Deck 9: Nucleophilic Substitution and Beta-Elimination
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/111
Play
Full screen (f)
Deck 9: Nucleophilic Substitution and Beta-Elimination
1
The reaction of tert-butyl bromide, (CH3)3CBr, with methanol in an inert solvent proceeds by an SN1 mechanism to give tert-butyl methyl ether, (CH3)3COCH3. What is the effect of doubling the concentration of methanol on the rate of the reaction?
A)the rate remains the same
B)the rate decreases by a factor of 2
C)the rate increases by a factor of 2
D)the rate increases by a factor of 4
A)the rate remains the same
B)the rate decreases by a factor of 2
C)the rate increases by a factor of 2
D)the rate increases by a factor of 4
the rate remains the same
2
What is the equation for the rate of formation of 2-methoxypropane, CH3CH(OCH3)CH3, from the reaction of 2-bromopropane (i-PrBr) with methanol?
A)Rate = k [i-PrBr]
B)Rate = k [i-PrBr]2
C)Rate = k [CH3OH]
D)Rate = k [i-PrBr][CH3OH]
A)Rate = k [i-PrBr]
B)Rate = k [i-PrBr]2
C)Rate = k [CH3OH]
D)Rate = k [i-PrBr][CH3OH]
Rate = k [i-PrBr]
3
Which of the following reactions corresponds to a substitution?
A)propene 1,2-dibromopropane
B)1-iodopropane propene
C)propene propane
D)1-iodopropane 1-bromopropane
A)propene 1,2-dibromopropane
B)1-iodopropane propene
C)propene propane
D)1-iodopropane 1-bromopropane
1-iodopropane 1-bromopropane
4
What is the equation for the rate of formation of tert-butyl alcohol from the reaction of tert-butyl bromide (t-BuBr) with water by an SN1 mechanism?
A)Rate = k [t-BuBr]
B)Rate = k [t-BuBr][H2O]
C)Rate = k [H2O]
D)Rate = k [t-BuBr]2
A)Rate = k [t-BuBr]
B)Rate = k [t-BuBr][H2O]
C)Rate = k [H2O]
D)Rate = k [t-BuBr]2

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
5
Which of the following alkyl halides undergoes the fastest SN2 reaction with sodium azide, NaN3?
A)1-fluorohexane
B)1-chlorohexane
C)1-bromohexane
D)1-iodohexane
A)1-fluorohexane
B)1-chlorohexane
C)1-bromohexane
D)1-iodohexane

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
6
Which of the following alkyl halides undergoes the fastest solvolysis reaction with formic acid, HCOOH?
A)tert-butyl fluoride
B)tert-butyl chloride
C)tert-butyl bromide
D)tert-butyl iodide
A)tert-butyl fluoride
B)tert-butyl chloride
C)tert-butyl bromide
D)tert-butyl iodide

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
7
Which of the following statements related to SN1 reactions is not true?
A)The SN1 reaction can be described as a heterolytic bond cleavage followed by nucleophilic attack
B)Carbocations are electrophilic
C)The charged carbon atom of a carbocation has an unfilled valence shell
D)Nucleophiles are Lewis acids
A)The SN1 reaction can be described as a heterolytic bond cleavage followed by nucleophilic attack
B)Carbocations are electrophilic
C)The charged carbon atom of a carbocation has an unfilled valence shell
D)Nucleophiles are Lewis acids

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
8
Which of the following reactions corresponds to a substitution?
A)tert-butanol tert-butyl chloride
B)tert-butanol 2-methylpropene
C)3,3-dimethyl-2-butanol 2,3-dimethyl-2-butene
D)cyclohexene 1,2-dichlorocyclohexane
A)tert-butanol tert-butyl chloride
B)tert-butanol 2-methylpropene
C)3,3-dimethyl-2-butanol 2,3-dimethyl-2-butene
D)cyclohexene 1,2-dichlorocyclohexane

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
9
Which of the following alkyl halides undergoes the fastest SN2 reaction with sodium methylthiolate, NaSMe?
A)methyl iodide
B)ethyl iodide
C)2-bromopropane
D)tert-butyl chloride
A)methyl iodide
B)ethyl iodide
C)2-bromopropane
D)tert-butyl chloride

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
10
The reaction of methyl iodide with sodium azide, NaN3, proceeds by an SN2 mechanism. What is the effect of doubling the concentration of NaN3 on the rate of the reaction?
A)the rate remains the same
B)the rate decreases by a factor of 2
C)the rate increases by a factor of 2
D)the rate increases by a factor of 4
A)the rate remains the same
B)the rate decreases by a factor of 2
C)the rate increases by a factor of 2
D)the rate increases by a factor of 4

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
11
Which of the following alkyl halides undergoes the fastest solvolysis reaction with methanol, CH3OH?
A)methyl chloride
B)ethyl chloride
C)2-chloropropane
D)tert-butyl chloride
A)methyl chloride
B)ethyl chloride
C)2-chloropropane
D)tert-butyl chloride

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
12
What is the equation for the rate of formation of 1-iodobutane from the reaction of 1-chlorobutane (BuCl) with NaI by an SN2 mechanism?
A)Rate = k [BuCl]
B)Rate = k [BuCl][NaI]
C)Rate = k [NaI]
D)Rate = k [BuCl]2
A)Rate = k [BuCl]
B)Rate = k [BuCl][NaI]
C)Rate = k [NaI]
D)Rate = k [BuCl]2

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
13
Which of the following is the best set of conditions for the preparation of tert-butyl methyl ether?
A)tert-butyl fluoride and NaOCH3 in CH3OH
B)methanol and sodium tert-butoxide in tert-butanol
C)fluoromethane and sodium tert-butoxide in tert-butanol
D)tert-butyl bromide and CH3OH
A)tert-butyl fluoride and NaOCH3 in CH3OH
B)methanol and sodium tert-butoxide in tert-butanol
C)fluoromethane and sodium tert-butoxide in tert-butanol
D)tert-butyl bromide and CH3OH

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
14
Which of the following is best set of conditions for the preparation of tert-butanol?
A)tert-butyl fluoride in water
B)tert-butyl bromide in water
C)tert-butyl fluoride and NaOH in DMSO
D)tert-butyl bromide and NaOH in DMSO
A)tert-butyl fluoride in water
B)tert-butyl bromide in water
C)tert-butyl fluoride and NaOH in DMSO
D)tert-butyl bromide and NaOH in DMSO

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
15
Which of the following alkyl halides undergoes the fastest solvolysis reaction with ethanol, CH3CH2OH?
A)methyl fluoride
B)ethyl bromide
C)2-chloropropane
D)tert-butyl bromide
A)methyl fluoride
B)ethyl bromide
C)2-chloropropane
D)tert-butyl bromide

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
16
The reaction of tert-butyl chloride, (CH3)3CCl, with water in an inert solvent gives tert-butyl alcohol, (CH3)3COH. What is the effect of doubling the concentration of water on the rate of the reaction?
A)the rate remains the same
B)the rate decreases by a factor of 2
C)the rate increases by a factor of 2
D)the rate increases by a factor of 4
A)the rate remains the same
B)the rate decreases by a factor of 2
C)the rate increases by a factor of 2
D)the rate increases by a factor of 4

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
17
Which of the following alkyl halides undergoes the fastest SN2 reaction with sodium cyanide, NaCN?
A)methyl iodide
B)ethyl iodide
C)2-iodopropane
D)tert-butyl iodide
A)methyl iodide
B)ethyl iodide
C)2-iodopropane
D)tert-butyl iodide

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
18
The reaction of 1-bromopropane with sodium iodide gives 1-iodopropane. What is the effect of doubling the concentration of NaI on the rate of the reaction?
A)the rate remains the same
B)the rate decreases by a factor of 2
C)the rate increases by a factor of 2
D)the rate increases by a factor of 4
A)the rate remains the same
B)the rate decreases by a factor of 2
C)the rate increases by a factor of 2
D)the rate increases by a factor of 4

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
19
Which of the following statements related to SN1 reactions is not true?
A)The heterolysis of a bond between atoms which do not bear formal charges always produces a cation and an anion
B)The charged carbon atom of a carbocation has a complete octet of valence shell electrons
C)Carbocations are Lewis acids
D)Nucleophiles seek centers of low electron density
A)The heterolysis of a bond between atoms which do not bear formal charges always produces a cation and an anion
B)The charged carbon atom of a carbocation has a complete octet of valence shell electrons
C)Carbocations are Lewis acids
D)Nucleophiles seek centers of low electron density

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
20
What is the equation for the rate of formation of 2-methoxypropane, (CH3CH(OCH3)CH3, from the reaction of 2-bromopropane (i-PrBr) with sodium methoxide (NaOCH3)?
A)Rate = k [i-PrBr]
B)Rate = k [i-PrBr]2
C)Rate = k [NaOCH3]
D)Rate = k [i-PrBr][NaOCH3]
A)Rate = k [i-PrBr]
B)Rate = k [i-PrBr]2
C)Rate = k [NaOCH3]
D)Rate = k [i-PrBr][NaOCH3]

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
21
What is the major product formed upon treatment of (R) 1-bromo-4-methylhexane with sodium cyanide?
A)(R) 1-cyano-4-methylhexane
B)(S) 1-cyano-4-methylhexane
C)(R) 4-methyl-1-hexene
D)(S) 4-methyl-1-hexene
A)(R) 1-cyano-4-methylhexane
B)(S) 1-cyano-4-methylhexane
C)(R) 4-methyl-1-hexene
D)(S) 4-methyl-1-hexene

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
22
What is(are) the major organic product(s) obtained from the following substitution reaction? 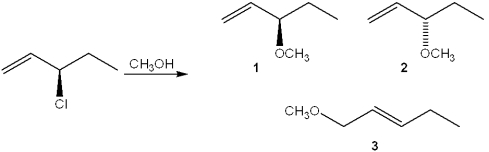
A)only 1
B)only 2
C)only 1 and 2
D)1, 2 and 3

A)only 1
B)only 2
C)only 1 and 2
D)1, 2 and 3

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
23
Which of the following solvents is the best choice for the reaction of 1-chlorohexane with sodium bromide?
A)water
B)N,N-dimethylformamide
C)hexane
D)toluene, PhCH3
A)water
B)N,N-dimethylformamide
C)hexane
D)toluene, PhCH3

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
24
What is the major organic product obtained from the following reaction? 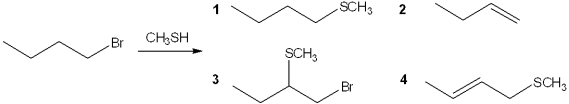
A)1
B)2
C)3
D)4

A)1
B)2
C)3
D)4

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
25
What is the major organic product obtained from the following reaction? 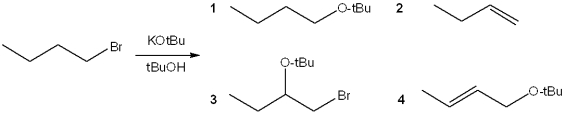
A)1
B)2
C)3
D)4

A)1
B)2
C)3
D)4

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
26
What is(are) the major organic product(s) obtained from the following substitution reaction? 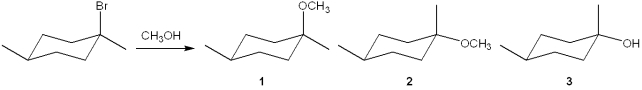
A)only 1
B)only 2
C)only 3
D)a mixture of 1 and 2

A)only 1
B)only 2
C)only 3
D)a mixture of 1 and 2

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
27
What is the major organic product(s) obtained from the following reaction? 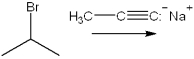
A)3-methyl-1-pentyne
B)3-methyl-2-pentyne
C)1-propene + propyne
D)4-methyl-2-pentene

A)3-methyl-1-pentyne
B)3-methyl-2-pentyne
C)1-propene + propyne
D)4-methyl-2-pentene

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
28
Which of the following anions is the most nucleophilic in polar protic solvents?
A)F-
B)Cl-
C)Br-
D)I-
A)F-
B)Cl-
C)Br-
D)I-

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
29
What is the major organic product obtained from the following reaction? 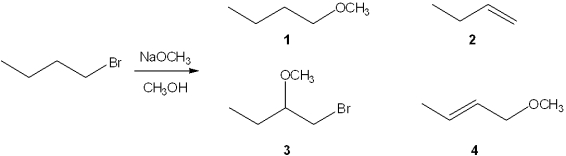
A)1
B)2
C)3
D)4

A)1
B)2
C)3
D)4

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
30
What is the major product formed upon treatment of (R) 2-bromohexane with sodium cyanide?
A)(R) 2-cyanohexane
B)(S) 2-cyanohexane
C)1-hexene
D)2-hexene
A)(R) 2-cyanohexane
B)(S) 2-cyanohexane
C)1-hexene
D)2-hexene

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
31
Which of the following anions is the most nucleophilic in polar aprotic solvents?
A)F-
B)Cl-
C)Br-
D)I-
A)F-
B)Cl-
C)Br-
D)I-

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
32
In which of the following solvents would the reaction of 1-bromobutane with sodium azide, NaN3, proceed the fastest?
A)acetic acid
B)ethanol
C)water
D)acetonitrile
A)acetic acid
B)ethanol
C)water
D)acetonitrile

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
33
What is the major organic product obtained from the following reaction? 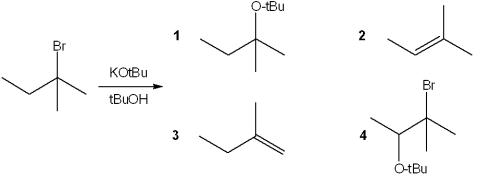
A)1
B)2
C)3
D)4

A)1
B)2
C)3
D)4

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
34
What is(are) the major organic product(s) obtained from the following substitution reaction? 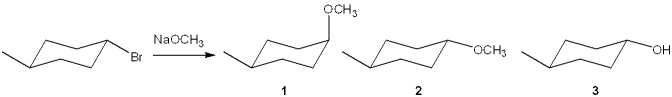
A)only 1
B)only 2
C)only 3
D)a mixture of 1 and 2

A)only 1
B)only 2
C)only 3
D)a mixture of 1 and 2

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
35
What is the major organic product(s) obtained from the following reaction? 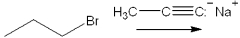
A)1-hexyne
B)2-hexyne
C)propene + propyne
D)propane + propyne
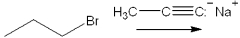
A)1-hexyne
B)2-hexyne
C)propene + propyne
D)propane + propyne

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
36
What is(are) the major organic product(s) obtained from the following substitution reaction? 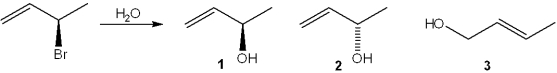
A)only 1
B)only 2
C)only 1 and 2
D)1, 2 and 3

A)only 1
B)only 2
C)only 1 and 2
D)1, 2 and 3

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
37
What is the major organic product obtained from the following reaction? 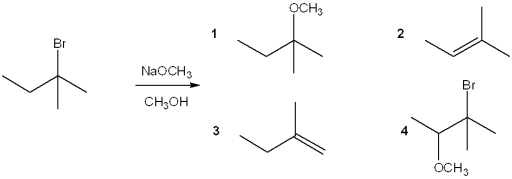
A)1
B)2
C)3
D)4

A)1
B)2
C)3
D)4

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
38
Which of the following is the most nucleophilic?
A)sodium ethoxide
B)acetic acid
C)methanol
D)water
A)sodium ethoxide
B)acetic acid
C)methanol
D)water

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
39
Which of the following compounds is the most nucleophilic in polar protic solvents?
A)H2O
B)NH3
C)H2S
D)PH3
A)H2O
B)NH3
C)H2S
D)PH3

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
40
Which of the following solvents is the best choice for the reaction of 1-chlorohexane with sodium bromide?
A)dimethylsulfoxide
B)water
C)hexane
D)toluene, PhCH3
A)dimethylsulfoxide
B)water
C)hexane
D)toluene, PhCH3

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
41
Which of the following is not a characteristic of SN1 reactions?
A)the electrophilic carbon undergoes inversion of stereochemistry
B)the rate is proportional to the concentration of substrate
C)the reaction proceeds faster in a more polar solvent
D)the rate is independent of the concentration of nucleophile
A)the electrophilic carbon undergoes inversion of stereochemistry
B)the rate is proportional to the concentration of substrate
C)the reaction proceeds faster in a more polar solvent
D)the rate is independent of the concentration of nucleophile

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
42
Which of the following alkyl bromides reacts the slowest with NaSCH3 in DMF? 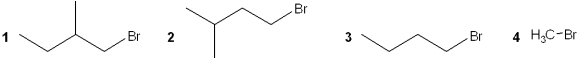
A)1
B)2
C)3
D)4

A)1
B)2
C)3
D)4

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
43
Which of the following represents the transition state of the rate-determining step in the SN1 reaction between tert-butyl chloride and water? 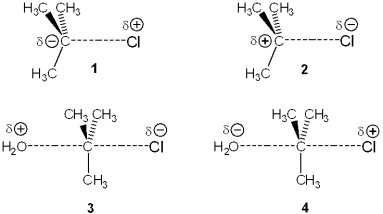
A)1
B)2
C)3
D)4

A)1
B)2
C)3
D)4

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
44
Which of the following statements is true regarding the reactivity of 1 and 2 with potassium tert-butoxide? 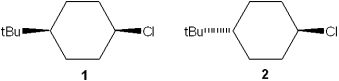
A)1 reacts faster than 2
B)1 and 2 give different dehydrochlorinated products
C)2 reacts by elimination whereas 1 reacts by substitution
D)1 reacts by elimination whereas 2 reacts by substitution

A)1 reacts faster than 2
B)1 and 2 give different dehydrochlorinated products
C)2 reacts by elimination whereas 1 reacts by substitution
D)1 reacts by elimination whereas 2 reacts by substitution

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
45
Which of the following energy diagrams represents the course of an exothermic E1 reaction? 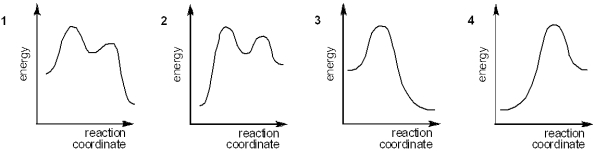
A)1
B)2
C)3
D)4

A)1
B)2
C)3
D)4

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
46
What is the major elimination product obtained from the following reaction? 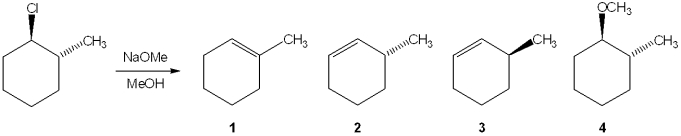
A)1
B)2
C)3
D)4

A)1
B)2
C)3
D)4

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
47
What is the role of tetrabutylammonium chloride as a phase transfer catalyst in the reaction of 1-chlorooctane and sodium cyanide in a mixture of water and CH2Cl2?
A)it transfers 1-chlorooctane into the aqueous phase
B)it transfers cyanide anion into the organic phase
C)it makes water and dichloromethane miscible
D)it transfers the sodium cation into the organic phase
A)it transfers 1-chlorooctane into the aqueous phase
B)it transfers cyanide anion into the organic phase
C)it makes water and dichloromethane miscible
D)it transfers the sodium cation into the organic phase

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
48
Which of the following is most likely to undergo rearrangement during reaction with methanol? 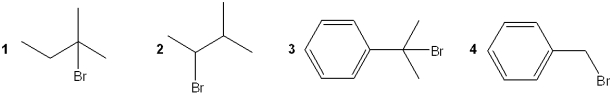
A)1
B)2
C)3
D)4

A)1
B)2
C)3
D)4

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
49
Which of the following anions is the best leaving group in an SN1 reaction?
A)F-
B)HO-
C)NH2-
D)Cl-
A)F-
B)HO-
C)NH2-
D)Cl-

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
50
Which of the following is the best leaving group in an SN2 reaction?
A)F-
B)H3C-
C)CH3O2SO-
D)HO-
A)F-
B)H3C-
C)CH3O2SO-
D)HO-

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
51
Which of the following energy diagrams represents the course of an exothermic SN1 reaction? 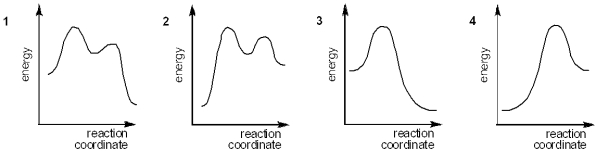
A)1
B)2
C)3
D)4

A)1
B)2
C)3
D)4

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
52
Which of the following energy diagrams represents the course of an exothermic E2 reaction? 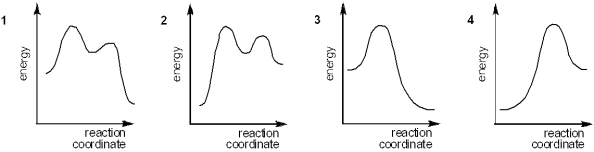
A)1
B)2
C)3
D)4

A)1
B)2
C)3
D)4

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
53
Which of the following statements is true regarding the reaction of tert-butyl bromide with water?
A)the rate is proportional to the concentration of tert-butyl bromide
B)the rate is proportional to the concentration of water
C)the rate is independent of the identity of the solvent
D)the rate of the reaction is independent of the temperature
A)the rate is proportional to the concentration of tert-butyl bromide
B)the rate is proportional to the concentration of water
C)the rate is independent of the identity of the solvent
D)the rate of the reaction is independent of the temperature

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
54
Which of the following is not a characteristic of SN2 reactions?
A)the electrophilic carbon undergoes inversion of stereochemistry
B)the rate is proportional to the concentration of substrate
C)the rate is proportional to the concentration of nucleophile
D)the rate is independent of the solvent
A)the electrophilic carbon undergoes inversion of stereochemistry
B)the rate is proportional to the concentration of substrate
C)the rate is proportional to the concentration of nucleophile
D)the rate is independent of the solvent

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
55
Which of the following compounds undergoes the most rapid hydrolysis reaction? 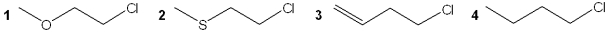
A)1
B)2
C)3
D)4
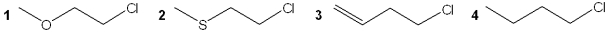
A)1
B)2
C)3
D)4

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
56
Which of the following statements is not true regarding SN2 reactions?
A)A carbocation intermediate is formed.
B)The mechanism has only one step.
C)Aprotic solvents are good choices for SN1 reactions.
D)The stereochemical outcome is inversion at the carbon bearing the leaving group.
A)A carbocation intermediate is formed.
B)The mechanism has only one step.
C)Aprotic solvents are good choices for SN1 reactions.
D)The stereochemical outcome is inversion at the carbon bearing the leaving group.

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
57
Which of the following statements is not true regarding SN1 reactions?
A)A carbocation intermediate is formed.
B)The mechanism has only one step.
C)Polar, protic solvents are good choices for SN1 reactions.
D)The stereochemical outcome is racemization at the carbon bearing the leaving group.
A)A carbocation intermediate is formed.
B)The mechanism has only one step.
C)Polar, protic solvents are good choices for SN1 reactions.
D)The stereochemical outcome is racemization at the carbon bearing the leaving group.

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
58
Which of the following statements is not true regarding the SN2 reaction of (R)-2-bromobutane with sodium cyanide?
A)the reaction proceeds with inversion of configuration
B)the rate is proportional to the concentration of sodium cyanide
C)the rate is proportional to the concentration of (R)-2-bromobutane
D)the rate of the reaction is independent of the identity of the solvent
A)the reaction proceeds with inversion of configuration
B)the rate is proportional to the concentration of sodium cyanide
C)the rate is proportional to the concentration of (R)-2-bromobutane
D)the rate of the reaction is independent of the identity of the solvent

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
59
Which of the following energy diagrams represents the course of an exothermic SN2 reaction? 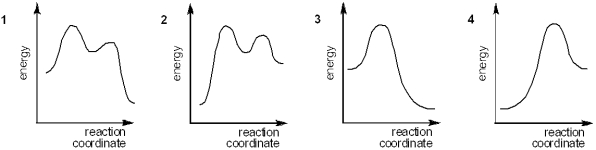
A)1
B)2
C)3
D)4

A)1
B)2
C)3
D)4

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
60
What is the major organic product obtained from the following reaction? 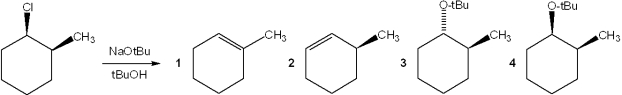
A)1
B)2
C)3
D)4

A)1
B)2
C)3
D)4

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
61
Which of the following represents the transition state of the rate-determining step in the reaction between tert-butyl iodide and water leading to elimination? 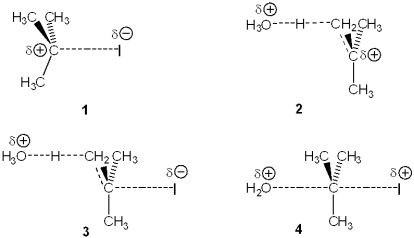
A)1
B)2
C)3
D)4

A)1
B)2
C)3
D)4

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
62
Which of the following sets consists of only polar aprotic solvents?
A)water, hexane, methanol
B)acetic acid, DMF, toluene
C)DMSO, ethanol, acetonitrile
D)DMF, acetonitrile, DMSO
A)water, hexane, methanol
B)acetic acid, DMF, toluene
C)DMSO, ethanol, acetonitrile
D)DMF, acetonitrile, DMSO

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
63
What is the best choice of reagent to perform the following transformation? 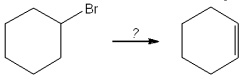
A)H2SO4
B)H2O
C)NaOCH3
D)KOtBu

A)H2SO4
B)H2O
C)NaOCH3
D)KOtBu

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
64
Which of the following sets consists of only polar protic solvents?
A)water, DMF, DMSO
B)acetic acid, methanol, water
C)DMSO, ethanol, acetonitrile
D)DMF, acetonitrile, DMSO
A)water, DMF, DMSO
B)acetic acid, methanol, water
C)DMSO, ethanol, acetonitrile
D)DMF, acetonitrile, DMSO

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
65
The rate law for the following reaction would be of the form Rate = k[A][B]. ![The rate law for the following reaction would be of the form Rate = k[A][B].](https://storage.examlex.com/TB1813/11ea7d75_f5fc_00ae_b9bd_63b901bf68bf_TB1813_00_TB1813_00.jpg)
![The rate law for the following reaction would be of the form Rate = k[A][B].](https://storage.examlex.com/TB1813/11ea7d75_f5fc_00ae_b9bd_63b901bf68bf_TB1813_00_TB1813_00.jpg)

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
66
The following energy diagram could represent an SN2 reaction. 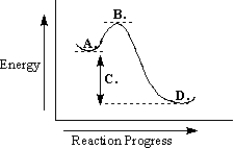


Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
67
The following reaction would be classified as an elimination. 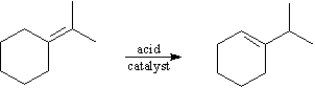


Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
68
Which of the following most favors elimination rather substitution in a reaction with sodium methoxide?
A)bromomethane
B)bromoethane
C)1-bromopropane
D)2-bromopropane
A)bromomethane
B)bromoethane
C)1-bromopropane
D)2-bromopropane

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
69
Which of the following represents the transition state of the rate-determining step in the reaction between tert-butyl bromide and methanol? 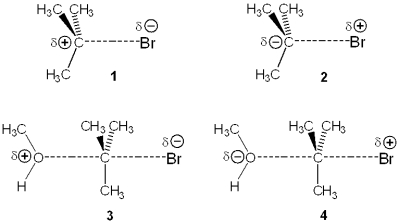
A)1
B)2
C)3
D)4

A)1
B)2
C)3
D)4

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
70
The following reaction would have a faster rate in a higher polarity solvent than one of low polarity. 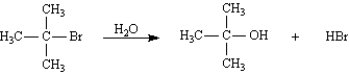


Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
71
In the following reaction the cyanide ion is both a nucleophile and a Lewis acid. 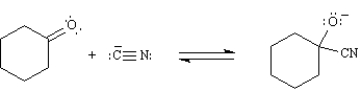
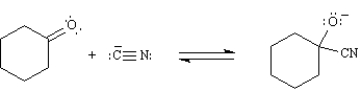

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
72
HCl is the electrophile in the following reaction. 


Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
73
Which of the following represents the transition state of the reaction between methyl iodide and ammonia? 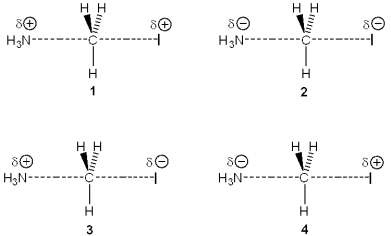
A)1
B)2
C)3
D)4

A)1
B)2
C)3
D)4

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
74
The first step in the mechanism for the following reaction would be the formation of a secondary carbocation. 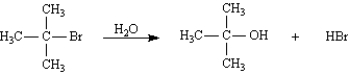


Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
75
Which of the following represents the transition state of the rate-determining step in the reaction between 2-chloropropane and sodium amide leading to elimination? 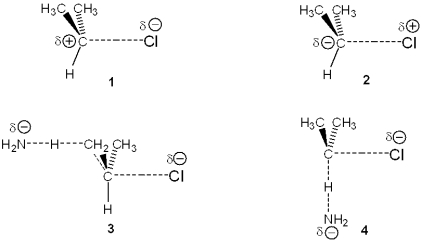
A)1
B)2
C)3
D)4

A)1
B)2
C)3
D)4

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
76
Which of the following represents the transition state of the rate-determining step in the reaction between tert-butyl bromide and methanol leading to elimination? 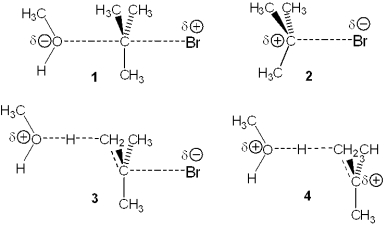
A)1
B)2
C)3
D)4

A)1
B)2
C)3
D)4

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
77
What is the best choice of reagent to perform the following transformation? 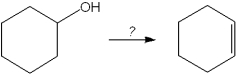
A)H2SO4
B)H2O
C)NaOCH3
D)KOtBu

A)H2SO4
B)H2O
C)NaOCH3
D)KOtBu

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
78
Which of the following most favors elimination rather substitution in a reaction with 2-bromopropane?
A)sodium methoxide
B)sodium ethoxide
C)sodium isoproxide
D)sodium tert-butoxide
A)sodium methoxide
B)sodium ethoxide
C)sodium isoproxide
D)sodium tert-butoxide

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
79
Which of the following represents the transition state of the rate-determining step in the reaction between 2-bromopropane and sodium methoxide leading to elimination? 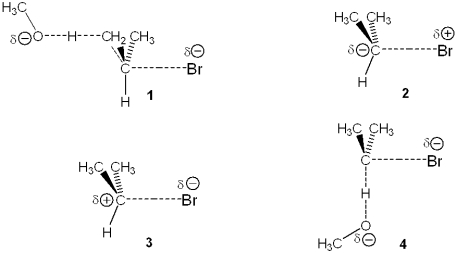
A)1
B)2
C)3
D)4

A)1
B)2
C)3
D)4

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck
80
Which of the following represents the transition state of the reaction between methyl bromide and sodium methylthiolate, NaSCH3? 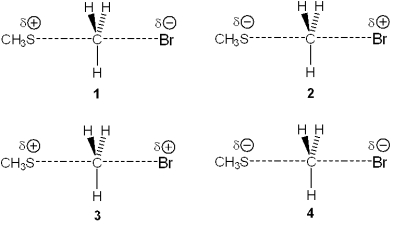
A)1
B)2
C)3
D)4

A)1
B)2
C)3
D)4

Unlock Deck
Unlock for access to all 111 flashcards in this deck.
Unlock Deck
k this deck


