Deck 5: Alkenes: Bonding, Nomenclature, and Properties
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/86
Play
Full screen (f)
Deck 5: Alkenes: Bonding, Nomenclature, and Properties
1
What is the approximate value of the C-C-C bond angle in propene?
A)90
B)109
C)120
D)180
A)90
B)109
C)120
D)180
120
2
What is the hybridization of carbon atoms labeled i - iii in the following structure? 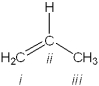
A)i = sp2; ii = sp2; iii = sp2
B)i = sp; ii = sp; iii = sp3
C)i = sp2; ii = sp; iii = sp3
D)i = sp2; ii = sp2; iii = sp3

A)i = sp2; ii = sp2; iii = sp2
B)i = sp; ii = sp; iii = sp3
C)i = sp2; ii = sp; iii = sp3
D)i = sp2; ii = sp2; iii = sp3
i = sp2; ii = sp2; iii = sp3
3
What is the index of hydrogen deficiency of a compound with a molecular formula of C5H10?
A)0
B)1
C)2
D)3
A)0
B)1
C)2
D)3
1
4
What type of molecular orbital is formed by the overlap of atomic orbitals shown? 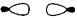
A)" bonding"
B)" antibonding"
C)" bonding"
D)" antibonding"
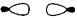
A)" bonding"
B)" antibonding"
C)" bonding"
D)" antibonding"

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
5
What is the approximate value of the length of the carbon-carbon bond in ethene?
A)121 pm
B)134 pm
C)142 pm
D)154 pm
A)121 pm
B)134 pm
C)142 pm
D)154 pm

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
6
What is the index of hydrogen deficiency of a compound with a molecular formula of C6H15N?
A)0
B)1
C)2
D)3
A)0
B)1
C)2
D)3

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
7
What is the index of hydrogen deficiency of a compound with a molecular formula of C6H8?
A)0
B)1
C)2
D)3
A)0
B)1
C)2
D)3

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
8
What is the approximate value of the H-C-H bonds angle in ethene?
A)90
B)109
C)120
D)180
A)90
B)109
C)120
D)180

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
9
Which of the following does not correspond to a molecular formula of a neutral compound?
A)C6H9NO2
B)C5H7N2O2
C)C8H12O3
D)C7H7N
A)C6H9NO2
B)C5H7N2O2
C)C8H12O3
D)C7H7N

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
10
What is the index of hydrogen deficiency of a compound with a molecular formula of C6H12N2?
A)0
B)1
C)2
D)3
A)0
B)1
C)2
D)3

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
11
What type of molecular orbital is formed by the overlap of atomic orbitals shown? 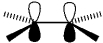
A)" bonding"
B)" antibonding"
C)" bonding"
D)" antibonding"

A)" bonding"
B)" antibonding"
C)" bonding"
D)" antibonding"

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
12
What type of molecular orbital is formed by the overlap of atomic orbitals shown? 
A)" bonding"
B)" antibonding"
C)" bonding"
D)" antibonding"
A)" bonding"
B)" antibonding"
C)" bonding"
D)" antibonding"

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
13
Which of the following compounds has a hydrogen deficiency of 3? 
A)only 1
B)only 2
C)only 3 and 4
D)only 1,3 and 4

A)only 1
B)only 2
C)only 3 and 4
D)only 1,3 and 4

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
14
Which of the following molecular formulae corresponds to a compound with a hydrogen deficiency of 1?
A)C5H8N2
B)C5H11N
C)C6H11N
D)C5H13NO2
A)C5H8N2
B)C5H11N
C)C6H11N
D)C5H13NO2

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
15
What is the index of hydrogen deficiency of a compound with a molecular formula of C5H9Br?
A)0
B)1
C)2
D)3
A)0
B)1
C)2
D)3

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
16
Which of the following compounds does not have a hydrogen deficiency of 5? 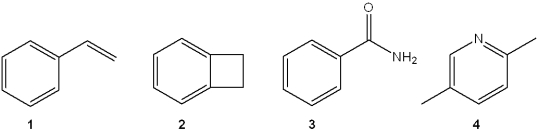
A)1
B)2
C)3
D)4

A)1
B)2
C)3
D)4

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
17
What is the hybridization of carbon atoms labeled i - iii in the following structure? 
A)i = sp; ii = sp; iii = sp
B)i = sp2; ii = p; iii = sp2
C)i = sp2; ii = sp; iii = sp2
D)i = sp2; ii = sp2; iii = sp2
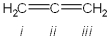
A)i = sp; ii = sp; iii = sp
B)i = sp2; ii = p; iii = sp2
C)i = sp2; ii = sp; iii = sp2
D)i = sp2; ii = sp2; iii = sp2

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
18
Which of the following molecular formulae corresponds to a compound with a hydrogen deficiency of 2?
A)C5H8O2
B)C5H10O
C)C6H15N
D)C5H10Br2
A)C5H8O2
B)C5H10O
C)C6H15N
D)C5H10Br2

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
19
Which of the following does not correspond to a molecular formula of a neutral compound?
A)C5H5Br
B)C5H7Cl2
C)C5H5Br3
D)C5H8Cl4
A)C5H5Br
B)C5H7Cl2
C)C5H5Br3
D)C5H8Cl4

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
20
What is the index of hydrogen deficiency of a compound with a molecular formula of C6H7Cl?
A)0
B)1
C)2
D)3
A)0
B)1
C)2
D)3

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
21
What is the name of the following compound? 
A)vinylcyclohexane
B)allylcyclohexane
C)methylenecyclohexane
D)propargylcyclohexane

A)vinylcyclohexane
B)allylcyclohexane
C)methylenecyclohexane
D)propargylcyclohexane

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
22
How many constitutional isomeric alkenes are there with the formula C6H12?
A)9
B)11
C)12
D)13
A)9
B)11
C)12
D)13

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
23
Which is the correct assignment of the names of the following groups? 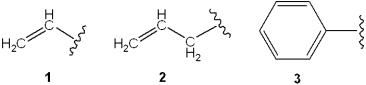
A)1 = methylene; 2 = vinyl; 3 = phenyl
B)1 = vinyl; 2 = propargyl; 3 = benzyl
C)1 = allyl; 2 = vinyl; 3 = benzyl
D)1 = vinyl; 2 = allyl; 3 = phenyl

A)1 = methylene; 2 = vinyl; 3 = phenyl
B)1 = vinyl; 2 = propargyl; 3 = benzyl
C)1 = allyl; 2 = vinyl; 3 = benzyl
D)1 = vinyl; 2 = allyl; 3 = phenyl

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
24
What is the IUPAC name of the following compound? 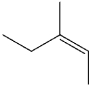
A)(E)-3-methyl-3-pentene
B)(Z)-3-methyl-3-pentene
C)(E)-3-methyl-2-pentene
D)(Z)-3-methyl-2-pentene

A)(E)-3-methyl-3-pentene
B)(Z)-3-methyl-3-pentene
C)(E)-3-methyl-2-pentene
D)(Z)-3-methyl-2-pentene

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
25
Which of the following substituents has the highest priority according to the Cahn-Ingold-Prelog system used in assigning E and Z configurations of carbon-carbon double bonds?
A)"-CH(CH3)2"
B)"-CH2OCH3"
C)"-CH2CH3"
D)"-CH2Br"
A)"-CH(CH3)2"
B)"-CH2OCH3"
C)"-CH2CH3"
D)"-CH2Br"

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
26
What is the IUPAC name of the following compound? 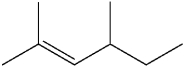
A)1,3-dimethylhexene
B)2,4-dimethyl-2-hexene
C)2,4-dimethyl-1-hexene
D)3,5-dimethyl-4-hexene

A)1,3-dimethylhexene
B)2,4-dimethyl-2-hexene
C)2,4-dimethyl-1-hexene
D)3,5-dimethyl-4-hexene

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
27
What is the IUPAC name of the following compound? 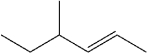
A)(E)-3-methyl-4-hexene
B)(Z)-3-methyl-4-hexene
C)(E)-4-methyl-2-hexene
D)(Z)-4-methyl-2-hexene

A)(E)-3-methyl-4-hexene
B)(Z)-3-methyl-4-hexene
C)(E)-4-methyl-2-hexene
D)(Z)-4-methyl-2-hexene

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
28
What is the IUPAC name of the following compound? 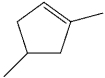
A)1,3-dimethylcyclopentene
B)1,4-dimethylcyclopentene
C)2,4-dimethylcyclopentene
D)1,3-dimethyl-5-cyclopentene

A)1,3-dimethylcyclopentene
B)1,4-dimethylcyclopentene
C)2,4-dimethylcyclopentene
D)1,3-dimethyl-5-cyclopentene

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
29
What is the IUPAC name of the following compound? 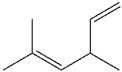
A)2-methyl-4-vinyl-2-pentene
B)3,5-dimethyl-1,4-hexadiene
C)2,4-dimethyl-3,5-hexadiene
D)1,1,3-trimethyl-1,4-pentadiene

A)2-methyl-4-vinyl-2-pentene
B)3,5-dimethyl-1,4-hexadiene
C)2,4-dimethyl-3,5-hexadiene
D)1,1,3-trimethyl-1,4-pentadiene

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
30
What is the IUPAC name of the following compound? ![<strong>What is the IUPAC name of the following compound? </strong> A)bicyclo[5.4.3]-2-octene B)bicyclo[5.4.3]-3-octene C)bicyclo[3.2.1]-2-octene D)2,6-methyleno-1-cyclooctene](https://storage.examlex.com/TB1813/11ea7d75_f5e8_9f90_b9bd_c542051492f5_TB1813_00_TB1813_00.jpg)
A)bicyclo[5.4.3]-2-octene
B)bicyclo[5.4.3]-3-octene
C)bicyclo[3.2.1]-2-octene
D)2,6-methyleno-1-cyclooctene
![<strong>What is the IUPAC name of the following compound? </strong> A)bicyclo[5.4.3]-2-octene B)bicyclo[5.4.3]-3-octene C)bicyclo[3.2.1]-2-octene D)2,6-methyleno-1-cyclooctene](https://storage.examlex.com/TB1813/11ea7d75_f5e8_9f90_b9bd_c542051492f5_TB1813_00_TB1813_00.jpg)
A)bicyclo[5.4.3]-2-octene
B)bicyclo[5.4.3]-3-octene
C)bicyclo[3.2.1]-2-octene
D)2,6-methyleno-1-cyclooctene

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
31
Which of the following does not correspond to a molecular formula of a neutral compound?
A)C4H8BrNO
B)C5H9ClN2O
C)C7H14BrO3
D)C8H14ClN
A)C4H8BrNO
B)C5H9ClN2O
C)C7H14BrO3
D)C8H14ClN

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
32
Which is the correct assignment of the common names of the following compounds? 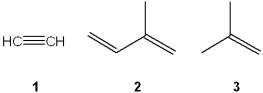
A)1 = ethylene; 2 = isobutylene; 3 = isoprene
B)1 = acetylene; 2 = isoprene; 3 = isobutylene
C)1 = ethylene; 2 = isoprene; 3 = isobutylene
D)1 = acetylene; 2 = isobutylene; 3 = isoprene

A)1 = ethylene; 2 = isobutylene; 3 = isoprene
B)1 = acetylene; 2 = isoprene; 3 = isobutylene
C)1 = ethylene; 2 = isoprene; 3 = isobutylene
D)1 = acetylene; 2 = isobutylene; 3 = isoprene

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
33
Which of the following substituents has the highest priority according to the Cahn-Ingold-Prelog system used in assigning E and Z configurations of carbon-carbon double bonds?
A)"-COOH"
B)"-Cl"
C)"-CH2OH"
D)"-CH3"
A)"-COOH"
B)"-Cl"
C)"-CH2OH"
D)"-CH3"

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
34
What is the name of the following compound? 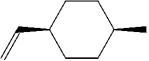
A)cis-4-methyl-1-allylcyclohexane
B)cis-4-allyl-1-methylcyclohexane
C)trans-4-allyl-1-methylcyclohexane
D)cis-4-methyl-1-vinylcyclohexane

A)cis-4-methyl-1-allylcyclohexane
B)cis-4-allyl-1-methylcyclohexane
C)trans-4-allyl-1-methylcyclohexane
D)cis-4-methyl-1-vinylcyclohexane

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
35
How many alkenes have the formula C5H10?
A)4
B)5
C)6
D)7
A)4
B)5
C)6
D)7

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
36
What is the IUPAC name of the following compound? ![<strong>What is the IUPAC name of the following compound? </strong> A)bicyclo[6.2]oct-2-ene B)bicyclo[4.4.4]oct-2-ene C)bicyclo[2.2.2]-2-octene D)bicyclo[2.2]-2-octene](https://storage.examlex.com/TB1813/11ea7d75_f5e8_787f_b9bd_75d087e48e6b_TB1813_00_TB1813_00.jpg)
A)bicyclo[6.2]oct-2-ene
B)bicyclo[4.4.4]oct-2-ene
C)bicyclo[2.2.2]-2-octene
D)bicyclo[2.2]-2-octene
![<strong>What is the IUPAC name of the following compound? </strong> A)bicyclo[6.2]oct-2-ene B)bicyclo[4.4.4]oct-2-ene C)bicyclo[2.2.2]-2-octene D)bicyclo[2.2]-2-octene](https://storage.examlex.com/TB1813/11ea7d75_f5e8_787f_b9bd_75d087e48e6b_TB1813_00_TB1813_00.jpg)
A)bicyclo[6.2]oct-2-ene
B)bicyclo[4.4.4]oct-2-ene
C)bicyclo[2.2.2]-2-octene
D)bicyclo[2.2]-2-octene

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
37
Which is the correct assignment of the names of the following groups? 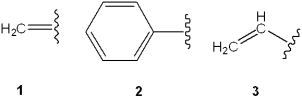
A)1 = vinyl; 2 = benzyl; 3 = allyl
B)1 = allyl; 2 = phenyl; 3 = vinyl
C)1 = methylene; 2 = phenyl; 3 = vinyl
D)1 = methylene; 2 = benzyl; 3 = allyl

A)1 = vinyl; 2 = benzyl; 3 = allyl
B)1 = allyl; 2 = phenyl; 3 = vinyl
C)1 = methylene; 2 = phenyl; 3 = vinyl
D)1 = methylene; 2 = benzyl; 3 = allyl

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
38
What is the IUPAC name of the following compound? 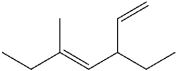
A)(Z)-3-ethyl-5-methyl-1,4-heptadiene
B)(E)-3-ethyl-5-methyl-1,4-heptadiene
C)(E)-3-methyl-5-vinyl-3-heptene
D)(Z)-1,3-diethyl-1-methyl-1,4-pentadiene

A)(Z)-3-ethyl-5-methyl-1,4-heptadiene
B)(E)-3-ethyl-5-methyl-1,4-heptadiene
C)(E)-3-methyl-5-vinyl-3-heptene
D)(Z)-1,3-diethyl-1-methyl-1,4-pentadiene

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
39
What is the IUPAC name of the following compound? 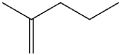
A)2-methylenepentane
B)2-methyl-1-pentene
C)2-vinylpentane
D)2-propyl-1-propene

A)2-methylenepentane
B)2-methyl-1-pentene
C)2-vinylpentane
D)2-propyl-1-propene

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
40
What is the IUPAC name of the following compound? 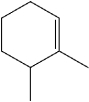
A)1,2-dimethylcyclohexene
B)2,3-dimethyl-1-cyclohexene
C)1,2-dimethyl-2-cyclohexene
D)1,6-dimethylcyclohexene

A)1,2-dimethylcyclohexene
B)2,3-dimethyl-1-cyclohexene
C)1,2-dimethyl-2-cyclohexene
D)1,6-dimethylcyclohexene

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
41
How many isomers of 1,6-heptadiene exist?
A)1
B)2
C)3
D)4
A)1
B)2
C)3
D)4

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
42
The following molecular formula could represent an alkene.
C27H56
C27H56

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
43
How many isomers of 2,4-heptadiene exist?
A)1
B)2
C)3
D)4
A)1
B)2
C)3
D)4

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
44
In the calculation of the index of hydrogen deficiency, when the elements of Group 6 are found in a formula no correction to the number of hydrogen atoms is needed.

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
45
(3E)-3,7-dimethyl-1,3,6-octatriene can be represented by either of the following two formulas. 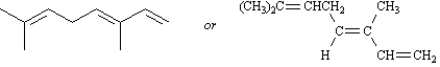
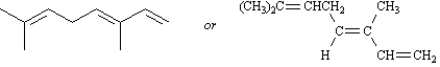

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
46
Which of the following is a terpene? 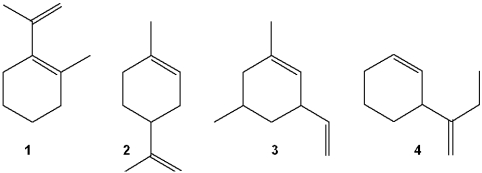
A)1
B)2
C)3
D)4

A)1
B)2
C)3
D)4

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
47
The presence of both rings and pi bonds contribute to the index of hydrogen deficiency in a compound.

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
48
In the carbon-carbon bond of an alkene, a bond is considerably weaker than a bond.

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
49
Which of the following is not a terpene? 
A)1
B)2
C)3
D)4

A)1
B)2
C)3
D)4

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
50
The following compound contains a carbon-carbon double bond with Z stereochemistry. 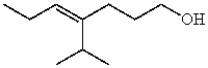


Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
51
Consider the following two structures. 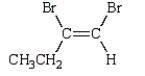
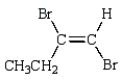 The structure on the left represents the more stable form of this compound.
The structure on the left represents the more stable form of this compound.

 The structure on the left represents the more stable form of this compound.
The structure on the left represents the more stable form of this compound.
Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
52
Which of the following compounds is most reactive (least stable)?
a.(Z)-cycloheptene
b.(E)-cycloheptene
c.(Z)-cyclooctene
d.(E)-cyclooctene
a.(Z)-cycloheptene
b.(E)-cycloheptene
c.(Z)-cyclooctene
d.(E)-cyclooctene

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
53
How many isomers of 1,3-heptadiene exist?
A)1
B)2
C)3
D)4
A)1
B)2
C)3
D)4

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
54
What types of units make up terpenes?
A)isoprene
B)propene
C)vitamin A
D)pentadiene
A)isoprene
B)propene
C)vitamin A
D)pentadiene

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
55
Which of the following is not true regarding alkenes?
A)Alkenes are non polar.
B)Alkenes burn in air to give H2O and CO2.
C)Alkenes are highly miscible with water.
D)The strongest intermolecular force between alkene molecules is the van der Waals interaction.
A)Alkenes are non polar.
B)Alkenes burn in air to give H2O and CO2.
C)Alkenes are highly miscible with water.
D)The strongest intermolecular force between alkene molecules is the van der Waals interaction.

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
56
The structure for 2,3-dimethyl-2-pentene is shown below. 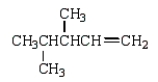


Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
57
What is the smallest trans cycloalkene that is stable at room temperature?
A)(E)-cyclohexene
B)(E)-cycloheptene
C)(E)-cyclooctene
D)(E)-cyclodecene
A)(E)-cyclohexene
B)(E)-cycloheptene
C)(E)-cyclooctene
D)(E)-cyclodecene

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
58
The following compound contains an allyl group. 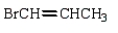
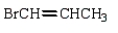

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
59
The following could be named as either trans-2-methylhex-3-ene or (Z)-2-methylhex-3-ene . 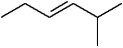


Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
60
How many isomers of 1,3,5-heptatriene exist?
A)1
B)2
C)4
D)8
A)1
B)2
C)4
D)8

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
61
What is the value of the index of hydrogen deficiency of a molecule with the formula C8H19N?

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
62
What is the IUPAC name of the following compound? 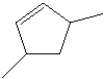


Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
63
What is the IUPAC name of the following compound? 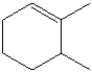


Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
64
What is the IUPAC name of the following compound? 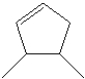


Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
65
What is the value of the index of hydrogen deficiency of aspartame, shown below? 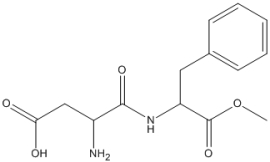


Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
66
What is the IUPAC name of the following compound? 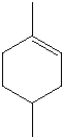


Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
67
What is the IUPAC name of the following compound? 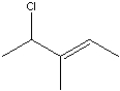


Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
68
What is the IUPAC name of the following compound? 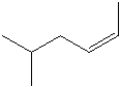


Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
69
What is the value of the index of hydrogen deficiency of a molecule with the formula C8H12?

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
70
What is the value of the index of hydrogen deficiency of a molecule with the formula C9H10?

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
71
Using the EZ system, the following would designated as the ____isomer. 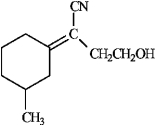


Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
72
A compound with the following molecular formula contains two double bonds. The correct subscript for H in the formula is ________.
C10H?ClN2O
C10H?ClN2O

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
73
What is the IUPAC name of the following compound? 


Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
74
Consider the compounds named below. Use the letter of the substance to answer the following questions. Some letters may be used more than once. Some questions may have more than one correct answer. In the latter case, only one letter need be given.
The compound with the lowest boiling point is represented by the letter ______.
A)propene
B)1-butene
C)2-butene
D)1-pentene
E)2-pentene
The compound with the lowest boiling point is represented by the letter ______.
A)propene
B)1-butene
C)2-butene
D)1-pentene
E)2-pentene

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
75
What is the value of the index of hydrogen deficiency of Ritalin, shown below? 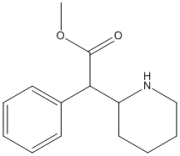


Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
76
What is the IUPAC name of the following compound? 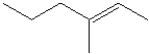


Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
77
Consider the compounds named below. Use the letter of the substance to answer the following questions. Some letters may be used more than once. Some questions may have more than one correct answer. In the latter case, only one letter need be given.
A compound that can exhibit cis-trans isomerism is represented by the letter ______.
A)propene
B)1-butene
C)2-butene
D)1-pentene
E)2-pentene
A compound that can exhibit cis-trans isomerism is represented by the letter ______.
A)propene
B)1-butene
C)2-butene
D)1-pentene
E)2-pentene

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
78
The following substance contains ______ terpene units. 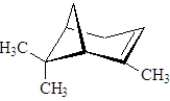


Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
79
Consider the compounds named below. Use the letter of the substance to answer the following questions. Some letters may be used more than once. Some questions may have more than one correct answer. In the latter case, only one letter need be given.
A compound likely to be a liquid at room temperature is represented by the letter ______.
A compound likely to be a liquid at room temperature is represented by the letter ______.

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck
80
What is the value of the index of hydrogen deficiency of a molecule with the formula C7H15N?

Unlock Deck
Unlock for access to all 86 flashcards in this deck.
Unlock Deck
k this deck


