Deck 2: Alkanes and Cycloalkanes
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/95
Play
Full screen (f)
Deck 2: Alkanes and Cycloalkanes
1
What is the name of the linear hydrocarbon with the molecular formula C7H16?
A)hexane
B)heptane
C)decane
D)undecane
A)hexane
B)heptane
C)decane
D)undecane
heptane
2
What is the correct assignment of common names for the following molecules? 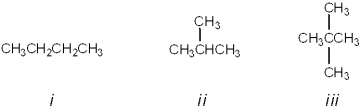
A)i = butane; ii = neopentane; iii = isopentane
B)i = neobutane; ii = isobutane; iii = pentane
C)i = butane; ii = isobutane; iii = isopentane
D)i = butane; ii = isobutane; iii = neopentane

A)i = butane; ii = neopentane; iii = isopentane
B)i = neobutane; ii = isobutane; iii = pentane
C)i = butane; ii = isobutane; iii = isopentane
D)i = butane; ii = isobutane; iii = neopentane
i = butane; ii = isobutane; iii = neopentane
3
What is the approximate C-C-C bond angle in propane?
A)90
B)109
C)120
D)180
A)90
B)109
C)120
D)180
109
4
What is the IUPAC name of the following compound? 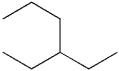
A)3-propylpentane
B)1,1-diethylpropane
C)3-ethylhexane
D)isooctane

A)3-propylpentane
B)1,1-diethylpropane
C)3-ethylhexane
D)isooctane

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
5
What is the IUPAC name of the following compound? 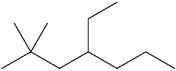
A)2,2-dimethyl-4-ethylheptane
B)4-ethyl-2,2-dimethyl-heptane
C)6,6-dimethyl-4-ethylheptane
D)4-ethyl-6,6-dimethyl-heptane

A)2,2-dimethyl-4-ethylheptane
B)4-ethyl-2,2-dimethyl-heptane
C)6,6-dimethyl-4-ethylheptane
D)4-ethyl-6,6-dimethyl-heptane

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
6
How many hydrogen atoms are there in nonane, the linear hydrocarbon with nine carbon atoms?
A)16
B)18
C)20
D)22
A)16
B)18
C)20
D)22

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
7
What is the IUPAC name of the following compound? 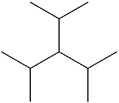
A)2,4-dimethyl-3-isopropyl-pentane
B)3-isopropyl-1,5-dimethylpentane
C)3-isopropyl-2,4-dimethylpentane
D)triisopropylmethane

A)2,4-dimethyl-3-isopropyl-pentane
B)3-isopropyl-1,5-dimethylpentane
C)3-isopropyl-2,4-dimethylpentane
D)triisopropylmethane

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
8
How many hydrogen atoms are there in dodecane, the linear hydrocarbon with twelve carbon atoms?
A)12
B)20
C)24
D)26
A)12
B)20
C)24
D)26

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
9
What is the IUPAC name of the following compound? 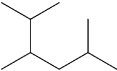
A)2-isopropyl-5-methylpentane
B)5-isopropyl-2-methylpentane
C)2,3,5-trimethylhexane
D)1,2-diisopropylpropane

A)2-isopropyl-5-methylpentane
B)5-isopropyl-2-methylpentane
C)2,3,5-trimethylhexane
D)1,2-diisopropylpropane

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
10
What is the IUPAC name of the following compound? 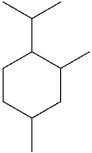
A)1-isopropyl-4,6-dimethylcyclohexane
B)1-isopropyl-2,4-dimethylcyclohexane
C)4-isopropyl-1,3-dimethylcyclohexane
D)4-isopropyl-1,5-dimethylcyclohexane

A)1-isopropyl-4,6-dimethylcyclohexane
B)1-isopropyl-2,4-dimethylcyclohexane
C)4-isopropyl-1,3-dimethylcyclohexane
D)4-isopropyl-1,5-dimethylcyclohexane

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
11
Approximately how long is a C-C single bond of an alkane?
A)111 pm
B)134 pm
C)142 pm
D)153 pm
A)111 pm
B)134 pm
C)142 pm
D)153 pm

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
12
What is the correct assignment of common names for the following molecules? 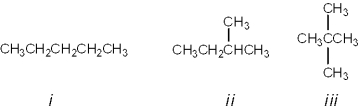
A)i = pentane; ii = isopentane; iii = neopentane
B)i = neopentane; ii = isopentane; iii = pentane
C)i = pentane; ii = neopentane; iii = isopentane
D)i = neopentane; ii = pentane; iii = isopentane

A)i = pentane; ii = isopentane; iii = neopentane
B)i = neopentane; ii = isopentane; iii = pentane
C)i = pentane; ii = neopentane; iii = isopentane
D)i = neopentane; ii = pentane; iii = isopentane

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
13
Which of the following compounds has only 1 and 3 carbon atoms?
A)hexane
B)2-methylpentane
C)3-methylpentane
D)2,3-dimethylbutane
A)hexane
B)2-methylpentane
C)3-methylpentane
D)2,3-dimethylbutane

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
14
How many constitutional isomers are there with the molecular formula C5H12?
A)2
B)3
C)4
D)5
A)2
B)3
C)4
D)5

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
15
What is the IUPAC name of the following compound? 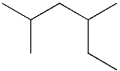
A)2-ethyl-4-methylpentane
B)2,4-dimethylhexane
C)3,5-dimethylhexane
D)1,1,3-trimethylpentane

A)2-ethyl-4-methylpentane
B)2,4-dimethylhexane
C)3,5-dimethylhexane
D)1,1,3-trimethylpentane

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
16
How many constitutional isomers are there with the molecular formula C4H10?
A)2
B)3
C)4
D)5
A)2
B)3
C)4
D)5

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
17
What is the name of the linear hydrocarbon with the molecular formula C11H24?
A)heptane
B)decane
C)undecane
D)eicosane
A)heptane
B)decane
C)undecane
D)eicosane

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
18
How many constitutional isomers are there with the molecular formula C6H14?
A)3
B)4
C)5
D)8
A)3
B)4
C)5
D)8

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
19
What is the IUPAC name of the following compound? 
A)5,5-dimethyl-3-ethylheptane
B)5-ethyl-3,3-dimethyl-heptane
C)3,3-dimethyl-5-ethylheptane
D)3-ethyl-5,5-dimethyl-heptane

A)5,5-dimethyl-3-ethylheptane
B)5-ethyl-3,3-dimethyl-heptane
C)3,3-dimethyl-5-ethylheptane
D)3-ethyl-5,5-dimethyl-heptane

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
20
Which of the following compounds has 1 , 2 , 3 and 4 carbon atoms?
A)hexane
B)2-methylhexane
C)2,2-dimethylhexane
D)2,2,3-trimethylhexane
A)hexane
B)2-methylhexane
C)2,2-dimethylhexane
D)2,2,3-trimethylhexane

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
21
Which one of the following structures represents a different compound from the other three? 
A)1
B)2
C)3
D)4

A)1
B)2
C)3
D)4

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
22
What is the IUPAC name of the following compound? ![<strong>What is the IUPAC name of the following compound? </strong> A)1-methylbicyclo[2.2.1]heptane B)2-methylbicyclo[2.2.1]heptane C)3-methylbicyclo[2.2.1]heptane D)4-methylbicyclo[2.2.1]heptane](https://storage.examlex.com/TB1813/11ea7d75_f5dc_42cb_b9bd_f551d639aacd_TB1813_00_TB1813_00.jpg)
A)1-methylbicyclo[2.2.1]heptane
B)2-methylbicyclo[2.2.1]heptane
C)3-methylbicyclo[2.2.1]heptane
D)4-methylbicyclo[2.2.1]heptane
![<strong>What is the IUPAC name of the following compound? </strong> A)1-methylbicyclo[2.2.1]heptane B)2-methylbicyclo[2.2.1]heptane C)3-methylbicyclo[2.2.1]heptane D)4-methylbicyclo[2.2.1]heptane](https://storage.examlex.com/TB1813/11ea7d75_f5dc_42cb_b9bd_f551d639aacd_TB1813_00_TB1813_00.jpg)
A)1-methylbicyclo[2.2.1]heptane
B)2-methylbicyclo[2.2.1]heptane
C)3-methylbicyclo[2.2.1]heptane
D)4-methylbicyclo[2.2.1]heptane

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
23
Which of the following cycloalkanes has the least ring strain?
A)cyclopropane
B)cyclopentane
C)cyclohexane
D)cycloheptane
A)cyclopropane
B)cyclopentane
C)cyclohexane
D)cycloheptane

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
24
Which of the following Newman projections represents 2,4-dimethylpentane? 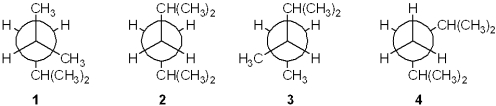
A)1
B)2
C)3
D)4

A)1
B)2
C)3
D)4

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
25
What is the IUPAC name for the following compound? ![<strong>What is the IUPAC name for the following compound? </strong> A)bicyclo[5.4.3]octane B)bicyclo[3.2.1]octane C)bicyclo[3.2.1]hexane D)bicyclo[2.2.1]octane](https://storage.examlex.com/TB1813/11ea7d75_f5dc_69de_b9bd_dfc5affc62fd_TB1813_00_TB1813_00.jpg)
A)bicyclo[5.4.3]octane
B)bicyclo[3.2.1]octane
C)bicyclo[3.2.1]hexane
D)bicyclo[2.2.1]octane
![<strong>What is the IUPAC name for the following compound? </strong> A)bicyclo[5.4.3]octane B)bicyclo[3.2.1]octane C)bicyclo[3.2.1]hexane D)bicyclo[2.2.1]octane](https://storage.examlex.com/TB1813/11ea7d75_f5dc_69de_b9bd_dfc5affc62fd_TB1813_00_TB1813_00.jpg)
A)bicyclo[5.4.3]octane
B)bicyclo[3.2.1]octane
C)bicyclo[3.2.1]hexane
D)bicyclo[2.2.1]octane

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
26
Which of the following cycloalkanes has the most ring strain?
A)cyclopropane
B)cyclobutane
C)cyclopentane
D)cyclohexane
A)cyclopropane
B)cyclobutane
C)cyclopentane
D)cyclohexane

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
27
Which of the following Newman projections represents the most stable conformation of 2,3-dimethylbutane? 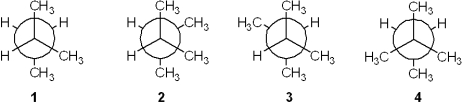
A)1
B)2
C)3
D)4

A)1
B)2
C)3
D)4

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
28
Which of the following structures represents trans-1,2-dimethylcyclohexane? 
A)1
B)2
C)3
D)4

A)1
B)2
C)3
D)4

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
29
Which of the following is the most stable conformation of trans-1-ethyl-3-methylcyclohexane? 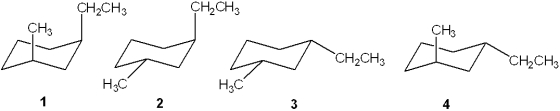
A)1
B)2
C)3
D)4

A)1
B)2
C)3
D)4

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
30
What is the IUPAC name of the following compound? 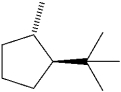
A)trans-1-isopropyl-4-methylcyclopentane
B)cis-1-tert-butyl-2-methylcyclopentane
C)trans-1-tert-butyl-2-methylcyclopentane
D)cis-1-isopropyl-2-methylcyclopentane

A)trans-1-isopropyl-4-methylcyclopentane
B)cis-1-tert-butyl-2-methylcyclopentane
C)trans-1-tert-butyl-2-methylcyclopentane
D)cis-1-isopropyl-2-methylcyclopentane

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
31
Which of the following is the most stable conformation of cis-1-isopropyl-3-methylcyclohexane? 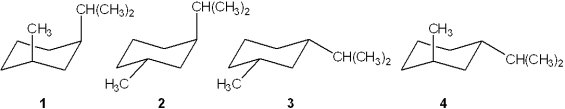
A)1
B)2
C)3
D)4

A)1
B)2
C)3
D)4

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
32
Which of the following structures represents trans-1,3-dimethylcyclohexane? 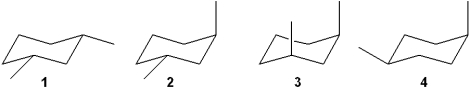
A)1
B)2
C)3
D)4

A)1
B)2
C)3
D)4

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
33
Which of the following Newman projections represents the most stable conformation of 2-methylbutane? 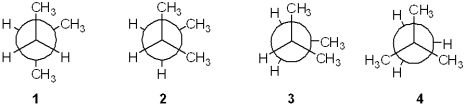
A)1
B)2
C)3
D)4

A)1
B)2
C)3
D)4

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
34
Which of the following alkanes has the highest boiling point?
A)2,3-dimethylbutane
B)2-methylpentane
C)3-methylpentane
D)hexane
A)2,3-dimethylbutane
B)2-methylpentane
C)3-methylpentane
D)hexane

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
35
Which of the following Newman projections does not represent 2-methylhexane? 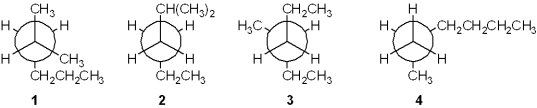
A)1
B)2
C)3
D)4

A)1
B)2
C)3
D)4

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
36
What is the IUPAC name for the following compound? ![<strong>What is the IUPAC name for the following compound? </strong> A)cycloheptane B)bicyclo[3.2.0]heptane C)bicyclo[5.4]heptane D)cyclobutylcyclopentane](https://storage.examlex.com/TB1813/11ea7d75_f5dc_69dd_b9bd_09857691b9d7_TB1813_00_TB1813_00.jpg)
A)cycloheptane
B)bicyclo[3.2.0]heptane
C)bicyclo[5.4]heptane
D)cyclobutylcyclopentane
![<strong>What is the IUPAC name for the following compound? </strong> A)cycloheptane B)bicyclo[3.2.0]heptane C)bicyclo[5.4]heptane D)cyclobutylcyclopentane](https://storage.examlex.com/TB1813/11ea7d75_f5dc_69dd_b9bd_09857691b9d7_TB1813_00_TB1813_00.jpg)
A)cycloheptane
B)bicyclo[3.2.0]heptane
C)bicyclo[5.4]heptane
D)cyclobutylcyclopentane

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
37
What is the IUPAC name of the following compound? ![<strong>What is the IUPAC name of the following compound? </strong> A)bicyclo[4.3]nonane B)bicyclo[4.3.0]nonane C)bicyclo[6.5]nonane D)bicyclo[6.5.0]nonane](https://storage.examlex.com/TB1813/11ea7d75_f5dc_69dc_b9bd_a543483b5fda_TB1813_00_TB1813_00.jpg)
A)bicyclo[4.3]nonane
B)bicyclo[4.3.0]nonane
C)bicyclo[6.5]nonane
D)bicyclo[6.5.0]nonane
![<strong>What is the IUPAC name of the following compound? </strong> A)bicyclo[4.3]nonane B)bicyclo[4.3.0]nonane C)bicyclo[6.5]nonane D)bicyclo[6.5.0]nonane](https://storage.examlex.com/TB1813/11ea7d75_f5dc_69dc_b9bd_a543483b5fda_TB1813_00_TB1813_00.jpg)
A)bicyclo[4.3]nonane
B)bicyclo[4.3.0]nonane
C)bicyclo[6.5]nonane
D)bicyclo[6.5.0]nonane

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
38
Which one of the following structures represents a different compound from the other three? 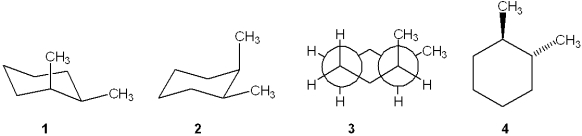
A)1
B)2
C)3
D)4

A)1
B)2
C)3
D)4

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
39
Which of the following alkanes has the highest boiling point?
A)propane
B)butane
C)pentane
D)hexane
A)propane
B)butane
C)pentane
D)hexane

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
40
What is the IUPAC name of the following compound? 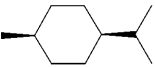
A)trans-1-isopropyl-4-methylcyclohexane
B)cis-1-isopropyl-4-methylcyclohexane
C)cis-2-isopropyl-5-methylcyclohexane
D)cis-1-tert-butyl-4-methylcyclohexane

A)trans-1-isopropyl-4-methylcyclohexane
B)cis-1-isopropyl-4-methylcyclohexane
C)cis-2-isopropyl-5-methylcyclohexane
D)cis-1-tert-butyl-4-methylcyclohexane

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
41
What is the approximate dihedral angle between the two chlorine atoms in the diaxial conformation of trans-1,2-dichlorocyclohexane?
A)0
B)60
C)120
D)180
A)0
B)60
C)120
D)180

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
42
In which of the following compounds are all of the carbon atoms in the same plane?
A)cyclopropane
B)cyclobutane
C)cyclopentane
D)cyclohexane
A)cyclopropane
B)cyclobutane
C)cyclopentane
D)cyclohexane

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
43
Which of the following structures is different from the other three? 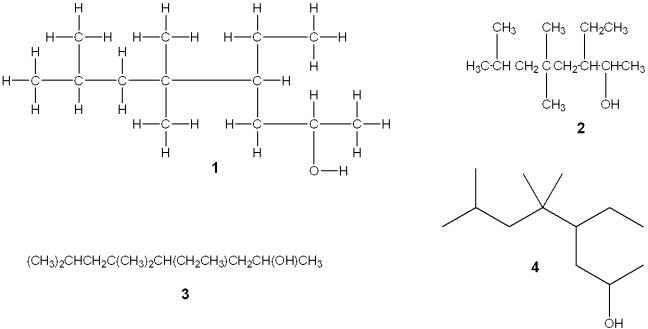
A)1
B)2
C)3
D)4

A)1
B)2
C)3
D)4

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
44
Which of the following structures is different from the other three? 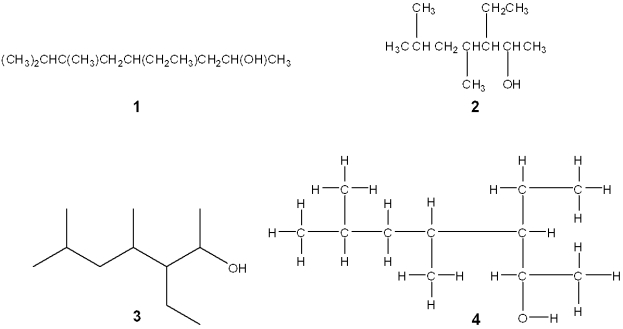
A)1
B)2
C)3
D)4

A)1
B)2
C)3
D)4

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
45
Which of the following compounds can adopt a chair conformation in which there are no axial methyl groups?
A)1,1-dimethylcyclohexane
B)cis-1,2-dimethylcyclohexane
C)trans-1,2-dimethylcyclohexane
D)cis-1,3-dimethylcyclohexane
A)1,1-dimethylcyclohexane
B)cis-1,2-dimethylcyclohexane
C)trans-1,2-dimethylcyclohexane
D)cis-1,3-dimethylcyclohexane

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
46
What is the approximate dihedral angle between the two chlorine atoms in the diequatorial conformation of trans-1,2-dichlorocyclohexane?
A)0
B)60
C)120
D)180
A)0
B)60
C)120
D)180

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
47
Which of the following cycloalkanes has the smallest heat of combustion per carbon atom?
A)cyclopropane
B)cyclopentane
C)cyclohexane
D)cycloheptane
A)cyclopropane
B)cyclopentane
C)cyclohexane
D)cycloheptane

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
48
Which of the following substituted cyclohexanes has the most negative value of G for ring flipping from the conformation in which the substituent is axial to the one where it is equatorial?
A)methylcyclohexane
B)chlorocyclohexane
C)isopropylcyclohexane
D)ethynylcyclohexane
A)methylcyclohexane
B)chlorocyclohexane
C)isopropylcyclohexane
D)ethynylcyclohexane

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
49
Which of the following undergoes the most exothermic combustion?
A)octane
B)2-methylheptane
C)2,2-dimethylhexane
D)2,2,3,3-tetramethylbutane
A)octane
B)2-methylheptane
C)2,2-dimethylhexane
D)2,2,3,3-tetramethylbutane

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
50
Which of the following compounds can adopt a chair conformation in which there are no axial methyl groups?
A)cis-1,2-dimethylcyclohexane
B)cis-1,3-dimethylcyclohexane
C)trans-1,3-dimethylcyclohexane
D)cis-1,4-dimethylcyclohexane
A)cis-1,2-dimethylcyclohexane
B)cis-1,3-dimethylcyclohexane
C)trans-1,3-dimethylcyclohexane
D)cis-1,4-dimethylcyclohexane

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
51
How many moles of molecular oxygen (O2) are consumed in the complete combustion of one mole of octane (C8H18)?
A)12.5
B)13
C)17
D)26
A)12.5
B)13
C)17
D)26

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
52
Which of the following statements is not true?
A)Combustion of an alkane is an exothermic reaction.
B)The heat of combustion of propane is three times that of methane.
C)The constitutional isomers of C7H16 have different heats of combustion from one another
D)The products of combustion of an alkane are H2O and CO2.
A)Combustion of an alkane is an exothermic reaction.
B)The heat of combustion of propane is three times that of methane.
C)The constitutional isomers of C7H16 have different heats of combustion from one another
D)The products of combustion of an alkane are H2O and CO2.

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
53
Which of the following is the steroid nucleus? 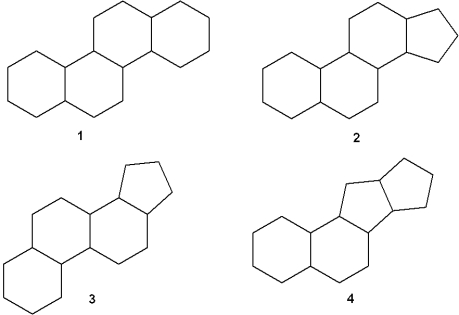
A)1
B)2
C)3
D)4

A)1
B)2
C)3
D)4

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
54
Which of the following cycloalkanes has the largest heat of combustion per carbon atom?
A)cyclopropane
B)cyclopentane
C)cyclohexane
D)cycloheptane
A)cyclopropane
B)cyclopentane
C)cyclohexane
D)cycloheptane

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
55
How many moles of molecular oxygen (O2) are consumed in the complete combustion of one mole of hexane (C6H14)?
A)6
B)9.5
C)12.5
D)14
A)6
B)9.5
C)12.5
D)14

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
56
What is the approximate dihedral angle between the two chlorine atoms in cis-1,2-dichlorocyclohexane?
A)0
B)60
C)120
D)180
A)0
B)60
C)120
D)180

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
57
Which of the following is not true regarding the properties of alkanes?
A)alkanes are nonpolar
B)alkanes burn in air to give H2O and CO2
C)alkanes are highly miscible with water
D)the strongest intermolecular force between alkane molecules is the van der Waals interaction
A)alkanes are nonpolar
B)alkanes burn in air to give H2O and CO2
C)alkanes are highly miscible with water
D)the strongest intermolecular force between alkane molecules is the van der Waals interaction

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
58
Which of the following statements is not true regarding the conformation of substituted cyclohexanes?
A)ring inversion of cyclohexane between two chair conformations takes place via a boat conformation
B)substituted cyclohexanes are destabilized by 1,3-diaxial interactions
C)the boat conformation of cyclohexane is usually more stable than the chair conformation
D)the relative amount of two conformations of substituted cyclohexanes can be determined from the difference in strain energy
A)ring inversion of cyclohexane between two chair conformations takes place via a boat conformation
B)substituted cyclohexanes are destabilized by 1,3-diaxial interactions
C)the boat conformation of cyclohexane is usually more stable than the chair conformation
D)the relative amount of two conformations of substituted cyclohexanes can be determined from the difference in strain energy

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
59
Which of the following substituted cyclohexanes has the most negative value of G for ring flipping from the conformation in which the substituent is axial to the one where it is equatorial?
A)fluorocyclohexane
B)methylcyclohexane
C)ethylcyclohexane
D)tert-butylcyclohexane
A)fluorocyclohexane
B)methylcyclohexane
C)ethylcyclohexane
D)tert-butylcyclohexane

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
60
Which of the following cycloalkanes has the largest heat of combustion?
A)cyclopropane
B)cyclobutane
C)cyclopentane
D)cyclohexane
A)cyclopropane
B)cyclobutane
C)cyclopentane
D)cyclohexane

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
61
The following Newman projection represents 2-methylhexane. 


Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
62
In the following conversion, the conformations are of equal stability. 


Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
63
The following pairs of Newman projections represent the same compound but in differing conformations. 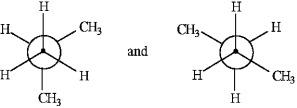
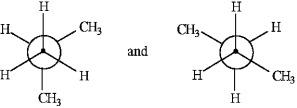

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
64
What is the IUPAC name of the following compound? 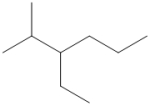


Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
65
Match the Newman projection for the conformation of 2-methylbutane to the indicated position on the potential energy diagram. 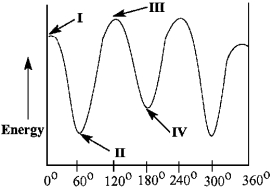 A)
A)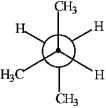 B)
B)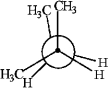 C)
C)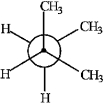
D)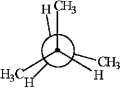
Conformation A is represented by Roman numeral ____.
 A)
A) B)
B) C)
C)
D)

Conformation A is represented by Roman numeral ____.

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
66
The tertiary carbon atom in the following structure is indicated by the letter_____. 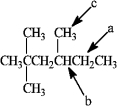


Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
67
What is the IUPAC name of the following compound? 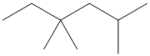


Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
68
What is the IUPAC name of the following compound? 


Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
69
The following structures represent, from left to right, a cis and a trans isomer. 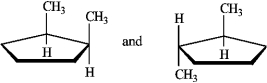


Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
70
What is the IUPAC name of the following compound? 


Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
71
In the following structure the positions labeled a and d are equatorial positions. 


Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
72
The correct IUPAC name for the following compound is___________________________. 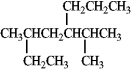


Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
73
Match the Newman projection for the conformation of 2-methylbutane to the indicated position on the potential energy diagram.  A)
A) B)
B) C)
C)
D)
Conformation C is represented by Roman numeral ____.
 A)
A) B)
B) C)
C)
D)

Conformation C is represented by Roman numeral ____.

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
74
Match the Newman projection for the conformation of 2-methylbutane to the indicated position on the potential energy diagram.  A)
A) B)
B) C)
C)
D)
Conformation B is represented by Roman numeral ____.
 A)
A) B)
B) C)
C)
D)

Conformation B is represented by Roman numeral ____.

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
75
The Newman projection of the gauche conformation of 1,2-dichloroethane is shown below. 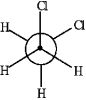


Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
76
Match the Newman projection for the conformation of 2-methylbutane to the indicated position on the potential energy diagram.  A)
A) B)
B) C)
C)
D)
Conformation D is represented by Roman numeral ____.
 A)
A) B)
B) C)
C)
D)

Conformation D is represented by Roman numeral ____.

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
77
What is the IUPAC name of the following compound? 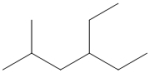


Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
78
There are four constitutional isomers for the molecular formula C6H14.

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
79
The most stable conformation of an alkane occurs when carbon-carbon bonds are staggered and bulky groups are anti.

Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck
80
The following structures represent a pair of constitutional isomers. 


Unlock Deck
Unlock for access to all 95 flashcards in this deck.
Unlock Deck
k this deck


