Deck 21: Benzene and the Concept of Aromaticity
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/85
Play
Full screen (f)
Deck 21: Benzene and the Concept of Aromaticity
1
What is the correct assignment of the names of the following fused arenes? ![<strong>What is the correct assignment of the names of the following fused arenes? </strong> A)1 = naphthalene; 2 = anthracene; 3 = pyrene B)1 = naphthalene; 2 = phenanthrene; 3 = coronene C)1 = naphthalene; 2 = anthracene; 3 = benzo[a]pyrene D)1 = anthracene; 2 = naphthalene; 3 = coronene](https://storage.examlex.com/TB1813/11ea7d75_f628_8e24_b9bd_a1873ea28e79_TB1813_00_TB1813_00.jpg)
A)1 = naphthalene; 2 = anthracene; 3 = pyrene
B)1 = naphthalene; 2 = phenanthrene; 3 = coronene
C)1 = naphthalene; 2 = anthracene; 3 = benzo[a]pyrene
D)1 = anthracene; 2 = naphthalene; 3 = coronene
![<strong>What is the correct assignment of the names of the following fused arenes? </strong> A)1 = naphthalene; 2 = anthracene; 3 = pyrene B)1 = naphthalene; 2 = phenanthrene; 3 = coronene C)1 = naphthalene; 2 = anthracene; 3 = benzo[a]pyrene D)1 = anthracene; 2 = naphthalene; 3 = coronene](https://storage.examlex.com/TB1813/11ea7d75_f628_8e24_b9bd_a1873ea28e79_TB1813_00_TB1813_00.jpg)
A)1 = naphthalene; 2 = anthracene; 3 = pyrene
B)1 = naphthalene; 2 = phenanthrene; 3 = coronene
C)1 = naphthalene; 2 = anthracene; 3 = benzo[a]pyrene
D)1 = anthracene; 2 = naphthalene; 3 = coronene
1 = naphthalene; 2 = anthracene; 3 = pyrene
2
Which is the correct assignment of the names of the following substituted benzenes? 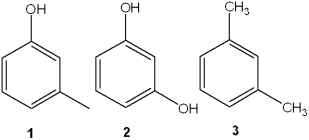
A)1 = phenol; 2 = m-cresol; 3 = toluene
B)1 = m-cresol; 2 = resorcinol; 3 = m-xylene
C)1 = anisole; 2 = catechol; 3 = m-xylene
D)1 = m-cresol; 2 = anisole; 3 = cumene

A)1 = phenol; 2 = m-cresol; 3 = toluene
B)1 = m-cresol; 2 = resorcinol; 3 = m-xylene
C)1 = anisole; 2 = catechol; 3 = m-xylene
D)1 = m-cresol; 2 = anisole; 3 = cumene
1 = m-cresol; 2 = resorcinol; 3 = m-xylene
3
What is the correct assignment of the names of the following substituted benzenes? 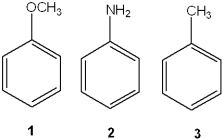
A)1 = phenol; 2 = aniline; 3 = anisole
B)1 = benzaldehyde; 2 = anisole; 3 = toluene
C)1 = anisole; 2 = xylene; 3 = toluene
D)1 = anisole; 2 = aniline; 3 = toluene

A)1 = phenol; 2 = aniline; 3 = anisole
B)1 = benzaldehyde; 2 = anisole; 3 = toluene
C)1 = anisole; 2 = xylene; 3 = toluene
D)1 = anisole; 2 = aniline; 3 = toluene
1 = anisole; 2 = aniline; 3 = toluene
4
Which of the following represents the energy levels of the molecular orbitals of benzene? 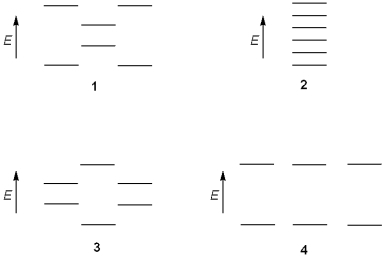
A)1
B)2
C)3
D)4

A)1
B)2
C)3
D)4

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
5
Which of the following statements is not true about the structure of benzene?
A)six atomic 2p-orbitals overlap to form six -molecular orbitals
B)there are three bonding -molecular orbitals and three -antibonding molecular orbitals
C)the ground state electronic configuration of benzene has six electrons in three -bonding molecular orbitals
D)the bonds alternate in length around the ring
A)six atomic 2p-orbitals overlap to form six -molecular orbitals
B)there are three bonding -molecular orbitals and three -antibonding molecular orbitals
C)the ground state electronic configuration of benzene has six electrons in three -bonding molecular orbitals
D)the bonds alternate in length around the ring

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
6
What is the hybridization of the nitrogen atom of pyridine?
A)s
B)sp
C)sp2
D)sp3
A)s
B)sp
C)sp2
D)sp3

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
7
What is the correct assignment of the names of the following heterocycles? 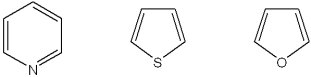
A)1 = pyrrole; 2 = thiophene; 3 = pyridine
B)1 = thiophene; 2 = furan; 3 = pyrrole
C)1 = pyridine; 2 = thiophene; 3 = furan
D)1 = pyridine; 2 = thiophene; 3 = pyrrole

A)1 = pyrrole; 2 = thiophene; 3 = pyridine
B)1 = thiophene; 2 = furan; 3 = pyrrole
C)1 = pyridine; 2 = thiophene; 3 = furan
D)1 = pyridine; 2 = thiophene; 3 = pyrrole

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
8
What is the hybridization of the oxygen atom of furan?
A)s
B)sp
C)sp2
D)sp3
A)s
B)sp
C)sp2
D)sp3

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
9
Which of the following compounds is aromatic?
A)ethane
B)cyclobutadiene
C)benzene
D)cyclooctatetraene
A)ethane
B)cyclobutadiene
C)benzene
D)cyclooctatetraene

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
10
What is the IUPAC name of the following compound? 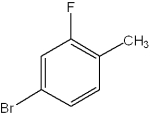
A)1-bromo-2-fluoro-4-toluene
B)4-bromo-2-fluorotoluene
C)2-bromo-4-fluorophenol
D)4-bromo-2-fluoroxylene

A)1-bromo-2-fluoro-4-toluene
B)4-bromo-2-fluorotoluene
C)2-bromo-4-fluorophenol
D)4-bromo-2-fluoroxylene

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
11
What is the IUPAC name of the following compound? 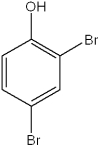
A)2,4-dibromotoluene
B)2,4-dibromophenol
C)2,4-dibromohydroxybenzene
D)4,6-dibromophenol

A)2,4-dibromotoluene
B)2,4-dibromophenol
C)2,4-dibromohydroxybenzene
D)4,6-dibromophenol

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
12
What is the correct assignment of the names of the following heterocycles? 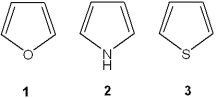
A)1 = pyrrole; 2 = thiophene; 3 = pyridine
B)1 = thiophene; 2 = furan; 3 = pyrrole
C)1 = furan; 2 = pyrrole; 3 = thiophene
D)1 = furan; 2 = thiophene; 3 = pyrrole

A)1 = pyrrole; 2 = thiophene; 3 = pyridine
B)1 = thiophene; 2 = furan; 3 = pyrrole
C)1 = furan; 2 = pyrrole; 3 = thiophene
D)1 = furan; 2 = thiophene; 3 = pyrrole

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
13
What is the correct assignment of the names of the following substituted benzenes? 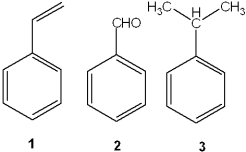
A)1 = styrene; 2 = benzaldehyde; 3 = cumene
B)1 = anisole; 2 = benzaldehyde; 3 = toluene
C)1 = styrene; 2 = xylene; 3 = toluene
D)1 = aniline; 2 = phenol; 3 = cumene

A)1 = styrene; 2 = benzaldehyde; 3 = cumene
B)1 = anisole; 2 = benzaldehyde; 3 = toluene
C)1 = styrene; 2 = xylene; 3 = toluene
D)1 = aniline; 2 = phenol; 3 = cumene

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
14
Which orbital contains the lone pair on the nitrogen atom of pyrrole?
A)s
B)p
C)sp
D)sp2
A)s
B)p
C)sp
D)sp2

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
15
Which of the following is not one of Hückel's criteria for aromaticity?
A)The compound must be cyclic
B)The compound must have one p-orbital on each atom of a ring
C)The compound must be planar or nearly planar so that there is a continuous overlap of p orbitals
D)The compound must have a closed loop of six pi electrons
A)The compound must be cyclic
B)The compound must have one p-orbital on each atom of a ring
C)The compound must be planar or nearly planar so that there is a continuous overlap of p orbitals
D)The compound must have a closed loop of six pi electrons

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
16
What is the IUPAC name of the following compound? 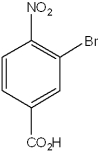
A)2-nitro-6-carboxybromobenzene
B)2-bromo-1-nitro-4-benzoic acid
C)2-bromo-4-carboxynitrobenzene
D)3-bromo-4-nitrobenzoic acid

A)2-nitro-6-carboxybromobenzene
B)2-bromo-1-nitro-4-benzoic acid
C)2-bromo-4-carboxynitrobenzene
D)3-bromo-4-nitrobenzoic acid

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
17
Which of the following statements is not true about the structure of benzene?
A)the carbon-carbon bonds are all the same length
B)the structure rapidly transforms between two resonance contributors
C)the structure is an average of two resonance contributors
D)the ring of six carbon atoms is planar
A)the carbon-carbon bonds are all the same length
B)the structure rapidly transforms between two resonance contributors
C)the structure is an average of two resonance contributors
D)the ring of six carbon atoms is planar

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
18
Which orbitals contain the lone pairs on the nitrogen atoms labeled i and ii in the imidazole ring? 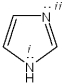
A)i = sp2;; ii = sp3
B)i = p;; ii = sp3
C)i = p;; ii = sp3
D)i = p;; ii = sp2

A)i = sp2;; ii = sp3
B)i = p;; ii = sp3
C)i = p;; ii = sp3
D)i = p;; ii = sp2

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
19
Which orbital contains the lone pair on the nitrogen atom of pyridine?
A)s
B)p
C)sp
D)sp2
A)s
B)p
C)sp
D)sp2

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
20
What is the approximate length of the carbon-carbon bonds in benzene?
A)110 pm
B)121 pm
C)139 pm
D)156 pm
A)110 pm
B)121 pm
C)139 pm
D)156 pm

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
21
Which of the following has the compounds shown in the correct order of decreasing acidity (i.e., more acidic > less acidic)? 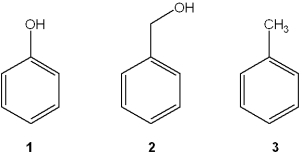
A)1 > 2 > 3
B)2 > 1 > 3
C)3 > 2 > 1
D)1 > 3 > 2

A)1 > 2 > 3
B)2 > 1 > 3
C)3 > 2 > 1
D)1 > 3 > 2

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
22
What is the best choice of reagent to achieve the following reaction? 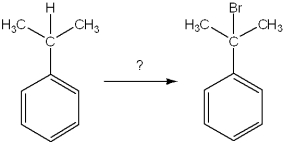
A)Br2, CCl4
B)HBr, H2O
C)Br2, light
D)NaBr

A)Br2, CCl4
B)HBr, H2O
C)Br2, light
D)NaBr

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
23
Which of the following compounds is antiaromatic?
A)ethane
B)cyclobutadiene
C)benzene
D)cyclooctatetraene
A)ethane
B)cyclobutadiene
C)benzene
D)cyclooctatetraene

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
24
Which of the following ions is aromatic? 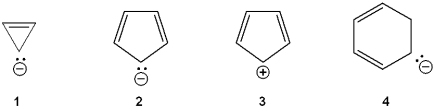
A)1
B)2
C)3
D)4

A)1
B)2
C)3
D)4

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
25
Which of the following has the compounds shown in the correct order of decreasing acidity (i.e., more acidic > less acidic)? 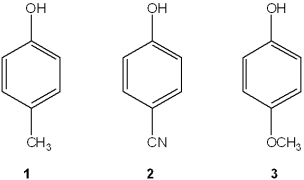
A)1 > 2 > 3
B)2 > 3 > 1
C)3 > 2 > 1
D)2 > 1 > 3

A)1 > 2 > 3
B)2 > 3 > 1
C)3 > 2 > 1
D)2 > 1 > 3

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
26
Which of the following has the compounds shown in the correct order of decreasing acidity (i.e., more acidic > less acidic)? 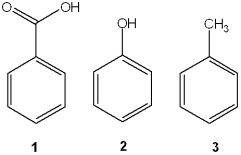
A)1 > 2 > 3
B)2 > 3 > 1
C)3 > 2 > 1
D)1 > 3 > 2

A)1 > 2 > 3
B)2 > 3 > 1
C)3 > 2 > 1
D)1 > 3 > 2

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
27
What is the major organic product obtained from the following reaction? 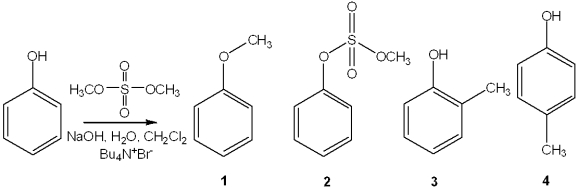
A)1
B)2
C)3
D)4

A)1
B)2
C)3
D)4

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
28
Which of the following heterocycles is not aromatic? 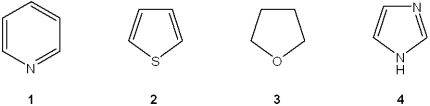
A)1
B)2
C)3
D)4

A)1
B)2
C)3
D)4

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
29
What is the major organic product obtained from the following reaction? 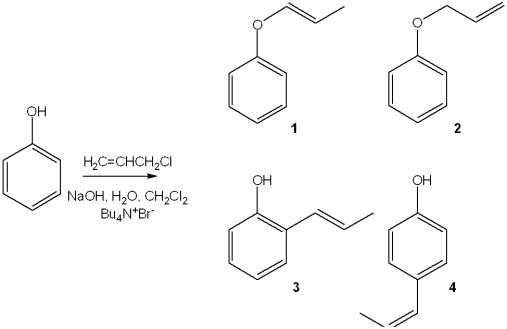
A)1
B)2
C)3
D)4

A)1
B)2
C)3
D)4

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
30
What is the best choice of reagent to achieve the following reaction? 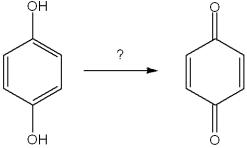
A)H2SO4
B)NaOH, H2O
C)K2Cr2O7, H2SO4
D)LiAlH4

A)H2SO4
B)NaOH, H2O
C)K2Cr2O7, H2SO4
D)LiAlH4

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
31
Which of the following molecules is the most acidic? 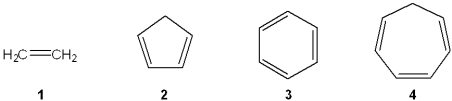
A)1
B)2
C)3
D)4

A)1
B)2
C)3
D)4

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
32
What is the major organic product obtained from the following reaction? 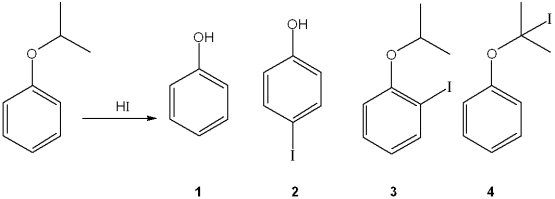
A)1
B)2
C)3
D)4

A)1
B)2
C)3
D)4

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
33
Which of the following represents the energy levels of the molecular orbitals of cyclopentadienyl anion? 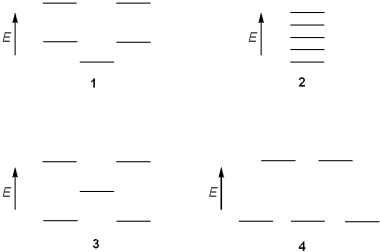
A)1
B)2
C)3
D)4

A)1
B)2
C)3
D)4

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
34
Which of the following has the compounds shown in the correct order of decreasing acidity (i.e., more acidic > less acidic)? 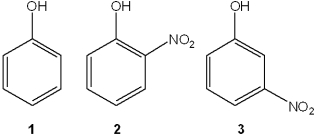
A)1 > 2 > 3
B)2 > 3 > 1
C)3 > 2 > 1
D)1 > 3 > 2

A)1 > 2 > 3
B)2 > 3 > 1
C)3 > 2 > 1
D)1 > 3 > 2

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
35
What is the best choice of reagent to achieve the following reaction? 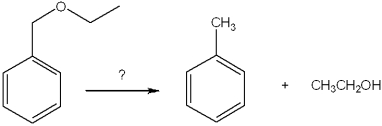
A)H2SO4
B)NaOH, H2O
C)K2Cr2O7, H2SO4
D)H2/Pd

A)H2SO4
B)NaOH, H2O
C)K2Cr2O7, H2SO4
D)H2/Pd

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
36
What is the major organic product obtained from the following reaction? 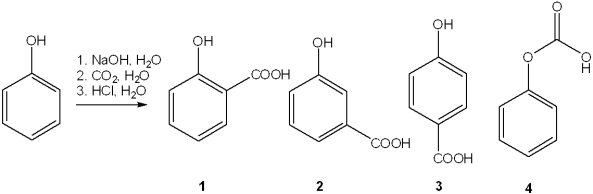
A)1
B)2
C)3
D)4

A)1
B)2
C)3
D)4

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
37
What is the major organic product obtained from the following reaction? 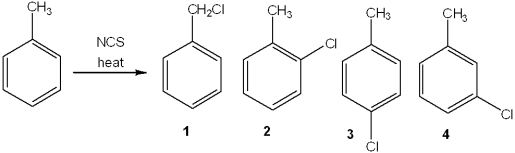
A)1
B)2
C)3
D)4

A)1
B)2
C)3
D)4

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
38
Which of the following compounds undergoes heterolytic carbon-halogen bond cleavage to form a stable organic cation? 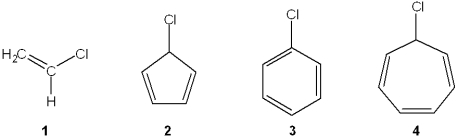
A)1
B)2
C)3
D)4

A)1
B)2
C)3
D)4

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
39
Which of the following has the compounds shown in the correct order of decreasing acidity (i.e., more acidic > less acidic)? 
A)1 > 2 > 3
B)2 > 1 > 3
C)3 > 2 > 1
D)1 > 3 > 2

A)1 > 2 > 3
B)2 > 1 > 3
C)3 > 2 > 1
D)1 > 3 > 2

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
40
What is the major organic product obtained from the following reaction? 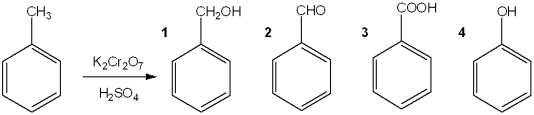
A)1
B)2
C)3
D)4

A)1
B)2
C)3
D)4

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
41
The following compound is named (Z)-2,3-diphenyl-2-hexene. 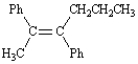


Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
42
Which of the following represents the energy levels of the molecular orbitals of cyclobutadiene? 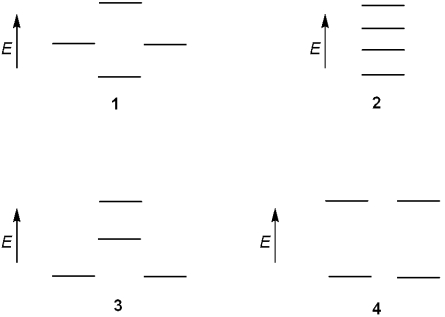
A)1
B)2
C)3
D)4

A)1
B)2
C)3
D)4

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
43
What are the relative positions of the substituents in the following structure? 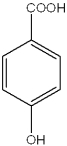
A)anti
B)meta
C)ortho
D)para

A)anti
B)meta
C)ortho
D)para

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
44
What are the relative positions of the substituents in the following structure? 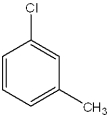
A)anti
B)meta
C)ortho
D)para

A)anti
B)meta
C)ortho
D)para

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
45
Furan has the structure shown below. 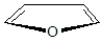 The orbitals in furan are shown below.
The orbitals in furan are shown below. 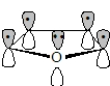
 The orbitals in furan are shown below.
The orbitals in furan are shown below. 

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
46
The product of the following reaction would be a dicarboxylic acid. 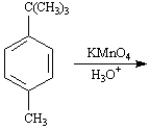


Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
47
Consider the following structures, 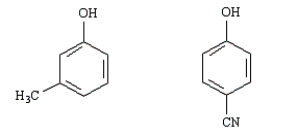 A B
A B
Structure A is more acidic than B.
 A B
A BStructure A is more acidic than B.

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
48
Consider the following structure. 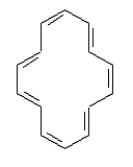 The 1H NMR spectrum of this compound -60 C shows a peak at 7.6 ppm, this would indicate aromaticity.
The 1H NMR spectrum of this compound -60 C shows a peak at 7.6 ppm, this would indicate aromaticity.
 The 1H NMR spectrum of this compound -60 C shows a peak at 7.6 ppm, this would indicate aromaticity.
The 1H NMR spectrum of this compound -60 C shows a peak at 7.6 ppm, this would indicate aromaticity. 
Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
49
The product of the following reaction would be 1-bromo-1-phenylethane. 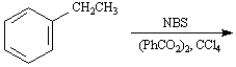
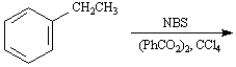

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
50
What are the relative positions of the substituents in the following structure? 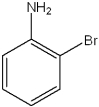
A)anti
B)meta
C)ortho
D)para

A)anti
B)meta
C)ortho
D)para

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
51
The following compound should be name as 1-((1S, 3R)-3-methylcyclohexyl)benzene. 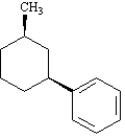


Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
52
How many p orbital electrons are present in cyclopentadienyl anion?
A)4
B)6
C)7
D)8
A)4
B)6
C)7
D)8

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
53
Which of the following is not true about [the bridged [10]annulene shown below? ![<strong>Which of the following is not true about [the bridged [10]annulene shown below? </strong> A)all of the carbon atoms of bridged [10]annulene are all in the same plane B)bridged [10]annulene is aromatic C)bridged [10]annulene undergoes substitution reactions similar to benzene D)bridged [10]annulene has 10 pi electrons](https://storage.examlex.com/TB1813/11ea7d75_f62a_8a0f_b9bd_25214fd27cc9_TB1813_00_TB1813_00.jpg)
A)all of the carbon atoms of bridged [10]annulene are all in the same plane
B)bridged [10]annulene is aromatic
C)bridged [10]annulene undergoes substitution reactions similar to benzene
D)bridged [10]annulene has 10 pi electrons
![<strong>Which of the following is not true about [the bridged [10]annulene shown below? </strong> A)all of the carbon atoms of bridged [10]annulene are all in the same plane B)bridged [10]annulene is aromatic C)bridged [10]annulene undergoes substitution reactions similar to benzene D)bridged [10]annulene has 10 pi electrons](https://storage.examlex.com/TB1813/11ea7d75_f62a_8a0f_b9bd_25214fd27cc9_TB1813_00_TB1813_00.jpg)
A)all of the carbon atoms of bridged [10]annulene are all in the same plane
B)bridged [10]annulene is aromatic
C)bridged [10]annulene undergoes substitution reactions similar to benzene
D)bridged [10]annulene has 10 pi electrons

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
54
Which of the following does not undergo oxidation in the presence of H2CrO4? 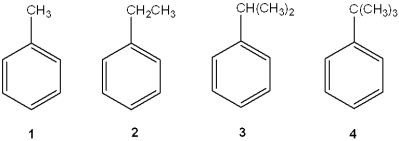
A)1
B)2
C)3
D)4

A)1
B)2
C)3
D)4

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
55
Which of the following is not true about [18]annulene? ![<strong>Which of the following is not true about [18]annulene? </strong> A)[18]annulene is planar B)[18]annulene is aromatic C)[18]annulene gives one peak in the <sup>1</sup>H NMR spectrum D)[18]annulene has 18 pi bonds](https://storage.examlex.com/TB1813/11ea7d75_f62a_62fd_b9bd_237c6774f0fd_TB1813_00_TB1813_00.jpg)
A)[18]annulene is planar
B)[18]annulene is aromatic
C)[18]annulene gives one peak in the 1H NMR spectrum
D)[18]annulene has 18 pi bonds
![<strong>Which of the following is not true about [18]annulene? </strong> A)[18]annulene is planar B)[18]annulene is aromatic C)[18]annulene gives one peak in the <sup>1</sup>H NMR spectrum D)[18]annulene has 18 pi bonds](https://storage.examlex.com/TB1813/11ea7d75_f62a_62fd_b9bd_237c6774f0fd_TB1813_00_TB1813_00.jpg)
A)[18]annulene is planar
B)[18]annulene is aromatic
C)[18]annulene gives one peak in the 1H NMR spectrum
D)[18]annulene has 18 pi bonds

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
56
What is the intermediate in the reaction of ethylbenzene with NBS in the presence of benzoyl peroxide to give 1-bromo-1-phenylethane?
A)Benzylic anion
B)Benzylic cation
C)Benzylic radical
D)Benzylic carbene
A)Benzylic anion
B)Benzylic cation
C)Benzylic radical
D)Benzylic carbene

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
57
How many p orbital electrons are present in furan?
A)4
B)6
C)7
D)8
A)4
B)6
C)7
D)8

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
58
Phenols are stronger acids than alcohols because of the resonance stabilization of alkoxide ions.

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
59
Which of the following is not true about cyclooctatetraene?
A)The planar conformation is antiaromatic
B)In the planar conformation there are two electrons in degenerate nonbonding pi orbitals.
C)In the tub conformation the pi system is not conjugated
D)The tub conformation is aromatic
A)The planar conformation is antiaromatic
B)In the planar conformation there are two electrons in degenerate nonbonding pi orbitals.
C)In the tub conformation the pi system is not conjugated
D)The tub conformation is aromatic

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
60
Which of the following is true about [10]annulene? ![<strong>Which of the following is true about [10]annulene? </strong> A)[10]annulene is planar B)[10]annulene is nonaromatic C)[10]annulene undergoes addition reactions similar to simple alkenes D)[10]annulene has 10 pi electrons](https://storage.examlex.com/TB1813/11ea7d75_f62a_62fe_b9bd_f5ee390c6844_TB1813_00_TB1813_00.jpg)
A)[10]annulene is planar
B)[10]annulene is nonaromatic
C)[10]annulene undergoes addition reactions similar to simple alkenes
D)[10]annulene has 10 pi electrons
![<strong>Which of the following is true about [10]annulene? </strong> A)[10]annulene is planar B)[10]annulene is nonaromatic C)[10]annulene undergoes addition reactions similar to simple alkenes D)[10]annulene has 10 pi electrons](https://storage.examlex.com/TB1813/11ea7d75_f62a_62fe_b9bd_f5ee390c6844_TB1813_00_TB1813_00.jpg)
A)[10]annulene is planar
B)[10]annulene is nonaromatic
C)[10]annulene undergoes addition reactions similar to simple alkenes
D)[10]annulene has 10 pi electrons

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
61
The following compound could be produced by reaction of phenol with bromobenzene. 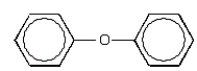


Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
62
What is the major organic product obtained from the following reaction? 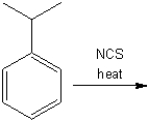


Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
63
Consider the following structure. ![Consider the following structure. If named as an annulene, the name would be [ __ ] annulene.](https://storage.examlex.com/TB1813/11ea7d75_f62b_e9af_b9bd_97701e65a992_TB1813_00_TB1813_00_TB1813_00_TB1813_00_TB1813_00_TB1813_00.jpg)
If named as an annulene, the name would be [ __ ] annulene.
![Consider the following structure. If named as an annulene, the name would be [ __ ] annulene.](https://storage.examlex.com/TB1813/11ea7d75_f62b_e9af_b9bd_97701e65a992_TB1813_00_TB1813_00_TB1813_00_TB1813_00_TB1813_00_TB1813_00.jpg)
If named as an annulene, the name would be [ __ ] annulene.

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
64
Consider the following structure. ![Consider the following structure. The <sup>1</sup>H NMR spectrum of [14]annulene at -60<sup> \circ </sup>C shows two peaks, one at 0 ppm and one at 7.6 ppm. -The NMR data would seem to indicate that this compound is ____________.](https://storage.examlex.com/TB1813/11ea7d75_f62c_10c0_b9bd_093820c2d3a1_TB1813_00_TB1813_00_TB1813_00_TB1813_00.jpg) The 1H NMR spectrum of [14]annulene at -60 C shows two peaks, one at 0 ppm and one at 7.6 ppm.
The 1H NMR spectrum of [14]annulene at -60 C shows two peaks, one at 0 ppm and one at 7.6 ppm.
-The NMR data would seem to indicate that this compound is ____________.
![Consider the following structure. The <sup>1</sup>H NMR spectrum of [14]annulene at -60<sup> \circ </sup>C shows two peaks, one at 0 ppm and one at 7.6 ppm. -The NMR data would seem to indicate that this compound is ____________.](https://storage.examlex.com/TB1813/11ea7d75_f62c_10c0_b9bd_093820c2d3a1_TB1813_00_TB1813_00_TB1813_00_TB1813_00.jpg) The 1H NMR spectrum of [14]annulene at -60 C shows two peaks, one at 0 ppm and one at 7.6 ppm.
The 1H NMR spectrum of [14]annulene at -60 C shows two peaks, one at 0 ppm and one at 7.6 ppm.-The NMR data would seem to indicate that this compound is ____________.

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
65
Consider the following structure. 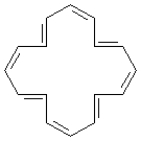
-This structure contains ____ electrons.

-This structure contains ____ electrons.

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
66
Consider the following general structure. 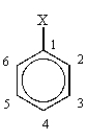 Positions 1,4 and 2,6 and 3,5 are termed para, ortho, and meta, respectively.
Positions 1,4 and 2,6 and 3,5 are termed para, ortho, and meta, respectively.
 Positions 1,4 and 2,6 and 3,5 are termed para, ortho, and meta, respectively.
Positions 1,4 and 2,6 and 3,5 are termed para, ortho, and meta, respectively.
Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
67
Answer the following questions concerning sulfathiazole below by filling ieach blank with the appropriate response. 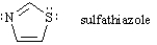
The hybridization of the nitrogen atom in sulfathiazole is ______.
A. sp
B. sp2
C. sp3
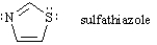
The hybridization of the nitrogen atom in sulfathiazole is ______.
A. sp
B. sp2
C. sp3

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
68
Answer the following questions concerning sulfathiazole below by filling ieach blank with the appropriate response. 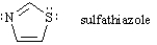
According to Hückel criteria, sulfathiazole is predicted to be ___________.
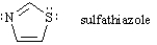
According to Hückel criteria, sulfathiazole is predicted to be ___________.

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
69
Consider the cycloheptatrienyl anion. Complete the following questions by filling in the blank with the appropriate word.
Consider the following compound. When treated with acidic K2Cr2O7 the product would be classified as a ___________.
When treated with acidic K2Cr2O7 the product would be classified as a ___________.
Consider the following compound.
 When treated with acidic K2Cr2O7 the product would be classified as a ___________.
When treated with acidic K2Cr2O7 the product would be classified as a ___________.
Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
70
Consider the following structure. 
Using the Hückel criteria, the compound would be ______________.

Using the Hückel criteria, the compound would be ______________.

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
71
What is the IUPAC name of the following compound? 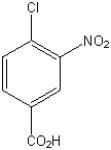


Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
72
Consider the cycloheptatrienyl anion. Complete the following questions by filling in the blank with the appropriate word.
-This anion has______ electrons.
-This anion has______ electrons.

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
73
What is the major organic product obtained from the following reaction? 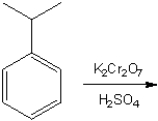


Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
74
Consider the cycloheptatrienyl anion. Complete the following questions by filling in the blank with the appropriate word.
Consider the following two compounds.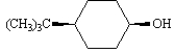
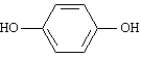 A B
A B
An ether solution containing these two substances was mixed with an aqueous 1 M NaOH solution. Substance _____ will be found in the basic layer.
Consider the following two compounds.

 A B
A BAn ether solution containing these two substances was mixed with an aqueous 1 M NaOH solution. Substance _____ will be found in the basic layer.

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
75
Consider the cycloheptatrienyl anion. Complete the following questions by filling in the blank with the appropriate word.
According the Hückel criteria this anion would be classified as ______________.
According the Hückel criteria this anion would be classified as ______________.

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
76
What is the IUPAC name of the following compound? 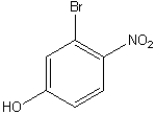


Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
77
Consider the following structure. ![Consider the following structure. The <sup>1</sup>H NMR spectrum of [14]annulene at -60<sup> \circ </sup>C shows two peaks, one at 0 ppm and one at 7.6 ppm. -The ratio of peak areas, respectively for the 7.6 ppm and 0 ppm, peaks would be_______.](https://storage.examlex.com/TB1813/11ea7d75_f62c_10c0_b9bd_093820c2d3a1_TB1813_00_TB1813_00_TB1813_00_TB1813_00.jpg) The 1H NMR spectrum of [14]annulene at -60 C shows two peaks, one at 0 ppm and one at 7.6 ppm.
The 1H NMR spectrum of [14]annulene at -60 C shows two peaks, one at 0 ppm and one at 7.6 ppm.
-The ratio of peak areas, respectively for the 7.6 ppm and 0 ppm, peaks would be_______.
![Consider the following structure. The <sup>1</sup>H NMR spectrum of [14]annulene at -60<sup> \circ </sup>C shows two peaks, one at 0 ppm and one at 7.6 ppm. -The ratio of peak areas, respectively for the 7.6 ppm and 0 ppm, peaks would be_______.](https://storage.examlex.com/TB1813/11ea7d75_f62c_10c0_b9bd_093820c2d3a1_TB1813_00_TB1813_00_TB1813_00_TB1813_00.jpg) The 1H NMR spectrum of [14]annulene at -60 C shows two peaks, one at 0 ppm and one at 7.6 ppm.
The 1H NMR spectrum of [14]annulene at -60 C shows two peaks, one at 0 ppm and one at 7.6 ppm.-The ratio of peak areas, respectively for the 7.6 ppm and 0 ppm, peaks would be_______.

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
78
Answer the following questions concerning sulfathiazole below by filling ieach blank with the appropriate response. 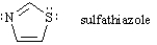
-Assuming that the sulfur atom is sp2-hybridized, there are _____ -electrons in the sulfathiazole ring.
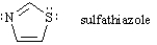
-Assuming that the sulfur atom is sp2-hybridized, there are _____ -electrons in the sulfathiazole ring.

Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
79
What is the IUPAC name of the following compound? 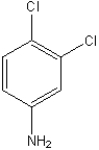


Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck
80
What is the IUPAC name of the following compound? 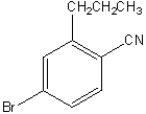


Unlock Deck
Unlock for access to all 85 flashcards in this deck.
Unlock Deck
k this deck


