide more frequently with the wall and the gas molecules collide more energetically with the wall
A)the average kinetic energy of the gas molecules decreases
B)the gas molecules collide more frequently with the wall
C)the gas molecules collide less frequently with the wall
D)the gas molecules collide more energetically with the wall
E)both the gas molecules collide more frequently with the wall and the gas molecules collide more energetically with the wall

Unlock Deck
Unlock for access to all 175 flashcards in this deck.
Unlock Deck
k this deck
31
Which one of the following gases would have the highest average molecular speed at 25 °C?
A)O2
B)N2
C)CO2
D)CH4
E)SF6
A)O2
B)N2
C)CO2
D)CH4
E)SF6

Unlock Deck
Unlock for access to all 175 flashcards in this deck.
Unlock Deck
k this deck
32
According to kinetic-molecular theory, in which of the following gases will the root-mean-square speed of the molecules be the highest at 200 °C?
A)HCl
B)Cl2
C)H2O
D)SF6
E)None. The molecules of all gases have the same root-mean-square speed at any given temperature.
A)HCl
B)Cl2
C)H2O
D)SF6
E)None. The molecules of all gases have the same root-mean-square speed at any given temperature.

Unlock Deck
Unlock for access to all 175 flashcards in this deck.
Unlock Deck
k this deck
33
Which of the following is not part of the kinetic-molecular theory?
A)Atoms are neither created nor destroyed by ordinary chemical reactions.
B)Attractive and repulsive forces between gas molecules are negligible.
C)Gases consist of molecules in continuous, random motion.
D)Collisions between gas molecules do not result in the loss of energy.
E)The volume occupied by all of the gas molecules in a container is negligible compared to the volume of the container.
A)Atoms are neither created nor destroyed by ordinary chemical reactions.
B)Attractive and repulsive forces between gas molecules are negligible.
C)Gases consist of molecules in continuous, random motion.
D)Collisions between gas molecules do not result in the loss of energy.
E)The volume occupied by all of the gas molecules in a container is negligible compared to the volume of the container.

Unlock Deck
Unlock for access to all 175 flashcards in this deck.
Unlock Deck
k this deck
34
A sample of an unknown volatile liquid was injected into a Dumas flask mflask = 27.0928 g, vflask = 0.1040 L)and heated until no visible traces of the liquid could be found. The flask and its contents were then rapidly cooled and reweighed (mflask + vapor = 27.4593 g). The atmospheric pressure and temperature during the experiment were 0.976 atm and 18.0 °C, respectively. The unknown volatile liquid was ________.
A)C6H12
B)C6H14
C)C7H14
D)C7H16
E)C6H6
A)C6H12
B)C6H14
C)C7H14
D)C7H16
E)C6H6

Unlock Deck
Unlock for access to all 175 flashcards in this deck.
Unlock Deck
k this deck
35
Arrange the following gases in order of increasing average molecular speed at 25 °C. Cl2, O2, F2, N2
A)Cl2 < F2 < O2 < N2
B)Cl2 < O2 < F2 < N2
C)N2 < F2 < Cl2 < O2
D)Cl2 < F2 < N2 < O2
E)F2 < O2 < N2 < Cl2
A)Cl2 < F2 < O2 < N2
B)Cl2 < O2 < F2 < N2
C)N2 < F2 < Cl2 < O2
D)Cl2 < F2 < N2 < O2
E)F2 < O2 < N2 < Cl2

Unlock Deck
Unlock for access to all 175 flashcards in this deck.
Unlock Deck
k this deck
36
Of the following gases, ________ will have the greatest rate of effusion at a given temperature.
A)NH3
B)CH4
C)Ar
D)HBr
E)HCl
A)NH3
B)CH4
C)Ar
D)HBr
E)HCl

Unlock Deck
Unlock for access to all 175 flashcards in this deck.
Unlock Deck
k this deck
37
A 255 mL round-bottom flask is weighed and found to have a mass of 114.85 g. A few milliliters of an easily vaporized liquid are added to the flask and the flask is immersed in a boiling water bath. All of the liquid vaporizes at the boiling temperature of water, filling the flask with vapor. When all of the liquid has vaporized, the flask is removed from the bath, cooled, dried, and reweighed. The new mass of the flask and the condensed vapor is 115.23 g. Which of the following compounds could the liquid be? (Assume the ambient pressure is 1 atm.)
A)C4H10
B)C3H7OH
C)C2H6
D)C2H5OH
E)C4H9OH
A)C4H10
B)C3H7OH
C)C2H6
D)C2H5OH
E)C4H9OH

Unlock Deck
Unlock for access to all 175 flashcards in this deck.
Unlock Deck
k this deck
38
At 333 K, which of the pairs of gases below would have the most nearly identical rates of effusion?
A)N2O and NO2
B)CO and N2
C)N2 and O2
D)CO and CO2
E)NO2 and N2O4
A)N2O and NO2
B)CO and N2
C)N2 and O2
D)CO and CO2
E)NO2 and N2O4

Unlock Deck
Unlock for access to all 175 flashcards in this deck.
Unlock Deck
k this deck
39
The density of air at STP is 1.285 g/L. Which of the following cannot be used to fill a balloon that will float in air at STP?
A)CH4
B)NO
C)Ne
D)NH3
E)HF
A)CH4
B)NO
C)Ne
D)NH3
E)HF

Unlock Deck
Unlock for access to all 175 flashcards in this deck.
Unlock Deck
k this deck
40
At STP, the ratio of the root-mean-square speed of CO2 to that of SO2 is ________.
A)2.001
B)2.119
C)1.000
D)1.207
E)1.456
A)2.001
B)2.119
C)1.000
D)1.207
E)1.456

Unlock Deck
Unlock for access to all 175 flashcards in this deck.
Unlock Deck
k this deck
41
A real gas will behave most like an ideal gas under conditions of ________.
A)high temperature and high pressure
B)high temperature and low pressure
C)low temperature and high pressure
D)low temperature and low pressure
E)STP
A)high temperature and high pressure
B)high temperature and low pressure
C)low temperature and high pressure
D)low temperature and low pressure
E)STP

Unlock Deck
Unlock for access to all 175 flashcards in this deck.
Unlock Deck
k this deck
42
A sample of a gas originally at 29 °C and 1.25 atm pressure in a 3.0 L container is allowed to contract until the volume is 2.2 L and the temperature is 11 °C. The final pressure of the gas is ________ atm.
A)2.9
B)2.8
C)1.6
D)2.1
E)0.38
A)2.9
B)2.8
C)1.6
D)2.1
E)0.38

Unlock Deck
Unlock for access to all 175 flashcards in this deck.
Unlock Deck
k this deck
43
If 50.75 g of a gas occupies 10.0 L at STP, 129.3 g of the gas will occupy ________ L at STP.
A)3.92
B)50.8
C)12.9
D)25.5
E)5.08
A)3.92
B)50.8
C)12.9
D)25.5
E)5.08

Unlock Deck
Unlock for access to all 175 flashcards in this deck.
Unlock Deck
k this deck
44
A sample of a gas (1.50 mol)is contained in a 15.0 L cylinder. The temperature is increased from 100 °C to 150 °C. The ratio of final pressure to initial pressure [ ![<strong>A sample of a gas (1.50 mol)is contained in a 15.0 L cylinder. The temperature is increased from 100 °C to 150 °C. The ratio of final pressure to initial pressure [ ] is ________.</strong> A)1.50 B)0.667 C)0.882 D)1.13 E)1.00](https://storage.examlex.com/TB2701/11ea7cce_1f4d_53fc_a2ab_83bb23c574fa_TB2701_11.jpg) ] is ________.
] is ________.
A)1.50
B)0.667
C)0.882
D)1.13
E)1.00
![<strong>A sample of a gas (1.50 mol)is contained in a 15.0 L cylinder. The temperature is increased from 100 °C to 150 °C. The ratio of final pressure to initial pressure [ ] is ________.</strong> A)1.50 B)0.667 C)0.882 D)1.13 E)1.00](https://storage.examlex.com/TB2701/11ea7cce_1f4d_53fc_a2ab_83bb23c574fa_TB2701_11.jpg) ] is ________.
] is ________.A)1.50
B)0.667
C)0.882
D)1.13
E)1.00

Unlock Deck
Unlock for access to all 175 flashcards in this deck.
Unlock Deck
k this deck
45
A gas at a pressure of 325 torr exerts a force of ________ N on an area of 5.5 m2.
A)1.8 × 103
B)59
C)2.4 × 105
D)0.018
E)2.4
A)1.8 × 103
B)59
C)2.4 × 105
D)0.018
E)2.4

Unlock Deck
Unlock for access to all 175 flashcards in this deck.
Unlock Deck
k this deck
46
A sample of a gas (5.0 mol)at 1.0 atm is expanded at constant temperature from 10 L to 15 L. The final pressure is ________ atm.
A)1.5
B)7.5
C)0.67
D)3.3
E)15
A)1.5
B)7.5
C)0.67
D)3.3
E)15

Unlock Deck
Unlock for access to all 175 flashcards in this deck.
Unlock Deck
k this deck
47
A sample of a gas originally at 25 °C and 1.00 atm pressure in a 2.5 L container is subject to a pressure of 0.85 atm and a temperature of 15 °C. The final volume of the gas is ________ L.
A)3.0
B)2.8
C)2.6
D)2.1
E)0.38
A)3.0
B)2.8
C)2.6
D)2.1
E)0.38

Unlock Deck
Unlock for access to all 175 flashcards in this deck.
Unlock Deck
k this deck
48
A gas vessel is attached to an open-end manometer containing a nonvolatile liquid of density 0.791 g/mL as shown below. 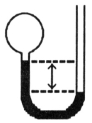 The difference in heights of the liquid in the two sides of the manometer is 43.4 cm when the atmospheric pressure is 755 mm Hg. Given that the density of mercury is 13.6 g/mL, the pressure of the enclosed gas is ________ atm.
The difference in heights of the liquid in the two sides of the manometer is 43.4 cm when the atmospheric pressure is 755 mm Hg. Given that the density of mercury is 13.6 g/mL, the pressure of the enclosed gas is ________ atm.
A)1.03
B)0.967
C)0.993
D)0.990
E)0.987
 The difference in heights of the liquid in the two sides of the manometer is 43.4 cm when the atmospheric pressure is 755 mm Hg. Given that the density of mercury is 13.6 g/mL, the pressure of the enclosed gas is ________ atm.
The difference in heights of the liquid in the two sides of the manometer is 43.4 cm when the atmospheric pressure is 755 mm Hg. Given that the density of mercury is 13.6 g/mL, the pressure of the enclosed gas is ________ atm.A)1.03
B)0.967
C)0.993
D)0.990
E)0.987

Unlock Deck
Unlock for access to all 175 flashcards in this deck.
Unlock Deck
k this deck
49
A mixture of carbon dioxide and an unknown gas was allowed to effuse from a container. The carbon dioxide took 1.25 times as long to escape as the unknown gas. Which one could be the unknown gas?
A)Cl2
B)CO
C)HCl
D)H2
E)SO2
A)Cl2
B)CO
C)HCl
D)H2
E)SO2

Unlock Deck
Unlock for access to all 175 flashcards in this deck.
Unlock Deck
k this deck
50
A gas at a pressure of 10.0 Pa exerts a force of ________ N on an area of 5.5 m2.
A)55
B)0.55
C)5.5
D)1.8
E)18
A)55
B)0.55
C)5.5
D)1.8
E)18

Unlock Deck
Unlock for access to all 175 flashcards in this deck.
Unlock Deck
k this deck
51
A mixture of two gases was allowed to effuse from a container. One of the gases escaped from the container 1.43 times as fast as the other one. The two gases could have been ________.
A)CO and SF6
B)O2 and Cl2
C)CO and CO2
D)Cl2 and SF6
E)O2 and SF6
A)CO and SF6
B)O2 and Cl2
C)CO and CO2
D)Cl2 and SF6
E)O2 and SF6

Unlock Deck
Unlock for access to all 175 flashcards in this deck.
Unlock Deck
k this deck
52
How much faster does 235UF6 effuse than 238UF6?
A)1.013 times as fast
B)1.009 times as fast
C)1.004 times as fast
D)1.006 times as fast
E)1.018 times as fast
A)1.013 times as fast
B)1.009 times as fast
C)1.004 times as fast
D)1.006 times as fast
E)1.018 times as fast

Unlock Deck
Unlock for access to all 175 flashcards in this deck.
Unlock Deck
k this deck
53
When gases are treated as real, via use of the van der Waals equation, the actual volume occupied by gas molecules ________ the pressure exerted and the attractive forces between gas molecules ________ the pressure exerted, as compared to an ideal gas.
A)decreases, increases
B)increases, increases
C)increases, decreases
D)does not affect, decreases
E)does not affect, increases
A)decreases, increases
B)increases, increases
C)increases, decreases
D)does not affect, decreases
E)does not affect, increases

Unlock Deck
Unlock for access to all 175 flashcards in this deck.
Unlock Deck
k this deck
54
An ideal gas differs from a real gas in that the molecules of an ideal gas ________.
A)have no attraction for one another
B)have appreciable molecular volumes
C)have a molecular weight of zero
D)have no kinetic energy
E)have an average molecular mass
A)have no attraction for one another
B)have appreciable molecular volumes
C)have a molecular weight of zero
D)have no kinetic energy
E)have an average molecular mass

Unlock Deck
Unlock for access to all 175 flashcards in this deck.
Unlock Deck
k this deck
55
Which one of the following gases would deviate the least from ideal gas behavior?
A)Ne
B)CH3Cl
C)Kr
D)CO2
E)F2
A)Ne
B)CH3Cl
C)Kr
D)CO2
E)F2

Unlock Deck
Unlock for access to all 175 flashcards in this deck.
Unlock Deck
k this deck
56
A gas vessel is attached to an open-end manometer filled with a nonvolatile liquid of density 0.993 g/mL as shown below. 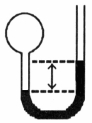 The difference in heights of the liquid in the two sides of the manometer is 32.3 mm when the atmospheric pressure is 765 mm Hg. Given that the density of mercury is 13.6 g/mL, the pressure of the enclosed gas is ________ atm.
The difference in heights of the liquid in the two sides of the manometer is 32.3 mm when the atmospheric pressure is 765 mm Hg. Given that the density of mercury is 13.6 g/mL, the pressure of the enclosed gas is ________ atm.
A)1.05
B)1.01
C)0.976
D)0.993
E)1.08
 The difference in heights of the liquid in the two sides of the manometer is 32.3 mm when the atmospheric pressure is 765 mm Hg. Given that the density of mercury is 13.6 g/mL, the pressure of the enclosed gas is ________ atm.
The difference in heights of the liquid in the two sides of the manometer is 32.3 mm when the atmospheric pressure is 765 mm Hg. Given that the density of mercury is 13.6 g/mL, the pressure of the enclosed gas is ________ atm.A)1.05
B)1.01
C)0.976
D)0.993
E)1.08

Unlock Deck
Unlock for access to all 175 flashcards in this deck.
Unlock Deck
k this deck
57
A sample of H2 gas (12.28 g)occupies 100.0 L at 400.0 K and 2.00 atm. A sample weighing 9.49 g occupies ________ L at 353 K and 2.00 atm.
A)109
B)68.2
C)54.7
D)147
E)77.3
A)109
B)68.2
C)54.7
D)147
E)77.3

Unlock Deck
Unlock for access to all 175 flashcards in this deck.
Unlock Deck
k this deck
58
In a Torricelli barometer, a pressure of one atmosphere supports a 760 mm column of mercury. If the original tube containing the mercury is replaced with a tube having twice the diameter of the original, the height of the mercury column at one atmosphere pressure is ________ mm.
A)380
B)760
C)1.52 × 103
D)4.78 × 103
E)121
A)380
B)760
C)1.52 × 103
D)4.78 × 103
E)121

Unlock Deck
Unlock for access to all 175 flashcards in this deck.
Unlock Deck
k this deck
59
Which noble gas is expected to show the largest deviations from the ideal gas behavior?
A)helium
B)neon
C)argon
D)krypton
E)xenon
A)helium
B)neon
C)argon
D)krypton
E)xenon

Unlock Deck
Unlock for access to all 175 flashcards in this deck.
Unlock Deck
k this deck
60
The van der Waals equation for real gases recognizes that ________.
A)gas particles have non-zero volumes and interact with each other
B)molar volumes of gases of different types are different
C)the non-zero volumes of gas particles effectively decrease the amount of "empty space" between them
D)the molecular attractions between particles of gas decreases the pressure exerted by the gas
E)all of the above statements are true
A)gas particles have non-zero volumes and interact with each other
B)molar volumes of gases of different types are different
C)the non-zero volumes of gas particles effectively decrease the amount of "empty space" between them
D)the molecular attractions between particles of gas decreases the pressure exerted by the gas
E)all of the above statements are true

Unlock Deck
Unlock for access to all 175 flashcards in this deck.
Unlock Deck
k this deck
61
The reaction of 50 mL of Cl2 gas with 50 mL of CH4 gas via the equation: Cl2 (g) + CH4 (g) → HCl (g) + CH3Cl (g)
Will produce a total of ________ mL of products if pressure and temperature are kept constant.
A)100
B)50
C)200
D)150
E)250
Will produce a total of ________ mL of products if pressure and temperature are kept constant.
A)100
B)50
C)200
D)150
E)250

Unlock Deck
Unlock for access to all 175 flashcards in this deck.
Unlock Deck
k this deck
62
A 0.133 mol sample of gas in a 525 mL container has a pressure of 312 torr. The temperature of the gas is ________ °C.
A)20.3
B)-253
C)-20.3
D)203
E)22.4
A)20.3
B)-253
C)-20.3
D)203
E)22.4

Unlock Deck
Unlock for access to all 175 flashcards in this deck.
Unlock Deck
k this deck
63
The molecular weight of a gas is ________ g/mol if 6.7 g of the gas occupies 6.3 L at STP.
A)24
B)3.6 × 103
C)27
D)3.0 × 102
E)1.8 × 10-2
A)24
B)3.6 × 103
C)27
D)3.0 × 102
E)1.8 × 10-2

Unlock Deck
Unlock for access to all 175 flashcards in this deck.
Unlock Deck
k this deck
64
The pressure of a sample of CH4 gas (6.022 g)in a 30.0 L vessel at 402 K is ________ atm.
A)2.42
B)6.62
C)0.413
D)12.4
E)22.4
A)2.42
B)6.62
C)0.413
D)12.4
E)22.4

Unlock Deck
Unlock for access to all 175 flashcards in this deck.
Unlock Deck
k this deck
65
The density of N2O at 1.53 atm and 45.2 °C is ________ g/L.
A)18.2
B)1.76
C)0.388
D)9.99
E)2.58
A)18.2
B)1.76
C)0.388
D)9.99
E)2.58

Unlock Deck
Unlock for access to all 175 flashcards in this deck.
Unlock Deck
k this deck
66
The amount of gas that occupies 36.52 L at 68.0 °C and 672 mm Hg is ________ mol.
A)127
B)1.15
C)878
D)24.4
E)12.7
A)127
B)1.15
C)878
D)24.4
E)12.7

Unlock Deck
Unlock for access to all 175 flashcards in this deck.
Unlock Deck
k this deck
67
The reaction of 25 mL of N2 gas with 75 mL of H2 gas to form ammonia via the equation: N2 (g) + 3H2 (g) → 2NH3 (g)
Will produce ________ mL of ammonia if pressure and temperature are kept constant.
A)250
B)50
C)200
D)150
E)100
Will produce ________ mL of ammonia if pressure and temperature are kept constant.
A)250
B)50
C)200
D)150
E)100

Unlock Deck
Unlock for access to all 175 flashcards in this deck.
Unlock Deck
k this deck
68
The amount of gas that occupies 60.82 L at 31.0 °C and 367 mm Hg is ________ mol.
A)1.18
B)0.850
C)894
D)11.6
E)0.120
A)1.18
B)0.850
C)894
D)11.6
E)0.120

Unlock Deck
Unlock for access to all 175 flashcards in this deck.
Unlock Deck
k this deck
69
The reaction of 50 mL of Cl2 gas with 50 mL of C2H4 gas via the equation: Cl2 (g) + C2H4 (g) → C2H4Cl2 (g)
Will produce a total of ________ mL of products if pressure and temperature are kept constant.
A)100
B)50
C)25
D)125
E)150
Will produce a total of ________ mL of products if pressure and temperature are kept constant.
A)100
B)50
C)25
D)125
E)150

Unlock Deck
Unlock for access to all 175 flashcards in this deck.
Unlock Deck
k this deck
70
The volume of 0.25 mol of a gas at 72.7 kPa and 15 °C is ________ m3.
A)8.1 × 10-5
B)1.2 × 10-4
C)4.3 × 10-4
D)8.2 × 10-3
E)2.2 × 10-1
A)8.1 × 10-5
B)1.2 × 10-4
C)4.3 × 10-4
D)8.2 × 10-3
E)2.2 × 10-1

Unlock Deck
Unlock for access to all 175 flashcards in this deck.
Unlock Deck
k this deck
71
The molecular weight of a gas is ________ g/mol if 3.5 g of the gas occupies 2.1 L at STP.
A)41
B)5.5 × 103
C)37
D)4.6 × 102
E)2.7 × 10-2
A)41
B)5.5 × 103
C)37
D)4.6 × 102
E)2.7 × 10-2

Unlock Deck
Unlock for access to all 175 flashcards in this deck.
Unlock Deck
k this deck
72
The reaction of 100 mL of Cl2 gas with 100 mL of CH4 gas via the equation: Cl2 (g) + CH4 (g) → HCl (g) + CH3Cl (g)
Will produce a total of ________ mL of products if pressure and temperature are kept constant.
A)100
B)50
C)200
D)150
E)250
Will produce a total of ________ mL of products if pressure and temperature are kept constant.
A)100
B)50
C)200
D)150
E)250

Unlock Deck
Unlock for access to all 175 flashcards in this deck.
Unlock Deck
k this deck
73
The mass of nitrogen dioxide contained in a 4.32 L vessel at 48 °C and 141600 Pa is ________ g.
A)5.35 × 104
B)53.5
C)10.5
D)70.5
E)9.46 × 10-2
A)5.35 × 104
B)53.5
C)10.5
D)70.5
E)9.46 × 10-2

Unlock Deck
Unlock for access to all 175 flashcards in this deck.
Unlock Deck
k this deck
74
A gas in a 325 mL container has a pressure of 695 torr at 19 °C. There are ________ mol of gas in the flask.
A)1.24 × 10-2
B)1.48 × 10-2
C)9.42
D)12.4
E)80.6
A)1.24 × 10-2
B)1.48 × 10-2
C)9.42
D)12.4
E)80.6

Unlock Deck
Unlock for access to all 175 flashcards in this deck.
Unlock Deck
k this deck
75
The molecular weight of a gas that has a density of 7.10 g/L at 25.0 °C and 1.00 atm pressure is ________ g/mol.
A)174
B)14.6
C)28.0
D)5.75 × 10-3
E)6.85 × 10-2
A)174
B)14.6
C)28.0
D)5.75 × 10-3
E)6.85 × 10-2

Unlock Deck
Unlock for access to all 175 flashcards in this deck.
Unlock Deck
k this deck
76
The molecular weight of a gas that has a density of 6.70 g/L at STP is ________ g/mol.
A)4.96 × 102
B)1.50 × 102
C)7.30 × 101
D)3.35
E)2.98 × 10-1
A)4.96 × 102
B)1.50 × 102
C)7.30 × 101
D)3.35
E)2.98 × 10-1

Unlock Deck
Unlock for access to all 175 flashcards in this deck.
Unlock Deck
k this deck
77
The density of ammonia gas in a 4.32 L container at 837 torr and 45.0 °C is ________ g/L.
A)3.86
B)0.719
C)0.432
D)0.194
E)4.22 × 10-2
A)3.86
B)0.719
C)0.432
D)0.194
E)4.22 × 10-2

Unlock Deck
Unlock for access to all 175 flashcards in this deck.
Unlock Deck
k this deck
78
The reaction of 50 mL of N2 gas with 150 mL of H2 gas to form ammonia via the equation: N2 (g) + 3H2 (g) → 2NH3 (g)
Will produce ________ mL of ammonia if pressure and temperature are kept constant.
A)250
B)50
C)200
D)150
E)100
Will produce ________ mL of ammonia if pressure and temperature are kept constant.
A)250
B)50
C)200
D)150
E)100

Unlock Deck
Unlock for access to all 175 flashcards in this deck.
Unlock Deck
k this deck
79
The temperature of a sample of CH4 gas (10.34 g)in a 50.0 L vessel at 1.33 atm is ________ °C.
A)984
B)-195
C)195
D)1260
E)-1260
A)984
B)-195
C)195
D)1260
E)-1260

Unlock Deck
Unlock for access to all 175 flashcards in this deck.
Unlock Deck
k this deck
80
The molecular weight of a gas that has a density of 5.75 g/L at STP is ________ g/mol.
A)3.90
B)129
C)141
D)578
E)1.73 × 10-3
A)3.90
B)129
C)141
D)578
E)1.73 × 10-3

Unlock Deck
Unlock for access to all 175 flashcards in this deck.
Unlock Deck
k this deck


