Deck 16: Reactions of Carboxylic Acids and Carboxylic Acid Derivatives
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question
Question

Unlock Deck
Sign up to unlock the cards in this deck!
Unlock Deck
Unlock Deck
1/128
Play
Full screen (f)
Deck 16: Reactions of Carboxylic Acids and Carboxylic Acid Derivatives
1
Provide the proper IUPAC name for the compound below. 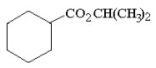

isopropyl cyclohexanecarboxylate
2
Which of the following compounds is g-butyrolactone? 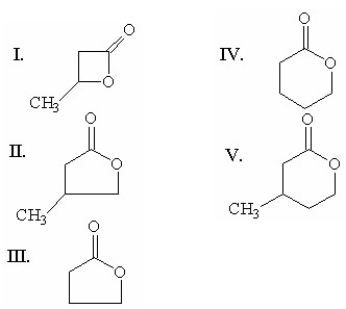
A)I
B)II
C)III
D)IV
E)V

A)I
B)II
C)III
D)IV
E)V
III
3
Which of the following compounds is an amide? 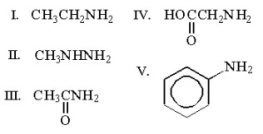
A)I
B)II
C)III
D)IV
E)V

A)I
B)II
C)III
D)IV
E)V
III
4
Provide the proper IUPAC name for the compound below. 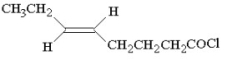


Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
5
Which of the following compounds is an anhydride? 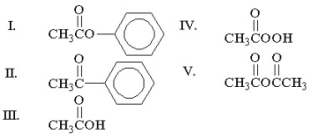
A)I
B)II
C)III
D)IV
E)V

A)I
B)II
C)III
D)IV
E)V

Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
6
What is the common name for the following compound? 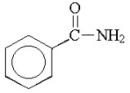
A)phenylcarbamide
B)benzamide
C)phenylmethanamide
D)benzeneamide
E)benzylamide

A)phenylcarbamide
B)benzamide
C)phenylmethanamide
D)benzeneamide
E)benzylamide

Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
7
Provide the proper IUPAC name for ClCH2CH2CONHCH3.

Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
8
Which of the following compounds is an acyl chloride? 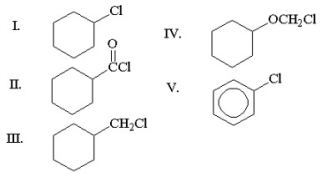
A)I
B)II
C)III
D)IV
E)V

A)I
B)II
C)III
D)IV
E)V

Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
9
Which of the following compounds is benzoyl chloride? 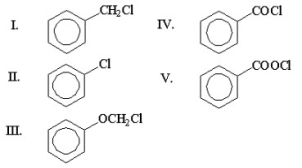
A)I
B)II
C)III
D)IV
E)V

A)I
B)II
C)III
D)IV
E)V

Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
10
Which of the following compounds is an ester? 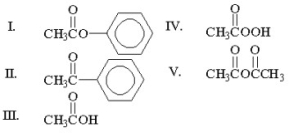
A)I
B)II
C)III
D)IV
E)V

A)I
B)II
C)III
D)IV
E)V

Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
11
Provide the structure of propanoic anhydride.

Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
12
What is the common name for the following compound? 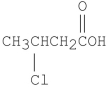
A)λ-chlorobutanoic acid
B)β-chlorobutanoic acid
C)β-chlorobutyric acid
D)λ-chlorobutyric acid
E)3-chlorobutyric acid

A)λ-chlorobutanoic acid
B)β-chlorobutanoic acid
C)β-chlorobutyric acid
D)λ-chlorobutyric acid
E)3-chlorobutyric acid

Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
13
What is the IUPAC name for the following compound? 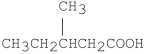
A)3-methylpentanoic acid
B)isohexanoic acid
C)β-methylvaleric acid
D)2-methylpentanoic acid
E)3-methylvaleric acid
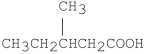
A)3-methylpentanoic acid
B)isohexanoic acid
C)β-methylvaleric acid
D)2-methylpentanoic acid
E)3-methylvaleric acid

Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
14
Which of the following compounds is methyl formate?
A)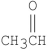
B)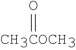
C)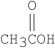
D)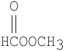
E)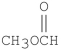
A)

B)
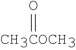
C)
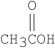
D)
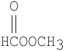
E)
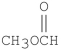

Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
15
Provide the proper IUPAC name for (CH3)3CCH2CH2CH(CN)CH3.

Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
16
Which of the following compounds is D-valerolactam? 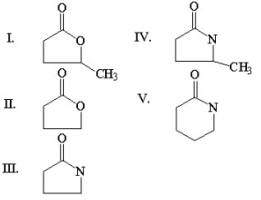
A)I
B)II
C)III
D)IV
E)V

A)I
B)II
C)III
D)IV
E)V

Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
17
What is the IUPAC name for the following compound? 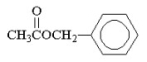
A)phenyl acetate
B)methyl benzoate
C)methyl phenylacetate
D)benzyl acetate
E)benzyl methylacetate

A)phenyl acetate
B)methyl benzoate
C)methyl phenylacetate
D)benzyl acetate
E)benzyl methylacetate

Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
18
What is the common name for the following compound? 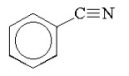
A)benzonitrile
B)benzenecyanide
C)phenylcyanide
D)cyanophenyl
E)benzocyanide

A)benzonitrile
B)benzenecyanide
C)phenylcyanide
D)cyanophenyl
E)benzocyanide

Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
19
What is the common name for the following compound? 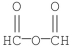
A)propanoic anhydride
B)formic anhydride
C)formyl formate
D)ethanoic anhydride
E)diformyl ether

A)propanoic anhydride
B)formic anhydride
C)formyl formate
D)ethanoic anhydride
E)diformyl ether

Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
20
Which of the following compounds is N,N-dimethylbenzamide? 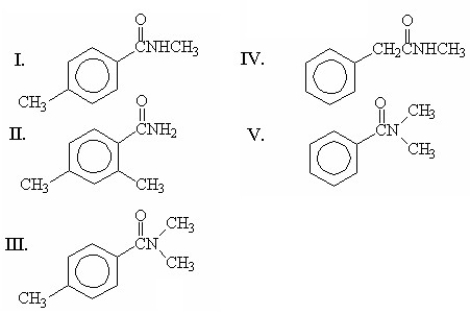
A)I
B)II
C)III
D)IV
E)V

A)I
B)II
C)III
D)IV
E)V

Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
21
Provide the IUPAC name for the organic compound below. 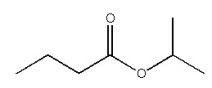


Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
22
Which C-N bond below is shorter, I or II? Explain. 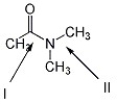


Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
23
Give the name of the structure. 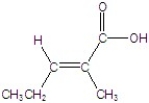
A)Z-2-methyl-2-pentanoic acid
B)E-2-methyl-2-pentanoic acid
C)Z-2-methyl-2-pentenoic acid
D)E-2-methyl-2-pentenoic acid
E)E-2-methyl-2-pentynoic acid

A)Z-2-methyl-2-pentanoic acid
B)E-2-methyl-2-pentanoic acid
C)Z-2-methyl-2-pentenoic acid
D)E-2-methyl-2-pentenoic acid
E)E-2-methyl-2-pentynoic acid

Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
24
Give the systematic name of the structure. 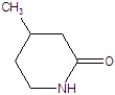


Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
25
Give the name of the structure. 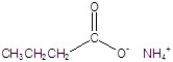
A)amino propanoate
B)ammonium butanoate
C)amino butanoate
D)ammonium propanoate
E)propyl amine

A)amino propanoate
B)ammonium butanoate
C)amino butanoate
D)ammonium propanoate
E)propyl amine

Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
26
Give the name of the structure. 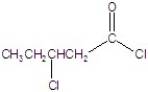
A)1,3-dichloropentanoate
B)3-chloropentanoyl chloride
C)2-chloropentyl chloride
D)2-chloropentanoyl chloride
E)2-chlorobutanoyl chloride

A)1,3-dichloropentanoate
B)3-chloropentanoyl chloride
C)2-chloropentyl chloride
D)2-chloropentanoyl chloride
E)2-chlorobutanoyl chloride

Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
27
Arrange the compounds below in order of increasing boiling point. 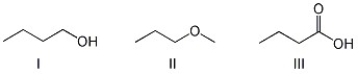


Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
28
Give the name of the structure. 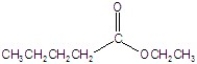
A)ethyl butanoate
B)butyl methyl ether
C)ethyl pentanoate
D)butyl ethanoate
E)pentyl ethanoate

A)ethyl butanoate
B)butyl methyl ether
C)ethyl pentanoate
D)butyl ethanoate
E)pentyl ethanoate

Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
29
Provide the IUPAC name for the organic compound below. 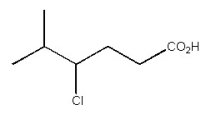


Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
30
Provide the IUPAC name for the organic compound below. 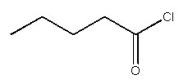


Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
31
What is the hybridization and geometry of the carbonyl carbon in carboxylic acids and their derivatives?
A)sp3, tetrahedral
B)sp2, trigonal planar
C)sp2, tetrahedral
D)sp3, trigonal planar
E)sp, trigonal planar
A)sp3, tetrahedral
B)sp2, trigonal planar
C)sp2, tetrahedral
D)sp3, trigonal planar
E)sp, trigonal planar

Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
32
Provide the name of the compound below. 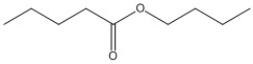


Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
33
Which of the following compounds has the lowest boiling point?
A)1-butanol
B)butanoic acid
C)butanenitrile
D)methyl propanoate
E)butanamide
A)1-butanol
B)butanoic acid
C)butanenitrile
D)methyl propanoate
E)butanamide

Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
34
Give the name of the structure. 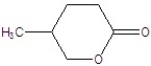
A)4-methyl-2-oxacyclohexanone
B)5-methyl-2-oxacyclohexanone
C)3-methyl-2-oxacyclohexanone
D)4-methyl-2-oxacyclopentanone
E)3-methyl-2-oxacyclopentanone

A)4-methyl-2-oxacyclohexanone
B)5-methyl-2-oxacyclohexanone
C)3-methyl-2-oxacyclohexanone
D)4-methyl-2-oxacyclopentanone
E)3-methyl-2-oxacyclopentanone

Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
35
Provide the systematic name of the compound shown below. 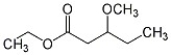


Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
36
Give the name of the structure. 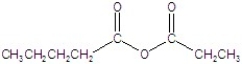
A)butanoic ethanoic anhydride
B)butanoic propanoic anhydride
C)ethanoic pentanoic anhydride
D)pentyl propyl anhydride
E)pentanoic propanoic anhydride

A)butanoic ethanoic anhydride
B)butanoic propanoic anhydride
C)ethanoic pentanoic anhydride
D)pentyl propyl anhydride
E)pentanoic propanoic anhydride

Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
37
Give the name of the structure. 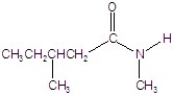
A)N,2-dimethylpentanamide
B)isopentyl methyl amide
C)N,2-dimethylbutanamide
D)N,3-dimethylbutanamide
E)N,3-dimethylpentanamide

A)N,2-dimethylpentanamide
B)isopentyl methyl amide
C)N,2-dimethylbutanamide
D)N,3-dimethylbutanamide
E)N,3-dimethylpentanamide

Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
38
Give the systematic name of the structure. 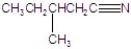
A)3-methylpentanenitrile
B)2-methylpentanenitrile
C)2-methylbutanenitrile
D)3-methylbutanenitrile
E)3-methylpentylnitrile
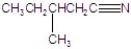
A)3-methylpentanenitrile
B)2-methylpentanenitrile
C)2-methylbutanenitrile
D)3-methylbutanenitrile
E)3-methylpentylnitrile

Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
39
In which of the following compounds does the carbonyl stretch in the IR spectrum occur at the lowest wavenumber?
A)cyclohexanone
B)ethyl acetate
C)propanoyl chloride
D)pentanamide
E)λ-butyrolactone
A)cyclohexanone
B)ethyl acetate
C)propanoyl chloride
D)pentanamide
E)λ-butyrolactone

Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
40
Which of the following statements is not true about fatty acids?
A)Fatty acids are carboxylic acids with long hydrocarbon side chains.
B)The double bonds in unsaturated fatty acids are always conjugated.
C)Most naturally occurring fatty acids contain even numbers of carbons and are unbranched.
D)Fatty acids can be saturated or unsaturated.
E)Physical properties of fatty acids depend on the length of the hydrocarbon chain and the degree of unsaturation.
A)Fatty acids are carboxylic acids with long hydrocarbon side chains.
B)The double bonds in unsaturated fatty acids are always conjugated.
C)Most naturally occurring fatty acids contain even numbers of carbons and are unbranched.
D)Fatty acids can be saturated or unsaturated.
E)Physical properties of fatty acids depend on the length of the hydrocarbon chain and the degree of unsaturation.

Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
41
Which of the following compounds is hydrolyzed most slowly in aqueous NaOH?
A)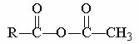
B)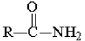
C)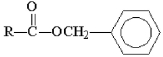
D)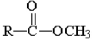
E)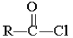
A)

B)
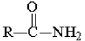
C)
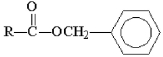
D)
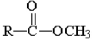
E)


Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
42
Which of the following compounds is most reactive toward nucleophilic acyl substitution?
A)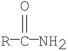
B)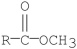
C)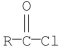
D)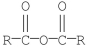
E)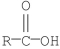
A)
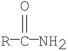
B)
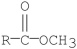
C)
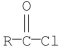
D)
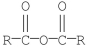
E)
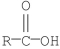

Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
43
Identify the compound that would be formed from the hydrogenation of linolenic acid.
A)palmitic acid
B)lauric acid
C)stearic acid
D)myristic acid
E)linoleic acid
A)palmitic acid
B)lauric acid
C)stearic acid
D)myristic acid
E)linoleic acid

Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
44
Do you expect the following reaction to go to completion? Why or why not? 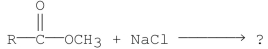
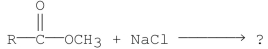

Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
45
Which reaction coordinate diagram best represents the reaction that occurs when NaNH2 reacts with CH3COCl to form acetamide?
A)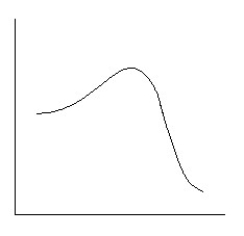
B)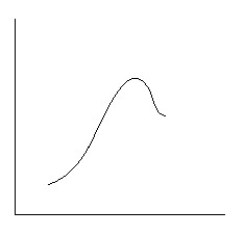
C)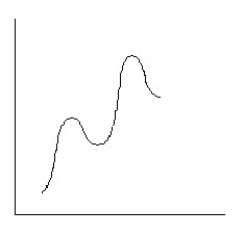
D)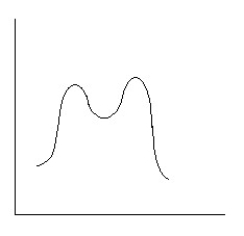
E)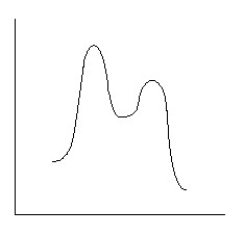
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
46
Which of the following statements is true for both cholesterol and vitamin A?
A)Both are vitamins.
B)Both are proteins.
C)Both are lipids.
D)Both are steroids.
E)Both are fatty acids.
A)Both are vitamins.
B)Both are proteins.
C)Both are lipids.
D)Both are steroids.
E)Both are fatty acids.

Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
47
In an MO depiction of the carbonyl's reactivity with an amine nucleophile, the most important MO overlap occurs between the amine's lone pair and the carbonyl's ________.
A)πc-o
B)π*c-o
C)σc-o
D)σ*c-o
E)no
A)πc-o
B)π*c-o
C)σc-o
D)σ*c-o
E)no

Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
48
In nucleophilic acyl substitution,
A)protonation of the carbonyl is followed immediately by loss of the leaving group.
B)loss of the leaving group is followed by rearrangement of the carbocation.
C)addition to the carbonyl by a nucleophile is followed by loss of the leaving group.
D)ester hydrolysis is followed by deprotonation.
E)an SN2 reaction occurs.
A)protonation of the carbonyl is followed immediately by loss of the leaving group.
B)loss of the leaving group is followed by rearrangement of the carbocation.
C)addition to the carbonyl by a nucleophile is followed by loss of the leaving group.
D)ester hydrolysis is followed by deprotonation.
E)an SN2 reaction occurs.

Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
49
What organic compounds are formed when linoleic acid, CH3(CH2)4CH  CHCH2CH
CHCH2CH  CH(CH2)7CO2H, is ozonolyzed and subsequently treated with H2O2?
CH(CH2)7CO2H, is ozonolyzed and subsequently treated with H2O2?
 CHCH2CH
CHCH2CH  CH(CH2)7CO2H, is ozonolyzed and subsequently treated with H2O2?
CH(CH2)7CO2H, is ozonolyzed and subsequently treated with H2O2?
Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
50
Acetyl chloride undergoes nucleophilic substitution at a faster rate than methyl acetate because ________.
A)the ester is more sterically hindered than the acid chloride
B)the acid chloride is more sterically hindered than the ester
C)the methoxide is a better leaving group than chloride
D)esters hydrolyze faster than acid chlorides
E)chloride is a better leaving group than methoxide
A)the ester is more sterically hindered than the acid chloride
B)the acid chloride is more sterically hindered than the ester
C)the methoxide is a better leaving group than chloride
D)esters hydrolyze faster than acid chlorides
E)chloride is a better leaving group than methoxide

Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
51
Which of the following compounds is the least reactive toward nucleophilic acyl substitution?
A)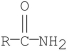
B)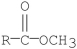
C)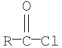
D)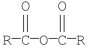
E)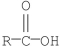
A)
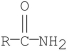
B)
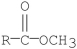
C)
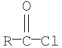
D)
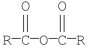
E)
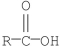

Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
52
Identify the compound with the highest melting point.
A)palmitic acid
B)lauric acid
C)stearic acid
D)myristic acid
E)linoleic acid
A)palmitic acid
B)lauric acid
C)stearic acid
D)myristic acid
E)linoleic acid

Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
53
Which of the following terms best describes the compound below? CH3(CH2)7CH  CH(CH2)7CO2H
CH(CH2)7CO2H
A)an unsaturated fatty acid
B)a triglyceride
C)a synthetic detergent
D)a micelle
E)isoprene
 CH(CH2)7CO2H
CH(CH2)7CO2HA)an unsaturated fatty acid
B)a triglyceride
C)a synthetic detergent
D)a micelle
E)isoprene

Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
54
Palmitic acid (hexadecanoic acid)has a melting point of 63°C. Palmitoleic acid (9-hexadecenoic acid)has a melting point of 32°C. Explain this difference in melting points.

Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
55
Why do most naturally occurring fatty acids contain an even number of carbon atoms?

Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
56
Which of the following best explains why the melting points of saturated fats increase with increasing molecular weight?
A)decreased polarity
B)decreased hydrogen bonding
C)increased hydrogen bonding
D)decreased intermolecular van der Waal's interactions
E)increased intermolecular van der Waal's interactions
A)decreased polarity
B)decreased hydrogen bonding
C)increased hydrogen bonding
D)decreased intermolecular van der Waal's interactions
E)increased intermolecular van der Waal's interactions

Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
57
Which of the following terms best describes the compound below? CH3(CH2)12CO2H
A)a fatty acid
B)an oil
C)a wax
D)a soap
E)a phosphatidic acid
A)a fatty acid
B)an oil
C)a wax
D)a soap
E)a phosphatidic acid

Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
58
Myristic acid has the formula CH3(CH2)12CO2H. Provide the structure of the triglyceride trimyristin.

Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
59
Which of the following fatty acids cannot be synthesized by mammals but must be included in the diet because it is needed to synthesize arachidonic acid which in turn will synthesize prostaglandins?
A)Lauric acid
B)Palmitic acid
C)Arachidic acid
D)Linoleic acid
E)Oleic acid
A)Lauric acid
B)Palmitic acid
C)Arachidic acid
D)Linoleic acid
E)Oleic acid

Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
60
Which of the following compounds is hydrolyzed most rapidly inaqueous NaOH?
A)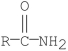
B)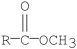
C)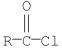
D)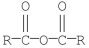
E)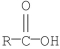
A)
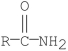
B)
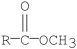
C)
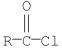
D)
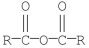
E)
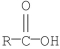

Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
61
Which of the following compounds would yield acetic acid when hydrolyzed under heat?
A)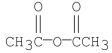
B)CH3C N
N
C)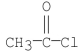
D)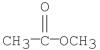
E)all the above
A)
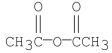
B)CH3C
 N
NC)
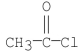
D)
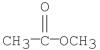
E)all the above

Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
62
Esters and amides are most easily made by nucleophilic acyl substitution reactions on ________.
A)alcohols
B)acid anhydrides
C)carboxylates
D)carboxylic acids
E)acid chlorides
A)alcohols
B)acid anhydrides
C)carboxylates
D)carboxylic acids
E)acid chlorides

Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
63
Provide the structure of the tetrahedral intermediate in the reaction of sodium methoxide with propanoyl chloride.

Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
64
A wax is an ester formed from a long-chain carboxylic acid and a long-chain alcohol. Show how a wax can be made by using CH3(CH2)15OH as the only organic starting material. 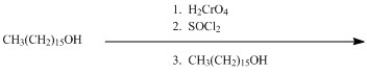
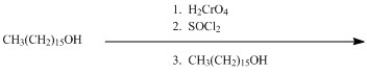

Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
65
Provide the major organic product of the following. 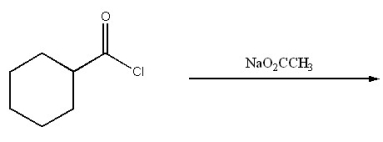


Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
66
Which of the following is the best method for preparing 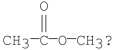
A)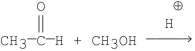
B)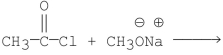
C)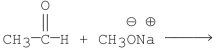
D)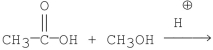
E)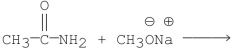
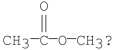
A)
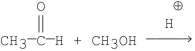
B)
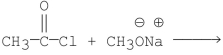
C)
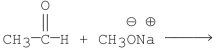
D)
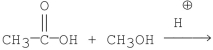
E)
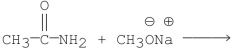

Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
67
Provide the major organic product of the reaction below. 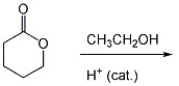


Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
68
Give the product of the reaction. 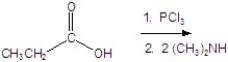
A)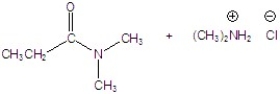
B)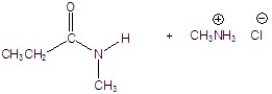
C)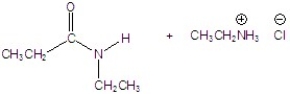
D)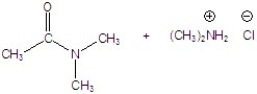
E)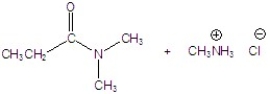
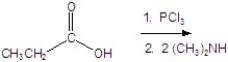
A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
69
Provide a detailed, stepwise mechanism for the reaction of propanoyl chloride with ammonia.

Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
70
Provide the major organic product of the following. 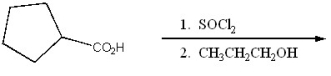
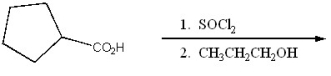

Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
71
Provide a detailed, stepwise mechanism for the reaction of acetyl chloride with methanol to produce methyl acetate and HCl.

Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
72
Give the product of the reaction. 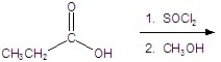
A)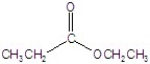
B)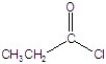
C)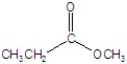
D)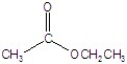
E)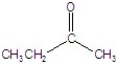

A)

B)

C)

D)

E)


Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
73
Typically, amides will hydrolyze under ________ conditions than esters.
A)milder
B)more dilute
C)more vigorous
D)less vigorous
E)more saline
A)milder
B)more dilute
C)more vigorous
D)less vigorous
E)more saline

Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
74
When benzoyl chloride is reacted with ammonia to prepare benzamide, two equivalents of ammonia must be used. Explain why this is the case.

Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
75
List the following carboxylic acid derivatives in decreasing order of reactivity in nucleophilic acyl substitution reactions: acetic anhydride, methyl acetate, acetamide, acetyl chloride.

Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
76
Provide the major organic product of the reaction below. 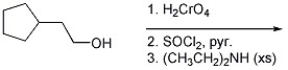


Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
77
Which of the following esters undergoes hydrolysis in base most easily?
A)CH3CH2CO2CH3
B)(CH3)2CHCH2CO2CH3
C)C6H5O2CCH3
D)p-CH3C6H4O2CCH3
E)p-NO2C6H4O2CCH3
A)CH3CH2CO2CH3
B)(CH3)2CHCH2CO2CH3
C)C6H5O2CCH3
D)p-CH3C6H4O2CCH3
E)p-NO2C6H4O2CCH3

Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
78
In the lab, a student tried to prepare N,N-diethylacetamide by heating acetyl chloride with two equivalents of triethylamine. Was the student successful? Explain.

Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
79
Which of the following is (are)formed in the reaction between ethyl butanoate and ethyl amine?
A)ethanol
B)1-butanol
C)N-ethylbutanamide
D)both A and C
E)both B and C
A)ethanol
B)1-butanol
C)N-ethylbutanamide
D)both A and C
E)both B and C

Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck
80
What are the products from the following reaction? 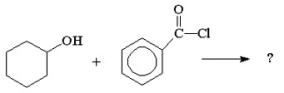


Unlock Deck
Unlock for access to all 128 flashcards in this deck.
Unlock Deck
k this deck


