Deck 4: Acids and Bases
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/94
Play
Full screen (f)
Deck 4: Acids and Bases
1
Which of the following compounds is the strongest acid?
A)CH4
B)CH3CH3
C)H2C=CH2
D)HC CH
A)CH4
B)CH3CH3
C)H2C=CH2
D)HC CH
HC CH
2
The pKa of acetic acid, CH3COOH, is 4.76. What is the value of the equilibrium constant Keq, for the following equilibrium? [The concentration of water in a dilute aqueous solution is 55 M] ![<strong>The pK<sub>a</sub> of acetic acid, CH<sub>3</sub>COOH, is 4.76. What is the value of the equilibrium constant K<sub>eq</sub>, for the following equilibrium? [The concentration of water in a dilute aqueous solution is 55 M] </strong> A)3.2 * 10<sup>7</sup> B)-3.2 * 10<sup>-</sup><sup>7</sup> C)-3.2 * 10<sup>7</sup> D)3.2 *10<sup>-</sup><sup>7</sup>](https://storage.examlex.com/TB1813/11ea7d75_f5e3_6f2f_b9bd_77533aa092af_TB1813_00_TB1813_00.jpg)
A)"3.2 * 107"
B)"-3.2 * 10-7"
C)"-3.2 * 107"
D)"3.2 *10-7"
![<strong>The pK<sub>a</sub> of acetic acid, CH<sub>3</sub>COOH, is 4.76. What is the value of the equilibrium constant K<sub>eq</sub>, for the following equilibrium? [The concentration of water in a dilute aqueous solution is 55 M] </strong> A)3.2 * 10<sup>7</sup> B)-3.2 * 10<sup>-</sup><sup>7</sup> C)-3.2 * 10<sup>7</sup> D)3.2 *10<sup>-</sup><sup>7</sup>](https://storage.examlex.com/TB1813/11ea7d75_f5e3_6f2f_b9bd_77533aa092af_TB1813_00_TB1813_00.jpg)
A)"3.2 * 107"
B)"-3.2 * 10-7"
C)"-3.2 * 107"
D)"3.2 *10-7"
"3.2 *10-7"
3
Which of the following is the strongest acid?
A)CH3OH
B)CH3CHO
C)CH3COCH3
D)CH3COOH
A)CH3OH
B)CH3CHO
C)CH3COCH3
D)CH3COOH
CH3COOH
4
Which of the following is the correct order of decreasing basicity (stronger base > weaker base)?
A)NH3 > MeNH2 > H2O > HF
B)MeNH2 > NH3 > MeOH > CH4
C)NH3 > Me3N > H2O > MeOH
D)CH3COONa > NaOH > NaOMe > NaNMe2
A)NH3 > MeNH2 > H2O > HF
B)MeNH2 > NH3 > MeOH > CH4
C)NH3 > Me3N > H2O > MeOH
D)CH3COONa > NaOH > NaOMe > NaNMe2

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
5
The pKa of HCl is -7. What is the value of the equilibrium constant, Keq, for the following equilibrium? [The concentration of water in a dilute aqueous solution is 55 M] ![<strong>The pK<sub>a</sub> of HCl is -7. What is the value of the equilibrium constant, K<sub>eq</sub>, for the following equilibrium? [The concentration of water in a dilute aqueous solution is 55 M] </strong> A)1.8 * 10<sup>5</sup> B)385 C)1.8 * 10<sup>-</sup><sup>5</sup> D)-1.8* 10<sup>5</sup>](https://storage.examlex.com/TB1813/11ea7d75_f5e3_6f2e_b9bd_d531d02ebf21_TB1813_00_TB1813_00.jpg)
A)"1.8 * 105"
B)"385"
C)"1.8 * 10-5"
D)"-1.8* 105"
![<strong>The pK<sub>a</sub> of HCl is -7. What is the value of the equilibrium constant, K<sub>eq</sub>, for the following equilibrium? [The concentration of water in a dilute aqueous solution is 55 M] </strong> A)1.8 * 10<sup>5</sup> B)385 C)1.8 * 10<sup>-</sup><sup>5</sup> D)-1.8* 10<sup>5</sup>](https://storage.examlex.com/TB1813/11ea7d75_f5e3_6f2e_b9bd_d531d02ebf21_TB1813_00_TB1813_00.jpg)
A)"1.8 * 105"
B)"385"
C)"1.8 * 10-5"
D)"-1.8* 105"

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
6
Which of the following compounds has the highest pKa?
A)SiH4
B)H2S
C)PH3
D)HCl
A)SiH4
B)H2S
C)PH3
D)HCl

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
7
Which of the following compounds is the strongest acid?
A)CH3CH2CH2COOH
B)CH3CH2CHClCOOH
C)CH3CHClCH2COOH
D)ClCH2CH2CH2COOH
A)CH3CH2CH2COOH
B)CH3CH2CHClCOOH
C)CH3CHClCH2COOH
D)ClCH2CH2CH2COOH

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
8
Which of the following compounds is the strongest acid?
A)CH3COOH
B)ClCH2COOH
C)CH3CH2OH
D)ClCH2CH2OH
A)CH3COOH
B)ClCH2COOH
C)CH3CH2OH
D)ClCH2CH2OH

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
9
Which of the following is the strongest acid?
A)HCl
B)HI
C)HF
D)HBr
A)HCl
B)HI
C)HF
D)HBr

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
10
Which of the following is the strongest acid?
A)CH3NH2
B)CH3PH2
C)CH3OH
D)CH3SH
A)CH3NH2
B)CH3PH2
C)CH3OH
D)CH3SH

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
11
Which of the following compounds is the strongest acid?
A)CH3COOH
B)FCH2COOH
C)ClCH2COOH
D)BrCH2COOH
A)CH3COOH
B)FCH2COOH
C)ClCH2COOH
D)BrCH2COOH

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
12
Which of the following is the strongest acid?
A)CH3CH3
B)CH3NH2
C)CH3OH
D)CH3F
A)CH3CH3
B)CH3NH2
C)CH3OH
D)CH3F

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
13
Which of the following compounds is the strongest acid?
A)CF3OH
B)CF3CH2 CH2OH
C)CF3CH2 CH2 CH2OH
D)CF3CH2 CH2 CH2CH2OH
A)CF3OH
B)CF3CH2 CH2OH
C)CF3CH2 CH2 CH2OH
D)CF3CH2 CH2 CH2CH2OH

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
14
Which of the following is the strongest base?
A)iodide anion, I-
B)fluoride anion, F-
C)bromide anion, Br1-
D)chloride anion, Cl-
A)iodide anion, I-
B)fluoride anion, F-
C)bromide anion, Br1-
D)chloride anion, Cl-

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
15
Which of the following compounds is the strongest acid?
A)CH3OCH3
B)CH3CH2OH
C)CH3CHO
D)CH3CO2H
A)CH3OCH3
B)CH3CH2OH
C)CH3CHO
D)CH3CO2H

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
16
Which of the following is the strongest base?
A)NaOH
B)NaCO3
C)H2O
D)CH3OH
A)NaOH
B)NaCO3
C)H2O
D)CH3OH

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
17
Which of the following compounds is the strongest acid?
A)HF
B)H2O
C)NH3
D)CH4
A)HF
B)H2O
C)NH3
D)CH4

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
18
Which of the following is easiest to deprotonate?
A)CH4
B)CH3CH3
C)CH2=CH2
D)HC CH
A)CH4
B)CH3CH3
C)CH2=CH2
D)HC CH

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
19
Which of the following anions is the strongest base?
A)CH3COO-
B)HO-
C)NH2-
D)Cl-
A)CH3COO-
B)HO-
C)NH2-
D)Cl-

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
20
Which of the following anions is the strongest base?
A)NH2-
B)NH3
C)CH3CH=N-
D)CH3C N
A)NH2-
B)NH3
C)CH3CH=N-
D)CH3C N

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
21
Which of the following is a feature of a Lewis acid?
A)proton donor
B)proton acceptor
C)electron pair donor
D)electron pair acceptor
A)proton donor
B)proton acceptor
C)electron pair donor
D)electron pair acceptor

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
22
Which sets of curved arrows accounts for the protonation of propene with HI? 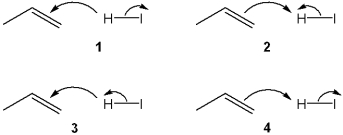
A)1
B)2
C)3
D)4

A)1
B)2
C)3
D)4

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
23
Which of the following terms describes the role of ethanol in the acid-base reaction shown? 
A)Brønsted-Lowry acid
B)Brønsted-Lowry base
C)Lewis acid
D)Lewis base

A)Brønsted-Lowry acid
B)Brønsted-Lowry base
C)Lewis acid
D)Lewis base

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
24
Which of the following is present in the highest concentration upon dissolution of H2SO4 in water?
A)H2SO4
B)H+
C)H3O+
D)HO-
A)H2SO4
B)H+
C)H3O+
D)HO-

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
25
Which set of curved arrows accounts for the deprotonation of an acid (A-H) by a base (:B)? 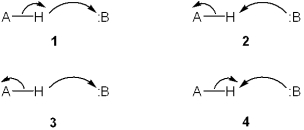
A)1
B)2
C)3
D)4

A)1
B)2
C)3
D)4

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
26
What is the value of the equilibrium constant, Keq, for the following reaction? 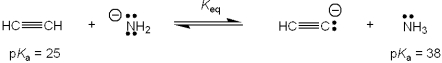
A)"1013"
B)"10-13"
C)"13"
D)"1/13"

A)"1013"
B)"10-13"
C)"13"
D)"1/13"

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
27
Which of the following terms describes the reactivity of trimethylamine, (CH3)3N?
A)Brønsted-Lowry acid and Lewis acid
B)Brønsted-Lowry base and Lewis base
C)Lewis acid and not a Brønsted-Lowry acid
D)Lewis base and not a Brønsted-Lowry base
A)Brønsted-Lowry acid and Lewis acid
B)Brønsted-Lowry base and Lewis base
C)Lewis acid and not a Brønsted-Lowry acid
D)Lewis base and not a Brønsted-Lowry base

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
28
Which of the following terms describes the role of ethene in the acid-base reaction shown? 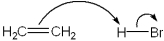
A)Brønsted-Lowry acid
B)Brønsted-Lowry base
C)Lewis acid
D)Lewis base

A)Brønsted-Lowry acid
B)Brønsted-Lowry base
C)Lewis acid
D)Lewis base

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
29
Which of the following is a feature of a Lewis base?
A)proton donor
B)proton acceptor
C)electron pair donor
D)electron pair acceptor
A)proton donor
B)proton acceptor
C)electron pair donor
D)electron pair acceptor

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
30
Which of the following is a Lewis acid but not a Brønsted-Lowry acid?
A)CH3COOH
B)AlCl3
C)H2O
D)CH3OH
A)CH3COOH
B)AlCl3
C)H2O
D)CH3OH

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
31
Which of the following terms describes the role of ethanol in the acid-base reaction shown? 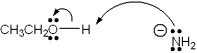
A)Brønsted-Lowry acid
B)Brønsted-Lowry base
C)Lewis acid
D)Lewis base

A)Brønsted-Lowry acid
B)Brønsted-Lowry base
C)Lewis acid
D)Lewis base

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
32
Which species is the conjugate acid in the following acid-base reaction? 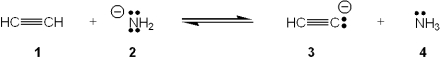
A)1
B)2
C)3
D)4

A)1
B)2
C)3
D)4

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
33
Which of the following compounds has the lowest pKa?
A)H2O
B)H2S
C)H2Se
D)H2Te
A)H2O
B)H2S
C)H2Se
D)H2Te

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
34
Which of the following compounds has the highest pKa?
A)NH3
B)H2O
C)HCl
D)CH4
A)NH3
B)H2O
C)HCl
D)CH4

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
35
What is the value of the equilibrium constant, Keq, for the following reaction? 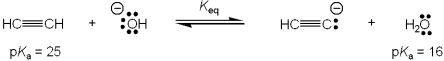
A)"109"
B)"10-9"
C)"9"
D)"1/9"

A)"109"
B)"10-9"
C)"9"
D)"1/9"

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
36
Which species is the conjugate acid in the following acid-base reaction? 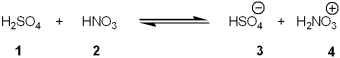
A)1
B)2
C)3
D)4

A)1
B)2
C)3
D)4

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
37
Which of the following compounds has the lowest pKa?
A)H2O
B)HBr
C)NH3
D)CH4
A)H2O
B)HBr
C)NH3
D)CH4

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
38
Which of the following terms describes the reactivity of boron tribromide, BBr3?
A)Brønsted-Lowry acid and Lewis acid
B)Brønsted-Lowry base and Lewis base
C)Lewis acid and not a Brønsted-Lowry acid
D)Lewis base and not a Brønsted-Lowry base
A)Brønsted-Lowry acid and Lewis acid
B)Brønsted-Lowry base and Lewis base
C)Lewis acid and not a Brønsted-Lowry acid
D)Lewis base and not a Brønsted-Lowry base

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
39
Which of the following terms describes the role of ethyne in the acid-base reaction shown? 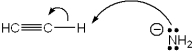
A)Brønsted-Lowry acid
B)Brønsted-Lowry base
C)Lewis acid
D)Lewis base

A)Brønsted-Lowry acid
B)Brønsted-Lowry base
C)Lewis acid
D)Lewis base

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
40
Which of the following is present in the highest concentration upon dissolution of acetic acid in water?
A)OH-
B)H3O+
C)CH3COOH
D)CH3COOH+
A)OH-
B)H3O+
C)CH3COOH
D)CH3COOH+

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
41
Which of the following has the highest bond dissociation energy?
A)HF
B)HCl
C)HBr
D)HI
A)HF
B)HCl
C)HBr
D)HI

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
42
Which of the following energy diagrams best represents the changes in energy during addition of HBr to an alkene? 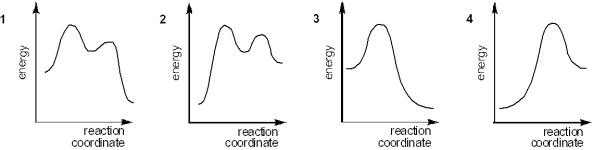
A)1
B)2
C)3
D)4

A)1
B)2
C)3
D)4

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
43
Which of the following has a pKa value of approximately 25?
A)CH3CH3
B)CH2=CH2
C)HC CH
D)CH3CH2OH
A)CH3CH3
B)CH2=CH2
C)HC CH
D)CH3CH2OH

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
44
Which of the following equations is correct?
A)" G = H - T S "
B)" H = G - T S "
C)" G = H - S "
D)" G = H - S /T"
A)" G = H - T S "
B)" H = G - T S "
C)" G = H - S "
D)" G = H - S /T"

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
45
Which of the following has a pKa value of approximately 16?
A)HBr
B)CH3COOH
C)CH3CH2OH
D)HC CH
A)HBr
B)CH3COOH
C)CH3CH2OH
D)HC CH

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
46
In the following list, there are two organic Lewis bases. 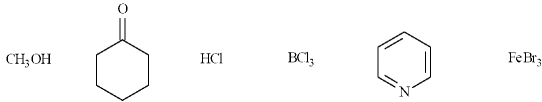


Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
47
Under which of the following conditions will a reaction be spontaneous when H > 0?
A)"T S = 0"
B)"T S > H "
C)"T S < 0 "
D)" G = 0"
A)"T S = 0"
B)"T S > H "
C)"T S < 0 "
D)" G = 0"

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
48
Which of the following concepts can be used to rationalize the observation that acetic acid is a stronger acid than methanol?
A)electronegativity
B)resonance
C)valence shell electron pair repulsion theory
D)Pauli exclusion principle
A)electronegativity
B)resonance
C)valence shell electron pair repulsion theory
D)Pauli exclusion principle

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
49
What is the approximate pKa value of HCl?
A)-7
B)5
C)16
D)51
A)-7
B)5
C)16
D)51

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
50
Which of the following statements is not true?
A)"The position of the equilibrium for an exergonic reaction favors products"
B)"The products of an exergonic reaction have a higher Gibbs free energy than the reactants."
C)"The equilibrium constant of a reaction for which G = 0 is 1."
D)" G = H -T S "
A)"The position of the equilibrium for an exergonic reaction favors products"
B)"The products of an exergonic reaction have a higher Gibbs free energy than the reactants."
C)"The equilibrium constant of a reaction for which G = 0 is 1."
D)" G = H -T S "

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
51
Which of the following is a definition of the rate-determining step of a reaction mechanism?
A)the first step
B)the last step
C)the step that crosses the highest energy barrier
D)the most exothermic step
A)the first step
B)the last step
C)the step that crosses the highest energy barrier
D)the most exothermic step

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
52
An acid with a low pKa is a strong acid and has a weak conjugate base.

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
53
Which of the following energy diagrams represents the fastest reaction? 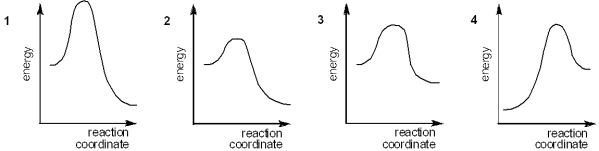
A)1
B)2
C)3
D)4

A)1
B)2
C)3
D)4

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
54
What is the approximate pKa value of acetic acid?
A)-7
B)5
C)16
D)51
A)-7
B)5
C)16
D)51

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
55
Which of the following is a feature of a Brønsted-Lowry base?
A)proton donor
B)proton acceptor
C)electron pair donor
D)electron pair acceptor
A)proton donor
B)proton acceptor
C)electron pair donor
D)electron pair acceptor

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
56
Which of the following is a feature of a Brønsted-Lowry acid?
A)proton donor
B)proton acceptor
C)electron pair donor
D)electron pair acceptor
A)proton donor
B)proton acceptor
C)electron pair donor
D)electron pair acceptor

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
57
In the following list, there are four Lewis acids, CH3OH, HCl, BCl3 and FeBr3. 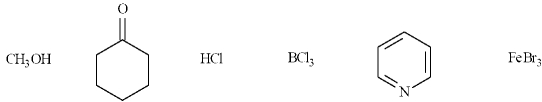


Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
58
Which atom in the following structure is preferentially protonated by a strong acid? 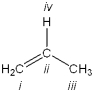
A)i
B)ii
C)iii
D)iv

A)i
B)ii
C)iii
D)iv

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
59
Which of the following energy diagrams represents the slowest reaction? 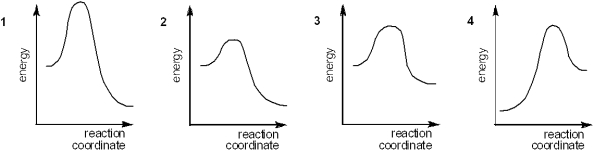
A)1
B)2
C)3
D)4

A)1
B)2
C)3
D)4

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
60
Which of the following is a definition of the activation energy of a reaction?
A)the difference in Gibbs free energy between the reactants and the transition state
B)the difference in Gibbs free energy between the reactants and the intermediate
C)the difference in Gibbs free energy between the reactants and the product
D)the difference in Gibbs free energy between the transition state and the product
A)the difference in Gibbs free energy between the reactants and the transition state
B)the difference in Gibbs free energy between the reactants and the intermediate
C)the difference in Gibbs free energy between the reactants and the product
D)the difference in Gibbs free energy between the transition state and the product

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
61
The following correctly shows the electron flow for the given reaction. 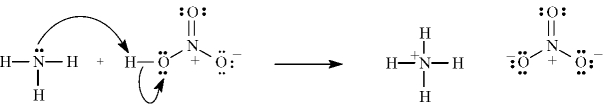


Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
62
A species that lies at an energy maximum during an individual step in a reaction is termed ____________________.

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
63
Complete the equation below for the protonation of cyclohexene with HCl. Show the movement of pairs of electrons with curved arrows and provide the structures of the conjugate acid and conjugate base. 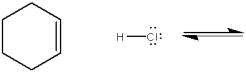
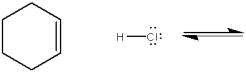

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
64
____________________ is defined by the following equation.
G = -RT ln Keq
G = -RT ln Keq

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
65
A reaction in which the free energy of the products is greater than the reactants is termed____________________ .

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
66
Use curved arrows to show the movement of pairs of electrons in the following acid-base reaction and show the structures of the conjugate acid and conjugate base. 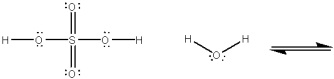


Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
67
Complete the equation below for the protonation of 2-butene with HBr. Show the movement of pairs of electrons with curved arrows and provide the structures of the conjugate acid and conjugate base. 


Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
68
Which acid has the strongest conjugate base?

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
69
Consider the following reaction coordinate diagram. 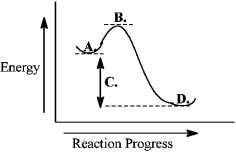 This reaction is endergonic.
This reaction is endergonic.
 This reaction is endergonic.
This reaction is endergonic.
Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
70
The following is generic depiction of a reaction using the curve arrow formalism. 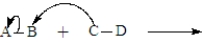 In this reaction electrons move from C to B and A will have a positive charge in the product.
In this reaction electrons move from C to B and A will have a positive charge in the product.
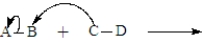 In this reaction electrons move from C to B and A will have a positive charge in the product.
In this reaction electrons move from C to B and A will have a positive charge in the product.
Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
71
A reaction where G is positive is _____________.

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
72
Which acid will be almost completely deprotonated by NaOH?

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
73
Consider the following reaction coordinate diagram. 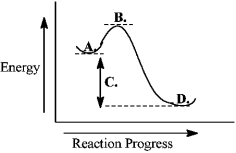 The free energy of activation is represented by the letter C.
The free energy of activation is represented by the letter C.
 The free energy of activation is represented by the letter C.
The free energy of activation is represented by the letter C.
Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
74
Use curved arrows to show the movement of pairs of electrons in the following acid-base reaction and show the structures of the conjugate acid and conjugate base. 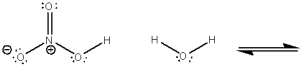


Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
75
Which acid has the smallest Ka?

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
76
The structure of the alkaloid ephedrine is shown below. When ephedrine is placed in the presence of an acid, the -OH group will be protonated. 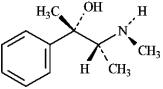


Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
77
Indole is pleasant smelling in highly dilute solutions and has been used in perfumery. 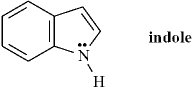 When indole is placed in the presence of a strong base the nitrogen atom will be deprotonated.
When indole is placed in the presence of a strong base the nitrogen atom will be deprotonated.
 When indole is placed in the presence of a strong base the nitrogen atom will be deprotonated.
When indole is placed in the presence of a strong base the nitrogen atom will be deprotonated.
Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
78
Consider the following reaction coordinate diagram. 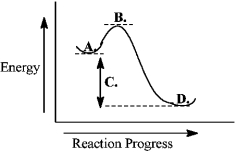 The transition state is represented by the letter B.
The transition state is represented by the letter B.
 The transition state is represented by the letter B.
The transition state is represented by the letter B.
Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
79
Indole is pleasant smelling in highly dilute solutions and has been used in perfumery. 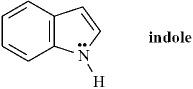 Indole can be classified as a Brønsted-Lowry base but not as a Lewis base..
Indole can be classified as a Brønsted-Lowry base but not as a Lewis base..
 Indole can be classified as a Brønsted-Lowry base but not as a Lewis base..
Indole can be classified as a Brønsted-Lowry base but not as a Lewis base..
Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck
80
The energy needed by reactants to reach the transition state is the____________________,

Unlock Deck
Unlock for access to all 94 flashcards in this deck.
Unlock Deck
k this deck


